Abstract
Rationale
Lack of access to conventional sources of reinforcement has been proposed as a risk factor for substance abuse in lower socio-economic populations. There is laboratory evidence that behavioral alternatives (enrichment or exercise) and alternative reinforcers (e.g., sweetened solutions) can reduce self-administration of a variety of drugs.
Objectives
To determine if drug self-administration could devalue wheel activity in an animal model.
Methods
Male Wistar rats were trained to self-administer 3,4-methylenedioxypyrovalerone (MDPV; “bath salts”), 0.05 mg/kg/infusion, i.v., with concurrent access to a running wheel that was either locked (LW) or unlocked (UW).
Results
MDPV intake steadily increased across the 20 session acquisition interval but did not differ significantly between UW and LW groups. Mean wheel rotations declined significantly across the acquisition interval in the UW group. Of the rats that acquired self-administration, 60% engaged in a binge-like behavior at the initiation of acquisition - intake was limited only by post-reinforcement timeout. The Binge rats had higher post acquisition levels of drug intake (even after excluding the binge session) and the UW Binge rats showed a precipitous post-acquisition drop in wheel activity that was not observed in the UW No-Binge rats.
Conclusions
These data confirm that MDPV is a powerful reward/reinforcer and show that a relatively high rate of intake at the onset of drug taking can devalue natural rewards (wheel activity) and can predict higher subsequent drug intake levels. Thus, limiting the intensity of initial drug exposure may attenuate subsequent drug abuse/addiction by preventing the devaluation of natural alternative rewards/reinforcers.
Keywords: stimulants, drug abuse, exercise, self-administration, cathinone, reward
1. Introduction
Impoverishment of access to conventional sources of reinforcement may be a risk factor for substance abuse, particularly in lower socio-economic populations (Richman 1977; Wall et al. 2011), possibly because drugs eventually come to out-compete available naturalistic rewards. Human substance dependence is a minority outcome within the population exposed to a given substance (Anthony et al. 1994; Schramm-Sapyta et al. 2009) and many, but not all, individuals who use drugs in adolescence stop as they develop into early adulthood. The dependent versus casual use trajectories are potentially differentiated by the relative importance of drug use versus other life goals and demands (Flory et al. 2004; Juon et al. 2011; Maume et al. 2005), thus it is of significant interest to model such factors in laboratory studies. A few parallel examples exist in rat studies. Home cage environmental enrichment has been shown to attenuate the acquisition of cocaine (Puhl et al. 2012) and alcohol self-administration (Deehan et al. 2011) and to diminish the rewarding effects of heroin in rats (El Rawas et al. 2009) and cocaine in mice (Solinas et al. 2008). It has even been shown that sexual rejection by previously-mated female flies increases the ethanol intake of male Drosophila melanogaster (Shohat-Ophir et al. 2012).
The opportunity to use an activity wheel is rewarding and reinforcing in laboratory rodents; wheel access will increase the probability of an operant response in rats (Hundt and Premack 1963; Premack et al. 1964) and will maintain lever pressing under a variety of schedules (Belke 2010; Belke and Hancock 2003; Collier and Hirsch 1971; Pierce et al. 1986). Concurrent access to cocaine intravenous self-administration (IVSA) and an activity wheel are mutually suppressing on female rats’ drug intake and activity (Cosgrove et al. 2002) and concurrent access to a wheel suppresses initial d-methamphetamine IVSA in male rats (Miller et al. 2012). In this latter study, once IVSA was established the introduction of wheel access did not affect drug taking, however, ongoing IVSA of MA gradually decreased the amount of wheel activity. Furthermore, rats introduced to the wheel after 7 or 14 IVSA sessions initially ran very little compared with drug-naïve controls. A contrast of rat strains which differed six-fold in spontaneous running showed that the effect of concurrent wheel access on MA IVSA was independent of distance traveled on the wheel. Thus the effect is most parsimoniously attributed to the rats’ spontaneous preference level, i.e., the reward value of wheel activity.
The present study was conducted to further test the hypothesis that co-option and devaluation of wheel running in rats is a consequence of establishing a consistent pattern of stimulant drug IVSA. The goal was to use conditions under which the reinforcer was highly efficacious and animals undergo an acquisition curve from zero to relatively stable intake across the test interval. The emerging (Benzie et al. 2011; Bluelight 2006; Borek and Holstege 2012; Ross et al. 2012; Wyman et al. 2013) substituted cathinone drug 3,4-methylenedioxypyrovalerone (MDPV) was selected as the stimulant drug model since it is readily self-administered by rats and may be more efficacious than MA (Aarde et al. 2013b; Watterson et al. 2014). MDPV is a potent monamine transporter inhibitor with high dopamine selectivity (Baumann et al. 2013; Cameron et al. 2013; Eshleman et al. 2013; Simmler et al. 2013). The Miller et al study (2012) used initial food-reinforced lever training which leads to relatively stable drug intake from the start of self-administration (e.g. (Aarde et al. 2013b; Miller et al. 2012) and may have advantaged drug-taking over wheel activity. Thus, the present study did not include any lever training before MDPV access.
2. Methods
2.1 Animals
Male Wistar rats (Charles River, New York; N=32) were housed in a humidity and temperature-controlled (23±1 °C) vivarium on a 12:1 2 hour light:dark cycle. Animals entered the laboratory at 10–11 weeks of age and weighed ~370 grams at the start of the study. All procedures were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and in a manner consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011; 8th ed).
2.2 Drugs
The racemic 3,4-methylenedioxypyrovelarone HCl used for this study was synthesized as described previously (Aarde et al. 2013b); doses are described as the salt. Drug was dissolved in physiological saline for injection.
2.3 Apparatus
Operant conditioning chambers (Med Associates Model ENV-045; Med-PC IV software) enclosed in sound-attenuating cubicles were used for concurrent wheel access and self-administration as previously described (Huang et al. 2012; Miller et al. 2012). Chambers were equipped with slanted activity wheels (~100 cm inner circumference, 30 degree tilt of running surface relative to the disk/back), which allowed wheel access while rats were tethered for self-administration.
2.4 Intravenous catheterization
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described previously (Aarde et al. 2013a; Aarde et al. 2013b). Briefly, the catheters consisted of a 14-cm length of polyurethane based tubing (Micro-Renathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal's back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB).
A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin; 0.4 mg/kg, i.m. Day 1, s.c. Day 2–3) and an analgesic (flunixin; 2.5 mg/kg, s.c.) were administered daily. During recovery, as well as during testing and training, intravenous catheters were flushed with heparinized saline (before sessions) and heparinized saline containing cefazolan (100 mg/mL; after sessions).
Catheter patency was assessed after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic Brevital sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec after infusion. In this study a total of N=26 survived to the end of the described studies with patent catheters.
2.5 Intravenous Self-Administration
All animals were drug and operant-training naïve prior to the start of this study. Subjects were transported to an experimental room (ambient temperature 23 ± 1 °C; illuminated by red light) and plac ed into the operant conditioning chambers for drug self-administration testing 7–8 hours after the beginning of the vivarium dark cycle. The catheter fittings on the animals' backs were connected to polyethylene tubing contained inside a protective spring suspended into the operant conditioning chamber from a liquid swivel attached to a balance arm. Each operant conditioning session started with the extension of two retractable levers into the chamber and lasted for 60 minutes. Following completion of one lever press (FR1) on the drug-associated lever (same side for all animals), a white stimulus light (above the lever) signaled delivery of the reinforcer (infusion volume = 0.1 ml; duration = 4 sec) and remained on during a 20-sec post-infusion timeout, during which responses were recorded but had no scheduled consequences. There were no consequences for responses on the alternate lever. After the first two operant conditioning sessions, a single priming infusion of drug was administered non-contingently for any session in which a rat had not responded on the drug-paired lever within the first 30 minutes.
2.6 Data Analysis
Measures included infusion rates (infusions/hour or infusions/5-min sampling interval), quarter wheel rotations (~25 cm), lever discrimination (% of presses on the drug-paired lever out of all lever presses, not including timeout), mean post-reinforcement pause (PRP), percent of PRPs equal to the 20 s timeout (PRP%20s), and timeout drug-paired-lever press rate. Factors included the within-subjects factors of self-administration session (1–20) and acquisition phase (Pre vs. Post) as well as the between-subjects factors of wheel-access group (Unlocked Wheel vs. Locked Wheel) and Acquisition-pattern group (Binge-like Acquisition Pattern vs. Non-Binge Acquisition Pattern). The binge-like pattern was operationally defined by >=8 maximum infusions in a given 5-min interval.
Repeated-measures analysis of variance (rmANOVA) was used to analyze infusion rate, lever discrimination, and quarter wheel rotations as a function of session as well as to analyze average infusion rate and average quarter wheel rotations as a function of acquisition phase. Post-acquisition measures did not include the “binge” session (addressed in separate analyses; see below). For two rats that acquired IVSA on the 1st session, pre-acquisition values were derived by mean unit imputation to permit the pre/post acquisition analyses. Post-hoc comparisons for means separation of effects confirmed by rmANOVA utilized Tukey’s honest significant difference method (Tukey’s HSD). Additionally, a Kaplan-Meier survival analysis was used to analyze the rate of acquisition. The acquisition criterion was defined as a sustained intake of >=6 infusion/hr/session and for the analysis, non-acquiring rats were right censored at 20 operant conditioning sessions and group differences were analyzed with the Mantel-Cox Logrank test. Comparisons of time-out lever pressing rate and post-reinforcement pause on the binge session (for Non-binge rats, the session for which acquisition criteria was reached was used for comparison) as a function of binge group and wheel group utilized non-parametric Mann-Whitney U tests (Bonferroni corrections; 4 comparisons per family; corrected for ties; two-tailed). Lastly, the comparison of PRP%20s on the binge session used a single t-test (unpaired, two-tailed) and was between wheel groups for the binge-like rats only (none of the Non-binge rats exhibited a 20 second PRP on their day of acquisition).
Analyses were conducted with GB-STATv7.0 (Dynamic Microsystems, Silver Spring MD) and StatView (SAS Institute, Inc., Cary, NC). Graphs were generated with Microsoft Excel (Microsoft, Redmond WA) and StatView and figures created with Microsoft PowerPoint (Microsoft, Redmond WA), Adobe Illustrator (Adobe Systems Incorporated; San Jose, CA) and Canvas (ACD Systems, Seattle WA).
3. Results
3.1 Wheel Group Assignment
All animals were initially tested over two sequential days in one-hour periods of access to the activity wheels (untethered). Subsequent self-administration wheel-access groups were confirmed to remain statistically indistinguishable (t(24) = 0.5, p = 0.6) even after the loss of 6 rats (3 from each wheel-access group) due to loss of catheter patency. Mean pre-self-administration wheel activity for the animals that remained patent was 915 quarter-rotations/hour (SD = 350; SEM=98) for the Unlocked-Wheel group and 840 quarter-rotations (SD = 350; SEM=97) for the Locked-Wheel group.
3.2 Unlocked vs. Locked Wheel (UW vs. LW)
3.2.1 MDPV Infusions
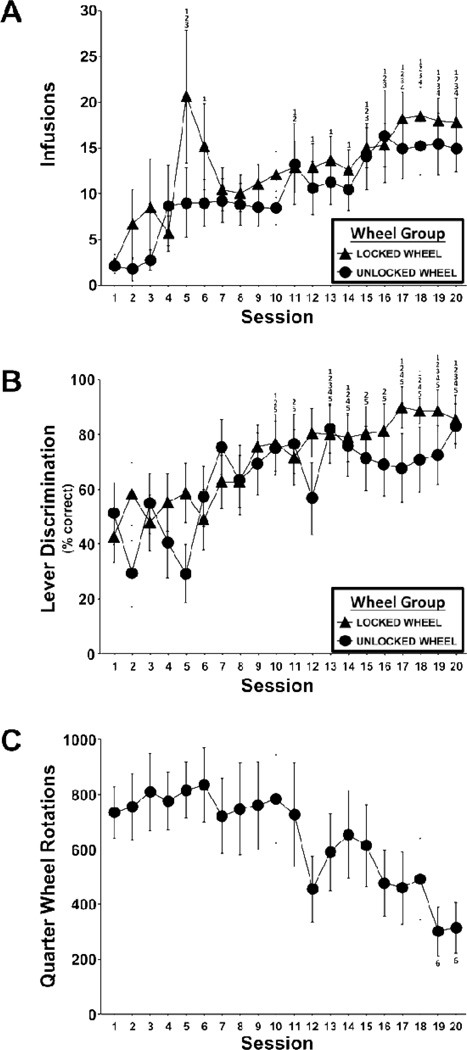
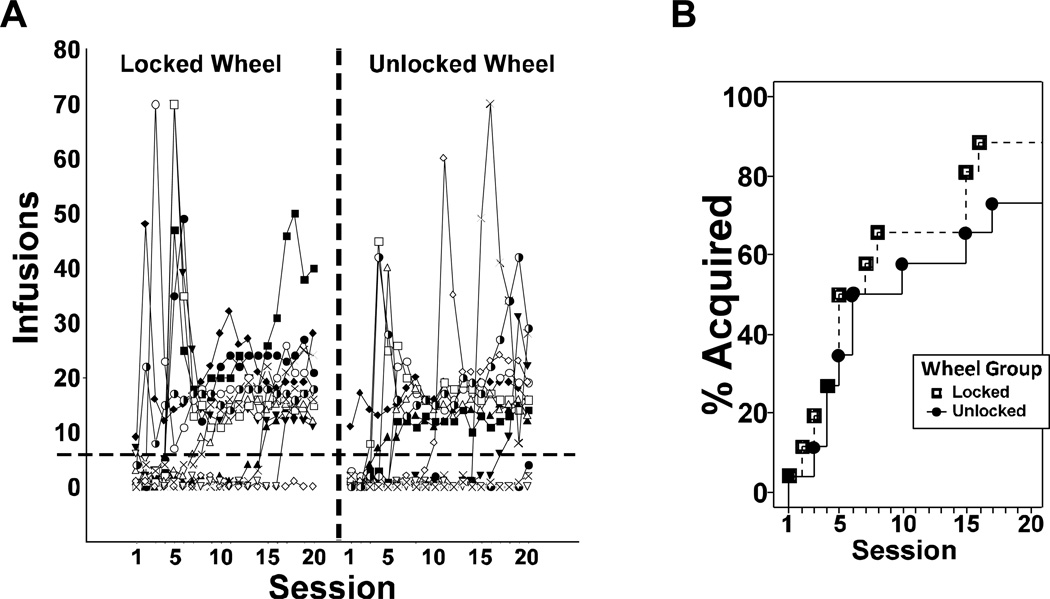
The mean number of infusions of MDPV (0.05 mg/kg/inf; Figure 1A) obtained increased across the 20-session acquisition interval (main effect of session: F19,456 = 5.9; p<0.0001). However, the groups did not differ in infusions; thus, there was no effect of wheel-access on infusions obtained. Post-hoc comparisons, collapsed across group, confirmed that infusions were higher in operant conditioning sessions 5–6 and 11–20 relative to 1, in sessions 5, 11, 15–20 relative to 2, in sessions 5, 15–20 relative to 3, and in sessions 17–20 relative to 4. No significant differences in infusions were confirmed for sessions 6–20. Lastly, there was no effect of wheel condition on the rate of acquisition (sessions to acquire for both groups: M = 6, SD = 5, SEM = 2) as shown in Figure 2.
Figure 1. Intravenous self-administration (IVSA) acquisition measures as a function of wheel group (Unlocked vs. Locked) and IVSA session.
Group (N=13 per group) means of A) infusion of 3,4-methylenedioxypyrovalerone (0.05 mg/kg/infusion; B) lever discrimination and C) quarter wheel rotations (Unlocked Group only) are presented. The numbers above session means indicate significant differences between that session and the session indicated by the number. Error bars represent ±SEM.
Figure 2. Acquisition Criterion.
A) Individual infusions are depicted by wheel group (Locked vs Unlocked). The acquisition criterion was defined as a sustained intake of >=6 infusion/hr/session, indicated by the horizontal dotted line. B) The proportion of the Unlocked Wheel and Locked Wheel groups that had met acquisition criteria on a given session.
3.2.2 Lever Discrimination
The proportion of lever presses on the drug-paired lever increased significantly across operant conditioning sessions (F19,456 = 5.9; p<0.0001); Figure 1B. The ANOVA did not confirm any significant effects of wheel-access group or interaction between group and session. Post-hoc comparisons, collapsed across group, confirmed that lever discrimination was higher in sessions 10, 13–14 and 17–20 relative to 1, in sessions 10–11, 13–20 relative to 2 and 5, in sessions 13, 19–20 relative to 3, and in sessions 13–14 and 17–20 relative to 4. No significant differences in lever discrimination were confirmed for sessions 7–20.
3.2.3 Wheel Activity
Mean quarter wheel rotations in the Unlocked Wheel group were initially 730 (SD= 336; SEM = 93) and significantly decreased across the 20 session acquisition period to below 300 quarter rotations in the final two operant conditioning sessions (main effect of session, F19,228 = 2.7; p<0.001; Figure 1C). Post-hoc comparisons confirmed that quarter-rotations were lower in sessions 19–20 relative to session 6.
3.3 Individual Wheel-Activity and Drug Intake
3.3.1 Across Operant Conditioning Sessions
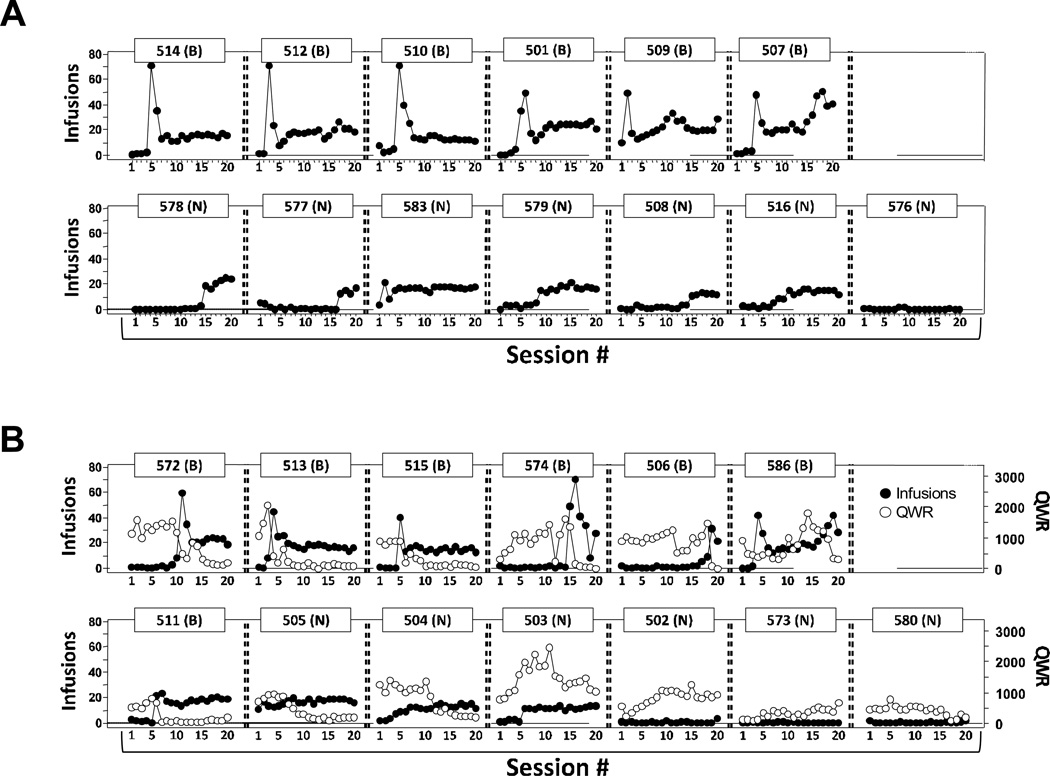
The individual patterns of wheel activity and drug-lever responding as a function of acquisition session were contrasted (Figure 3) in an effort to provide further insight into MDPV IVSA acquisition that would be obscured by the group-averaging approach. Indeed, self-administration for 6 of 13 individuals in the Locked Wheel group (rat #s 514, 512, 510, 501, 509, 507) and for 7 of 13 in the Unlocked Wheel group (rat #s 572, 513, 515, 574, 506, 586 and – though not clear at this level of analysis, see below and Figure 4 – rat # 511) exhibited a binge-like acquisition pattern. For these individuals, the maximum intake was observed early in each individual’s interval of stable intake and this initial high-intake “binge” was typically followed by stable intake at a lower or gradually increasing level thereafter. At this level of analysis, the distribution of MDPV intake patterns of the Unlocked Wheel and Locked Wheel groups were qualitatively similar.
Figure 3. Individual traces of infusions obtained and wheel rotations as a function of IVSA session.
A). Individual MDPV infusions earned (filled circles) are depicted for rats in the Locked Wheel group. B). Individual MDPV infusions earned (filled circles) and wheel activity emitted (Quarter Wheel-Rotations, QWR; unfilled circles) across the 20-session acquisition period are depicted the Unlocked Wheel group. For both groups the individual subject number and the acquisition-pattern grouping (B = Binge-like; N = Non-Binge) are indicated.
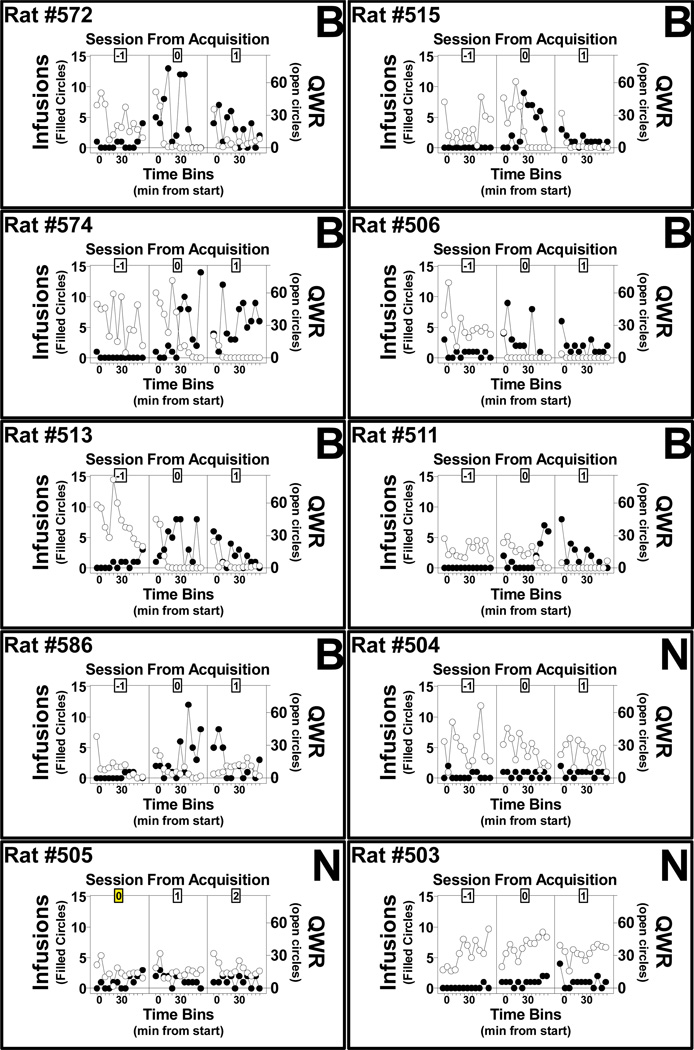
Figure 4.
Within-session infusions obtained and wheel rotations as a function of 5-min sampling interval and session (before, of, and after acquisition): MDPV infusions and quarter rotations (QWR) of the wheel per 5-min sampling intervals spanning the sessions around the time of acquisition (−1 = before, 0 = of and 1 = after; except for rat 505 as it acquired on its first session) for rats in the Unlocked wheel group (UW) that met the acquisition criteria. The acquisition-pattern group to which animals were assigned is indicated in the upper right corner (B = binge-like and N = non-binge-like). Six of the 7 Binge rats (572, 515, 574, 506, 513, and 511; top six panels) exhibited sustained, relatively precipitous drops in wheel activity around the time within the session when the infusion rate rose sharply. Wheel activity did not in general decrease across sessions prior to the spike in intake for Binge rats nor for any session in non- Binge rats.
Eight of the 13 Unlocked Wheel individuals exhibited behavior patterns in which wheel activity dropped notably across the acquisition interval (Figure 3). For 6 of these animals, this was precipitous and occurred simultaneously with a significant increase in drug intake (i.e., notable crossovers in the traces for infusions and wheel activity for rat #s 572, 513, 515, 574, 506, and 511) while the other two rats (i.e., rat #s 505 and 504) exhibited a comparatively more gradual reduction in wheel activity in the midst of a stable pattern of drug intake. Of the remaining 5 rats, the three that did not make acquisition criterion (i.e., rat #s 502, 573, and 580) showed stable or increasing wheel activity across most of the acquisition interval while the last two rats (i.e., rat #s 503 and 586) showed a multi-phasic pattern of wheel activity.
3.3.2 Within Operant Conditioning Sessions, Surrounding the Binge
Data that are averaged across individuals (Figure 1) and even across operant conditioning sessions within-individual (Figure 3) obscure the close temporal relationship that was observed between reductions in wheel activity and the acquisition of MDPV self-administration, and also underestimate the maximum infusion rate. As can be seen in the plots of within-session changes in infusion rate and wheel activity as a function of 5-min sampling interval (Figure 4), the appearance of a crossover from wheel activity to drug self-administration occurred in the time scale of minutes within the binge session – rather than gradually over a session or between sessions – for 6 of the 7 rats that exhibited a binge-like acquisition pattern. The within-session analysis also illustrates that when the binge-like pattern (defined by >=8 maximum infusions in a given 5-min interval) occurs late in the session (i.e., Rat #511) the overall session intake may not reflect the binge phenotype. Finally, the sustained reduction in wheel activity was not observed in rats for which no binge-like acquisition pattern was observed.
3.4 Binge-like vs. Non-Binge Acquisition Patterns
3.4.1 MDPV Infusions and Wheel Rotations – Pre- vs. Post-Acquisition
Independent of wheel condition (Unlocked Wheel, Locked Wheel), the animals that exhibited a binge-like pattern of MDPV self-administration acquisition (defined by >=8 maximum infusions in a given 5-min interval) had higher mean post-acquisition infusions per hour (M = 21, SD = 6, SEM = 2) than rats that did not show this pattern (M = 15, SD = 3, SEM = 1) (group by pre/post acquisition interaction; F1,20 = 9.3; p < 0.01) – even after excluding the spike in intake of the identified binge session (Figure 5A, left). Pre-acquisition infusion rates were not reliably different between binge-like (M = 1, SD = 1, SEM = 0.3) and non-binge (M = 2, SD = 1, SEM = 0.3) (p = 0.1) subgroups and the post-acquisition infusion rates were higher than pre-acquisition rates for both binge-like and non-binge (both p < 0.0001).
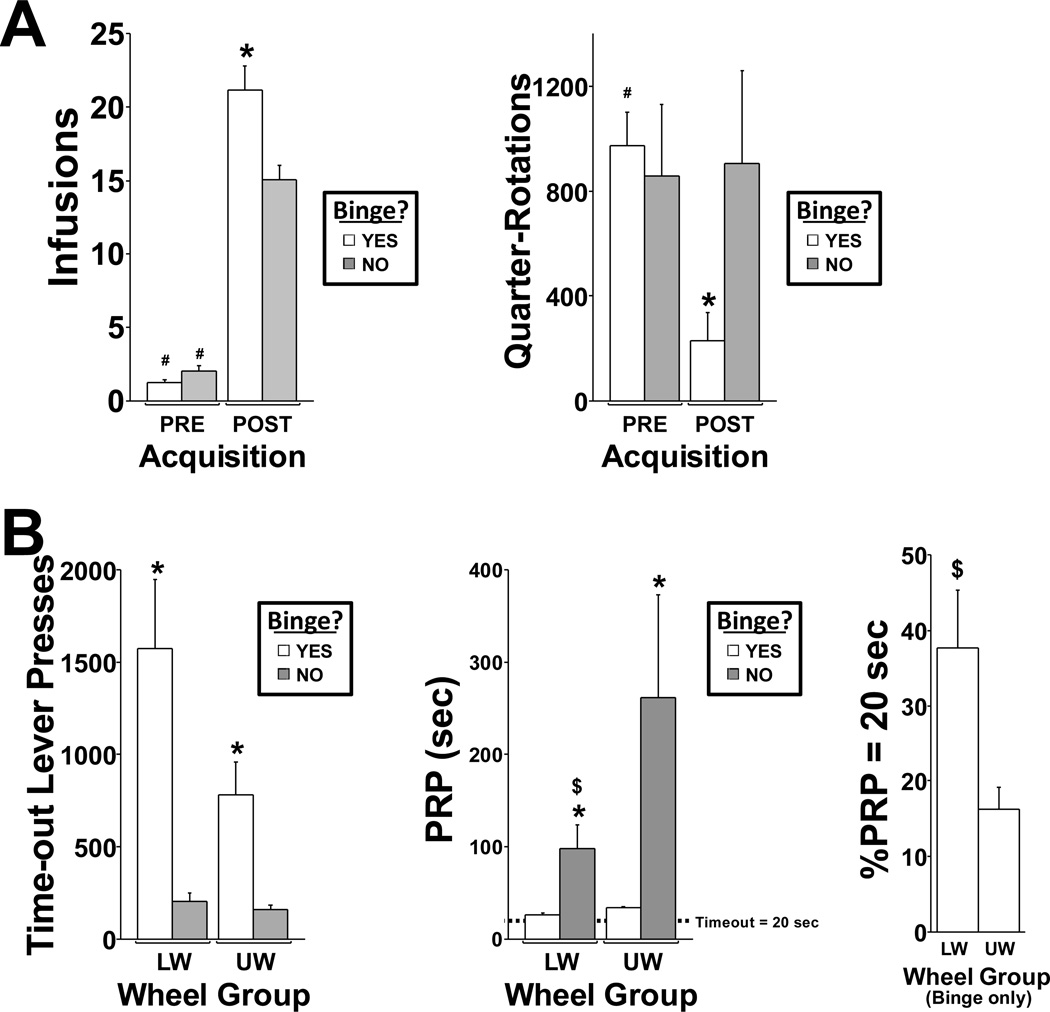
Figure 5. Behavior as a function of acquisition pattern.
A) Mean MDPV infusions (left) and mean quarter wheel rotations (right) as a function of Acquisition-pattern group (Binge? = “YES” or “NO”) and Acquisition phase (PRE vs. POST). Symbols for means separation (all p < 0.05): *YES ≠ NO; #PRE ≠ POST. Error bars represent SEM B) For the binge session (for “NO”-Binge rats, the 1st session wherein acquisition criterion was met was used for comparison), the measures of total timeout drug-paired-lever presses (left), mean post-reinforcement pause (PRP; middle), and % of PRP equal to the 20 second imposed timeout (%PRP=20sec; right) are plotted as a function of Wheel Group (Locked Wheel, LW; Unlocked Wheel, UW) and Acquisition Pattern group (except for %PRP=20sec for which only Binge rats are shown; %PRP=20sec was 0% for all “NO”-Binge rats). Symbols for means separation (all p < 0.05): *YES ≠ NO; $LW ≠ UW. Error bars represent SEM.
Within the Unlocked Wheel group, post-acquisition quarter-rotations per hour of the binge-like rats (M = 230, SD = 275, SEM = 104) was less than ¼ of that observed pre-acquisition (M = 974, SD = 339, SEM = 128), while wheel activity was similar for the non-binge rats post-acquisition (M = 904, SD = 610, SEM = 352) and pre-acquisition (M = 855, SD = 474, SEM = 274); Figure 5A, right. This interaction was confirmed by rmANOVA (acquisition-pattern group by acquisition phase interaction; F1,8 = 5.3; p < 0.05) and post hoc comparisons between groups confirmed that wheel activity was lower for binge-like than non-binge rats post-acquisition (p < 0.05) but was not different for pre-acquisition sessions. Lastly, post hoc comparisons between pre- and post-acquisition sessions confirmed that for binge-like rats, wheel quarter-rotations were lower post-acquisition than pre-acquisition (p < 0.01), but for non-binge rats the post- and pre-acquisition wheel activity was equivalent.
3.4.2 Timeout Lever Presses and Post-Reinforcement Pause (PRP) on the “Binge” Session
Examination of the behavioral events recorded in real time during the drug intake binge (again, this was defined by >=8 maximum infusions in a given 5-min interval) showed intervals wherein infusion rate was constrained only by the 20-second post-reinforcement timeout (during which drug-associated lever presses did not have scheduled consequences). Therefore, drug-associated-lever presses per hour during the imposed timeout, the mean duration of post-reinforcement pauses (PRPs) and the percentage of PRPs equal to 20 seconds (%PRP = 20s) were compared as a function of Acquisition-pattern group and Wheel group (Figure 5B), to further analyze and characterize behavioral responses.
For Locked Wheel rats, time-out lever pressing was higher for binge-like rats (M = 1574, SD = 921, SEM = 376) than non-binge rats (M = 201, SD = 123, SEM = 50) (UBAP(6) = 36, UNAP(6) = 0, Z = −2.9, p < 0.0125). However, for Unlocked Wheel rats, although time-out lever pressing for binge-like rats (M = 780, SD = 470, SEM = 178) was higher than that of non-binge rats (M = 162, SD = 31, SEM = 18), this difference did not reach the criterion for statistical reliability. Time-out lever pressing did not differ between Locked Wheel and Unlocked Wheel rats for either binge-like or non-binge subgroups.
Within the Locked Wheel rat group mean post-reinforcement pause duration was shorter for binge-like rats (M = 26, SD = 5, SEM = 2) than non-binge rats (M = 98, SD = 64, SEM = 26) (UBAP(6) = 0, UNAP(6) = 36, Z = −2.9, p < 0.0125). Although the mean post-reinforcement pause for binge-like rats within the Unlocked Wheel group (M = 34, SD = 5, SEM = 2) was shorter than that of the Unlocked Wheel non-binge rats (M = 261, SD = 193, SEM = 111), this difference was not statistically reliable. The post-reinforcement pause was reliably shorter for Locked Wheel than Unlocked Wheel binge-like rats (ULW(6) = 3, UUW(7) = 39, Z = −2.6, p < 0.0125), but across all of the non-binge rats, the Locked Wheel and Unlocked Wheel groupings were equivalent.
Lastly, for the binge-like rats (regardless of wheel condition), the percentage of post-reinforcement pauses equal to the minimum of 20 seconds was greater for the Locked Wheel rats (M = 38, SD = 19, SEM = 8) than the Unlocked Wheel rats (M = 16, SD = 8, SEM = 3) (t(11) = 2.8, p < 0.05). None of the non-binge rats had a post-reinforcement pause equal to 20 seconds on their session of acquisition.
4. Discussion
These data show that the initial acquisition and stabilization of intravenous self-administration (IVSA) of 3,4-methylenedioxypyrovalerone (MDPV) in rats reduces their activity on a wheel when the options are presented concurrently. Thus, the opportunity for drug self-administration replaces or diminishes the reward value of wheel activity, despite the fact that sufficient time exists within an operant conditioning session to engage in both behaviors at the observed rates. This appeared to be an abrupt phenomenon, occurring within a single session particularly when a binge was observed. Although the within-session effect on the binge day might reflect the induction of stereotypies or other competing behavior that precludes wheel running, this cannot account for the continued pattern of low wheel activity in subsequent sessions. This confirms and extends our prior study with MA self-administration (Miller et al. 2012). Mean wheel activity in the initial few sessions was nearly identical to that previously reported for the Wistar group in Miller et al (2012) and the mean number of infusions obtained the last 5 sessions was 16 infusions (SEM = 1) was similar to the mean of 19 infusions obtained by lever-trained Wistar rats responding under an FR5 contingency (Aarde et al. 2013b), which enhances comparison across studies. Similarly the mean lever discrimination ratio was 80% (SEM = 3) in the last 5 sessions which was similar to that reported previously for an FR5 schedule of reinforcement (86%) in a group previously lever trained (Aarde et al. 2013b). These studies therefore concur with the prior reports showing that MDPV is a highly effective reinforcer in rat self-administration models (Aarde et al. 2013b; Watterson et al. 2014).
Gradual acquisition of MDPV IVSA within the group (Figure 1A) was related to a progressive reduction in the amount of wheel activity (Figure 1C), in a pattern similar to the effects of MA self-administration on rats in our prior study (Miller et al. 2012). In that study, however, the use of lever pre-training resulted in substantial group MA intake from the very first session so it was not possible to observe any transition in drug intake and wheel activity within individuals. In this design, it was possible to observe such transition points and six of the 13 animals in the wheel-access group exhibited single-session transitions (Figure 3) in which relatively low drug intake and high wheel activity was replaced by increased drug IVSA and decreased wheel use. An additional three animals exhibited patterns in which wheel activity declined only after an interval of several operant conditioning sessions which consisted of stable and relatively high drug intake. Another three individuals maintained sustained wheel activity and failed to acquire MDPV IVSA and one individual sustained both robust wheel activity and relatively high MDPV intake. Thus, the plurality of individual trajectories support the conclusion that as MDPV IVSA was acquired within an individual, the wheel was devalued in most individuals. Furthermore, the emergence of a binge-like, single-session acquisition pattern in about half of the wheel-access rats was related to the greatest reduction in wheel activity (Figure 5). Although qualitatively it appeared that the binge may have been delayed in the group with unlocked wheels (Figure 2) the present design does not support an authoritative conclusion- additional investigation with larger groups might show whether wheel access delays the occurrence binge session.
The fact that MDPV IVSA was not reduced by concurrent wheel access, as has been shown for cocaine (Cosgrove et al. 2002) and MA (Miller et al. 2012), may be due to its relatively high efficacy as a reinforcer, i.e. compared with methamphetamine (Aarde et al. 2013b). Similarly, environmental enrichment protects against the escalation of cocaine intake under long-access conditions only when the per-infusion dose is relatively low (Gipson et al. 2011). These results are consistent with growing evidence that indicate that MDPV is a more efficacious and/or potent reinforcer than is MA in rat self-administration (Aarde et al. 2013b; Watterson et al. 2014). This relative advantage may explain why concurrent wheel activity caused a mean difference in initial MA intake (Miller et al. 2012) but not MDPV intake in the present study.
Individual differences in the effect of concurrent wheel access are critical, since only a subset of humans exposed to a wide variety of psychotropic drugs will become dependent (Anthony et al. 1994; Schramm-Sapyta et al. 2009). As Ahmed and colleagues have argued (Ahmed et al. 2013), animal models of the transition point beyond which drug taking supplants other sources of reinforcement may be advantageous to further our understanding of the individual differences in resilience against compulsive drug use in humans. Rats prefer saccharin or sucrose flavor to cocaine in a direct choice (Lenoir et al. 2007) and only with extended access to heroin will rats reverse their preference for saccharin and choose each reinforcer about equivalently (Lenoir et al. 2013). Given the ecological value of cues for high-value food, these paradigms may not be the most sensitive. While wheel activity is highly reinforcing in laboratory rats, the present data suggest it may present the ideal contrast with drug IVSA for such investigations.
The binge-like, single-session acquisition that was observed in half of the rats across the entire study differs from traditional concepts of drug self-administration binges. Roberts and colleagues (Morgan et al. 2005; Roberts et al. 2002) have defined binges as the opportunity for extended drug access across many hours to an entire day and Miczek and colleagues (Fowler et al. 2007; Tornatzky and Miczek 2000) similarly define a binge as high intake rates during a relatively long period of extended access (e.g., 24 hrs). However, in both cases, the binge occurs after acquisition and usually after a manipulation intended to increase intake levels (e.g., extended/long daily access sessions or social defeat stress). Indeed, the six-hour escalation paradigm pioneered by Ahmed and Koob (Ahmed and Koob 1998; 1999) has been shown to result in gradual mean increases in drug intake during the 1st hour of sessions that are sometimes characterized as an induced “loading-phase” binge. In contrast, the binge-like behavior in this study was observed spontaneously and was associated with the first days of acquisition. Thus, although the binge observed in these studies was characterized by relatively unrestrained, high intake levels – much as would occur for binge drinking or eating – the position of this binge near the beginning of acquisition and without prior intake-augmenting manipulations sets it apart from those traditionally described in drug self-administration studies.
Further experimentation will be necessary to determine if the binge experience causes the subsequent higher intake or simply serves as a marker for subsequent intake levels. Evidence that favors the former is that, in rats, the increased consumption levels of sweetened shortening that is produced by prior experience with a binge-inducing schedule (i.e., limited, intermittent access) is not observed if this binge experience is prevented by limiting the amount of shortening available (i.e., experiencing the binge-inducing schedule is not sufficient to increase later consumption – the binge experience must also occur) (Wojnicki et al. 2008). If these binge-eating results generalize to drug binging, than it is likely that similar binge-limiting interventions during the theorized initial binge/intoxication phase of addiction could prevent or retard the progression of addiction. This possibility increases the promise of immunological protection (e.g., antibodies with drug affinity (Miller et al. 2013) and gene therapies (e.g., transfection with genes for drug metabolizing enzymes (Anker et al. 2012) to decrease the incidence of addiction among at-risk individuals by interfering with initial, binge-like drug experiences.
It is also unknown if this binge-acquisition pattern is unique to MDPV or if it is a specific consequence of the high reward/reinforcer properties of MDPV. There are hints of a similar pattern in cocaine self-administration (Carroll and Lac 1993), but few reports that directly address this phenomenon are available. If the differing reward/reinforcer properties of different stimulants determines the frequency and magnitude of a binge-like acquisition then it is noteworthy that the reinforcer efficacy of a drug appears to be positively correlated with the relative actions of that drug upon dopamine and serotonin levels in the brain (Bauer et al. 2013) and furthermore, that MDPV tops this list of stimulants with regard to its DAT/SERT IC50 ratio with a ratio of ~300 (compared to cocaine at a ratio of ~3) (Simmler et al. 2013); also see (Baumann et al. 2013; Cameron et al. 2013). As responding for cocaine under a progressive-ratio schedule (a well validated measure of reinforcer efficacy) can be increased by serotonergic lesions of the medial forebrain bundle and amygdala (Loh and Roberts 1990), it is likely that MDPV’s high selectivity for enhancing dopamine levels over serotonin levels sets this drug apart from other stimulants as a reward/reinforcer. Indeed, recent evidence indicates that MDMA, a drug that is relatively weakly reinforceing (DAT/SERT IC50 ratio = 0.08; (Simmler et al. 2013)), is self-administered more readily after similar serotonergic lesions and in SERT knockout rats as compared to controls (Bradbury et al. 2013; Oakly et al. 2013). In other words, current data suggest that, as a consequence of its neurochemical specificities, MDPV may function as a “super reward” that elicits binge-like intakes at the onset of use (Aarde et al. 2013b; Simmler et al. 2013).
In summary, this study confirms the high reinforcing value, and therefore likely high abuse liability, of MDPV. In contrast to our prior findings with methamphetamine, concurrent wheel access did not significantly affect mean MDPV intake. This shows that there may be specific parameters of drug identity, behavioral training history, etc that may affect the shifts between drug self-administration and wheel activity. In the present study the acquisition of MDPV self-administration supplanted wheel activity as a reinforcer in rats, which is similar to the group-mean effects show for MA in Miller et al (2012). Examination of the individual patterns of wheel activity and MDPV infusions showed that this is essentially a one-trial effect in many individuals. Once a rat has experienced a relatively high-dose self-administration session, the wheel is subsequently used less than that individuals’ baseline thereby confirming that MDPV use can rapidly attenuate the appeal of a natural source of reward. Access to a wheel is otherwise highly reinforcing in laboratory rats (Belke and Heyman 1994; Hundt and Premack 1963) and therefore the present results show the rapid devaluation of this naturalistic reinforcer by the acquisition of stimulant drug IVSA. As such this paradigm models transition states in rats on both a group and individual basis.
Supplementary Material
Acknowledgements
This work was supported by USPHS grants DA024105 and DA024705; the NIH/NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The MDPV was synthesized by Garry R. Smith, PhD at Fox Chase Chemical Diversity Center (Doylestown, PA) from routes designed by T.J.D. under contract from T.J.D. and M.A.T. This is manuscript #24009 from The Scripps Research Institute. These experiments complied with the applicable laws of the USA.
Footnotes
Financial Disclosures
The authors report no financial conflicts that are relevant to the conduct of this study.
Literature Cited
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr., Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addiction biology. 2013a;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Current opinion in neurobiology. 2013 doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ, Carroll ME. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biological psychiatry. 2012;71:700–705. doi: 10.1016/j.biopsych.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: Basic findings from the national comorbidity survey. Exp Clin Pyschopharm. 1994;2:244–268. [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British journal of pharmacology. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW. Exclusive preference develops less readily on concurrent ratio schedules with wheel-running than with sucrose reinforcement. Journal of the experimental analysis of behavior. 2010;94:135–158. doi: 10.1901/jeab.2010.94-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Hancock SD. Responding for sucrose and wheel-running reinforcement: effects of sucrose concentration and wheel-running reinforcer duration. Journal of the experimental analysis of behavior. 2003;79:243–265. doi: 10.1901/jeab.2003.79-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Heyman GM. A matching law analysis of the reinforcing efficacy of wheel running in rats. Animal Learning & Behavior. 1994;22:267–274. Vol 22(3) [Google Scholar]
- Benzie F, Hekman K, Cameron L, Wade DR, Smolinske S. Emergency department visits after use of a drug sold as "bath salts" --- michigan, november 13, 2010--march 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:624–627. [PubMed] [Google Scholar]
- Bluelight MDPV Megathread. 2006 http://www.bluelight.ru/vb/threads/278421-MDPV-Megathread. [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and Multiorgan Failure After Abuse of "Bath Salts" Containing 3,4-Methylenedioxypyrovalerone. Annals of emergency medicine. 2012 doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte G, Schenk S. Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addiction biology. 2013 doi: 10.1111/adb.12069. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of "bath salts," produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013 doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Collier G, Hirsch E. Reinforcing properties of spontaneous activity in the rat. J Comp Physiol Psychol. 1971;77:155–160. doi: 10.1037/h0031588. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacology, biochemistry, and behavior. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr., Palmatier MI, Cain ME, Kiefer SW. Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behavioral neuroscience. 2011;125:184–193. doi: 10.1037/a0022627. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology. 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochemical pharmacology. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. Early adolescent through young adult alcohol and marijuana use trajectories: early predictors, young adult outcomes, and predictive utility. Development and psychopathology. 2004;16:193–213. doi: 10.1017/s0954579404044475. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Covington HE, 3rd, Miczek KA. Stereotyped and complex motor routines expressed during cocaine self-administration: results from a 24-h binge of unlimited cocaine access in rats. Psychopharmacology. 2007;192:465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of dmethamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and alcohol dependence. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundt AG, Premack D. Running as Both a Positive and Negative Reinforcer. Science. 1963;142:1087–1088. doi: 10.1126/science.142.3595.1087. [DOI] [PubMed] [Google Scholar]
- Juon HS, Fothergill KE, Green KM, Doherty EE, Ensminger ME. Antecedents and consequences of marijuana use trajectories over the life course in an African American population. Drug and alcohol dependence. 2011;118:216–223. doi: 10.1016/j.drugalcdep.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended Heroin Access Increases Heroin Choices Over a Potent Nondrug Alternative. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS one. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EA, Roberts DC. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology. 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- Maume MO, Ousey GC, Beaver K. Cutting the grass: A reexamination of the link between marital attachment, delinquent peers and desistance from marijuana use. J Quant Criminol. 2005;21:27–53. [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr., Janda KD, Taffe MA. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biological psychiatry. 2013;73:721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr., Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug and alcohol dependence. 2012;121:90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology. 2005;178:309–316. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- Oakly AC, Brox BW, Schenk S, Ellenbroek BA. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.75. [DOI] [PubMed] [Google Scholar]
- Pierce WD, Epling WF, Boer DP. Deprivation and satiation: The interrelations between food and wheel running. Journal of the experimental analysis of behavior. 1986;46:199–210. doi: 10.1901/jeab.1986.46-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Schaeffer RW, Hundt A. Reinforcement of Drinking by Running: Effect of Fixed Ratio and Reinforcement Time. Journal of the experimental analysis of behavior. 1964;7:91–96. doi: 10.1901/jeab.1964.7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behavioural pharmacology. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A. The epidemiology of drug abuse: current issues. Ecological studies of narcotic addiction. NIDA research monograph. 1977:173–196. [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug and alcohol dependence. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive "Bath Salts" Intoxication with Methylenedioxypyrovalerone. The American Journal of Medicine. 2012 doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration "binges": transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Wall M, Schmidt E, Sarang A, Atun R, Renton A. Sex, drugs and economic behaviour in Russia: a study of socio-economic characteristics of high risk populations. The International journal on drug policy. 2011;22:133–139. doi: 10.1016/j.drugpo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FH, Johnson DS, Corwin RL. Access conditions affect binge-type shortening consumption in rats. Physiology & behavior. 2008;95:649–657. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem Tissue Distribution of MDPV Following Lethal Intoxication by "Bath Salts". Journal of analytical toxicology. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.