Abstract
Tumor metastasis is the major cause of death among cancer patients, with more than 90% of cancer-related death attributable to the spreading of metastatic cells to secondary organs. Store-operated Ca2+ entry (SOCE) is the predominant Ca2+ entry mechanism in most cancer cells, and STIM1 is the endoplasmic reticulum (ER) Ca2+ sensor for store-operated channels (SOC). Here we reported that the STIM1 was overexpressed in colorectal cancer (CRC) patients. STIM1 overexpression in CRC was significantly associated with tumor size, depth of invasion, lymphnode metastasis status and serum levels of carcinoembryonic antigen. Furthermore, ectopic expression of STIM1 promoted CRC cell motility, while depletion of STIM1 with shRNA inhibited CRC cell migration. Our data further suggested that STIM1 promoted CRC cell migration through increasing the expression of cyclooxygenase-2 (COX-2) and production of prostaglandin E2 (PGE2). Importantly, ectopically expressed COX-2 or exogenous PGE2 were able to rescue migration defect in STIM1 knockdown CRC cells, and inhibition of COX-2 with ibuprofen and indomethacin abrogated STIM1-mediated CRC cell motility. In short, our data provided clinicopathological significance for STIM1 and store-operated Ca2+ entry in CRC progression, and implicated a role for COX-2 in STIM1-mediated CRC metastasis. Our studies also suggested a new approach to inhibit STIM1-mediated metastasis with COX-2 inhibitors.
Keywords: stromal interaction molecular 1 gene, store-operated calcium channel, colon cancer, metastasis, invasion, COX-2, colorectal cancer, cell migration
Introduction
Ca2+ is a ubiquitous and versatile second messenger regulating a variety of physiological and pathological process such as immune response, exocytosis, cell migration, axon guidance, and tumor dissemination1–5. In non-excitable cells, including majority of tumor cells, the store-operated Ca2+ channels (SOC) are the major Ca2+ entry mechanism3. Recently the stromal interaction molecule 1 (STIM1) and Orai1 were identified to be critical protein components in store-operated Ca2+ entry (SOCE) pathways6, 7. STIM1 and the closely related STIM2 are endoplasmic reticulum (ER) Ca2+ sensors activated by the decrease in ER Ca2+ concentration8. Orai1 is the major channel pore-forming unit for SOC in plasma membrane. Upon Ca2+ release from the ER, STIMs aggregate and translocate to the ER-plasma membrane to activate Orai19.
We previously reported that Ca2+ influx is important for tumor cell migration, and both Orai1 and STIM1 are essential for breast tumor cell migration, invasion and metastasis2, 4. STIM1 and Orai1 regulate focal adhesion turnover in migrating cells through Ras and Rac2. Since our initial report of the roles of STIM1 and Orai1 in breast cancer metastasis, these two proteins have been implicated in the migration, invasion, proliferation and metastasis of cervical cancer, non-small cell lung cancer, breast cancer and melanoma10–13. The regulation of the migration of non-cancer cells by STIM1 and Orai1 has also been reported14, 15. Store-independent activation of Orai1 by SPCA2 also contributes to breast cancer tumorigenesis through activation of MAPK16. There is now a growing body of literature supporting crucial roles of STIM1 and Orai1 in the invasion and metastasis of various cancers3. However, clinicopathological evidence supporting the roles of SOC in cancer progression is lacking, and the molecular mechanism by which SOCE promotes cancer cell motility, invasion and metastasis is not clear.
Colorectal cancer (CRC) is the fourth most common cancer in men and the third in women, with approximately 1 million new cases in 2002 (9.4% of the world total), and 529,000 deaths due to CRC are reported around the world annually17. Tumor metastasis is responsible for virtually all cancer-related death in CRC patients; however, the molecular mechanisms underlying CRC cancer dissemination are not fully understood. In this study we examined the clinicopathological roles of STIM1 in CRC progression and metastasis. Our data demonstrated that STIM1 was overexpressed in CRC specimens, and the expression levels of STIM1 positively correlated with tumor size, stage, depth of invasion, lymph node metastasis and the serum levels of carcinoembryonic antigen (CEA) in CRC patients. Ectopic expression of STIM1 in CRC cells induced the expression of pro-inflammatory and pro-metastasis enzyme cyclooxygenase-2 (COX-2). Importantly, inhibition of COX-2 with two non-steroidal anti-inflammatory drugs (NSAIDs), ibuprofen and indomethacin, abrogated STIM1-induced CRC cell migration. Our finding suggested that STIM1 overexpression in CRC patients promote invasion and metastasis through up-regulating COX-2 expression levels, and NSAIDs might be used to block STIM1-mediated CRC progression.
Results
STIM1 overexpression correlates with CRC tumor invasion, tumor size, and lymphnode metastasis
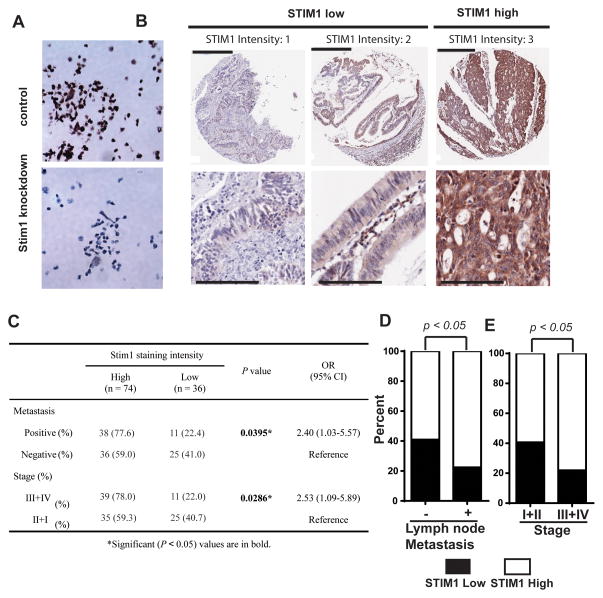
To investigate the clinicopathological role of STIM1 in CRC progression, we employed IHC staining using an anti-STIM1 antibody to examine the expression levels of STIM1 protein in CRC tissue specimens. The STIM1 antibody successfully stained paraffin embedded control DLD-1 colorectal cancer cells, but not DLD-1 cells expressing STIM1 shRNA, confirming the specificity of the antibody (Figure 1A). To examine the role of STIM1 in CRC progression, we stained a tissue microarray (TMA) consisting of 110 CRC samples for STIM1 expression. As shown in Figure 1B, STIM1 immunoreactivity appeared as diffuse cytoplasmic staining, consistent with the endoplasmic reticulum localization of STIM1. STIM1 was highly expressed in about two third (74 out of 110) of colorectal samples in the TMA, as demonstrated by the strong STIM1 immunoreactivity in these samples (Figure 1B and 1C). Tumors with positive lymph node metastasis and advanced stages also had higher levels of STIM1 expression (Figure 1C–1E). When the tumors were stratified into two groups based on STIM1 staining intensity, “STIM1 high” group had significantly higher incidence of advanced stage tumor (52.7%, 39 out of a total of 74) and tumor with lymphnode and/or distant metastasis (51.4%, 38 out of a total of 74) when compared to the “STIM1 low” group (30.6% and 30.6%, 11 and 11 out of a total of 36, respectively) (Figure 1C), suggesting that STIM1 overexpression might contribute to CRC progression and metastasis.
Figure 1. STIM1 overexpression in primary colorectal cancer.
A, IHC staining of paraffin-embedded DLD-1 colorectal cancer cells expressing control shRNA or STIM1 shRNA3. STIM1 knockdown significantly reduced the STIM1 IHC staining intensity, confirming the specificity of the antibody. B, Repressentives of primary colorectal cancers with STIM1 staining score 1, 2 and 3. Lower panel is magnified view of a region in the respective image in the upper panel. Scale bars are 400 μm in the upper panel and 100 μm in the lower panel. C–E, Statistical analysis showing that STIM1 expression levels in CRC surgical samples significantly correlate with metastasis (C and D) and tumor stage (C and E). p value was calculated by two-tailed Fisher’s exact test. OR and CI in C refer to Odds Ratio and Confidence Interval, respectively.
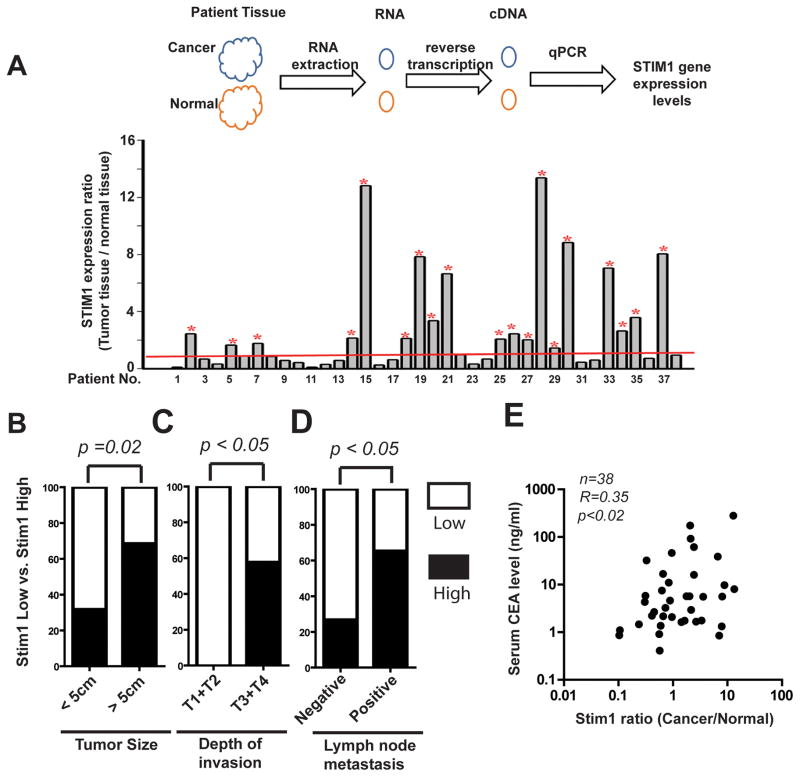
To further investigate the role of STIM1 in CRC progression and metastasis, we examined the expression levels of STIM1 in a cohort of 38 CRC patients treated at the Kaohsiung Medical University Hospital with a different approach (Table 1). Tumor tissues and paired adjacent normal tissues were collected from these patients according to an IRB approved protocol. Total mRNA from these samples was extracted and STIM1 expression levels in these samples were evaluated using quantitative real time PCR (qRT-PCR). As shown in Figure 2A, STIM1 expression levels in tumors were elevated in 19 out of the 38 CRC patients when compared to the paired adjacent normal tissue. We further used ELISA to compare the protein expression levels of STIM1 in another 20 paired CRC specimens and adjacent normal tissue. STIM1 protein levels increased in 90% of CRC samples (18 out of 20), suggesting the hyperactivation of store-operated calcium entry in CRC patients (supplementary Figure 1). To investigate the clinical significance of STIM1 overexpression in CRC progression, we studied the relationship between STIM1 expression levels and the clinicopathological data from CRC patients. As shown in Table 1, there were statistically significant correlation between STIM1 expression levels and the tumor size, depth of tumor invasion, lymph node metastasis status and UICC stages of CRC. The percentage of CRC samples with high STIM1 expression levels more than doubled in tumors equal or more than 5cm (68.6%) when compared to the less than 5cm group (31.6%) (Table 1, Fig. 2B). Intriguingly, all the 19 CRC cases with STIM1 overexpression had deep invasion (T3 and T4): either invasion completely through the muscularis propria into the serosa layer (T3) or in some cases had grown through the colon wall and invaded other organs or tissues (T4); no STIM1 overexpression was detected in the 5 CRC cases with shallower invasion (T1 and T2) (Table 1, Fig. 2C). Consistent with our IHC staining results (Figure 1), 65.2% of lymph node metastasis positive CRC patients also had high STIM1 levels; in contrast, STIM1 overexpression was only detected in 26.7% of lymph node negative patients. (Table 1, Fig. 2D). Patients with high levels of STIM1 expression were about twice as likely to develop metastasis (15 out of a total of 19 cases) as patients with low STIM1 levels (8 out of 19) (Table 1, Fig. 2D).
Table 1.
Correlations between clinicpathological features and STIM1 gene expression for 38 postoperative colorectal cancer patients
| Characteristics | Total cases N |
STIM1 overexpression
|
P | Adjusted P | |
|---|---|---|---|---|---|
| low N (%) |
high N (%) |
||||
| Gender | |||||
| Female | 17 | 7 (41.1) | 10 (58.8) | ||
| Male | 21 | 12 (57.1) | 9 (42.8) | 0.328 | |
| Age (years) | |||||
| <60 | 15 | 8 (53.3) | 7 (46.7) | ||
| ≥60 | 23 | 11 (47.8) | 12 (52.2) | 0.74 | |
| Tumor Size | |||||
| <5cm | 19 | 13 (68.4) | 6 (31.6) | ||
| ≥5cm | 19 | 6 (31.6) | 13 (68.4) | 0.023* | 0.004*† |
| Location | |||||
| Colon | 29 | 14 (48.3) | 15 (51.7) | ||
| Rectum | 9 | 5 (55.6) | 4 (44.4) | 1 | |
| Depth of tumor invasion | |||||
| T1+ T2 | 5 | 5 (100) | 0 (0) | ||
| T3+ T4 | 33 | 14 (42.4) | 19 (57.6) | 0.046* | 0.005*† |
| Lymph node metastasis | |||||
| No | 15 | 11 (73.3) | 4 (26.7) | ||
| Yes | 23 | 8 (34.8) | 15 (65.2) | 0.045* | 0.014*† |
| Stage (UICC)a | |||||
| I | 5 | 5 (100) | 0 (0) | ||
| II+III | 33 | 14 (42.45) | 19 (57.65) | 0.046* | 0.005*† |
| Histologyb | |||||
| WD+ MD | 31 | 17 (54.8) | 14 (45.2) | ||
| PD | 7 | 2 (28.6) | 5 (71.4) | 0.405 | 0.228† |
| Vascular invasion | |||||
| No | 22 | 12 (54.5) | 10 (45.4) | ||
| Yes | 16 | 7 (43.7) | 9 (56.3) | 0.511 | 0.346† |
| Perineural invasion | |||||
| No | 29 | 15 (51.7) | 14 (48.3) | ||
| Yes | 9 | 4 (44.4) | 5 (55.6) | 1 | 0.695† |
A P value of less than 0.05 was considered statistically significant.
International Union Against Cancer.
WD: Well differentiated; MD: Moderately well differentiated; PD: Poorly differentiated.
Adjusted the effects of age, sex and location (colon and rectum).
Figure 2. The correlation between STIM1 overexpression and tumor size, depth of invasion, lymphnode metastasis in a cohort of colorectal cancer patients.
A, 38 colon cancer patients’ RNA was isolated from tumor and normal tissues around the cancer tissue. Real-time PCR was applied to detect STIM1 gene expression levels. STIM1 gene expression levels in CRC tumor tissue was compared with paired normal adjacent colon tissues. Threshold of relative of STIM1 gene expression is set as 1. The patient is define as “STIM1 high” when the ratio is larger than one, and “STIM1 low” when the ratio equals or is lower than 1. * Indicatess relative of “STIM1 high” patients. B–D, The association between STIM1 expression levels and tumor size, invasion depth and lymphnode metastasis. p values were calculated by two-tailed Fisher’s exact test. E, Pearson correlation between STIM1 expression ratios and preoperative serum CEA in CRC patients.
When analyzed with multivariate analysis, the correlation between STIM1 expression levels and lymph node metastasis and tumor stages showed borderline significance (p=0.0527 and 0.08, respectively) (supplementary Table 1). Collectively, our data indicated STIM1 might promote CRC progression by mediating tumor invasion and metastasis.
Elevated levels of preoperative serum CEA in CRC patients with STIM1 overexpression
The levels of serum preoperative CEA in CRC patients is a critical prognosis marker, with significantly higher CEA levels in patients with advanced CRC poorer disease-free survival18. To further understand the pathological significance of STIM1 in CRC progression, we determined the serum CEA levels in the Kaoshiung cohort (Figure 2E). Peripheral blood samples from the CRC patients were collected less than 1 week prior to the operation, and the levels CEA in these samples were determined using an ELISA assay. The pre-operative CEA in the STIM1 high group (37.7±16.8 ng/ml) is about 5 times as high as the CEA in STIM1 low group (7.7±2.8 ng/ml). The STIM1 expression ratio (cancer vs. normal) significantly correlated with preoperative serum CEA in the cohort of 38 patients (Pearson correlation coefficient r=0.35, p<0.02) (Figure 2E). The significant correlation between STIM1 expression ratio and preoperative CEA suggested that patients with elevated STIM1 expression in tumor specimens might have poorer prognosis.
STIM1 is critical for CRC cell migration
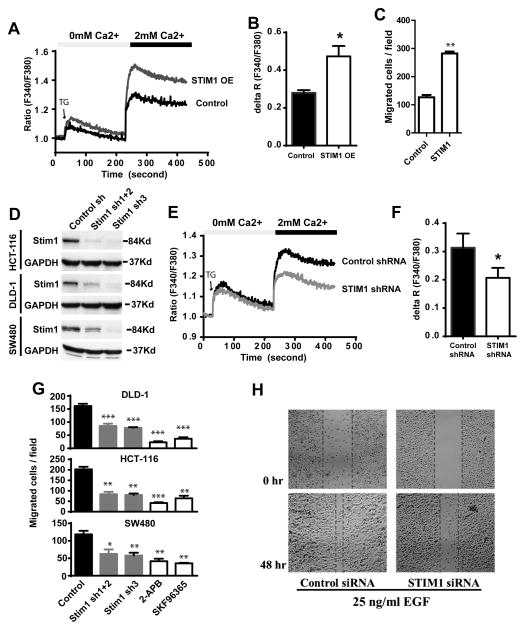
A critical signature of metastatic cancer cells is elevated mobility and invasiveness, which facilitate malignant cells to overcome multiple barriers during dissemination. To understand the mechanisms by which STIM1 promotes CRC progression and metastasis, we examined the effects of ectopic STIM1 overexpression on CRC cell migration. Ectopic expression of STIM1 in DLD-1 CRC cells promoted thapsigargin (TG) induced SOCE (Figure 3A and 3B). Next, the migration of DLD-1 cells expressing control vector of ectopic STIM1 was examined using a Boyden chamber assay. As shown in Figure 3C, activation of SOCE through ectopic expression of STIM1 promoted the migration of DLD-1 cells by about two folds. To determine whether STIM1 was required for CRC cell migration, we employed three shRNAs to knockdown STIM1 in three colon cancer cell lines (DLD-1, HCT116 and SW480), which decreased STIM1 protein levels by 50%–90% (Figure 3D) and inhibited TG-induced SOCE (Figure 3E and 3F). Depletion of STIM1 protein with shRNA or inhibition of SOCE with two pharmaceutical inhibitors 2-APB and SKF96365 robustly inhibited the migration of all three CRC cells (Figure 3G). In contrast, depletion of STIM1 with shRNA didn’t have noticeable effect on the proliferation of CRC cells (Supplementary Figure 2). Next we determined the role of STIM1 in epidermal growth factor (EGF)-induced cell migration using a wound-healing cell migration assay. In these assays, CRC cells were grown to a confluent monolayer, and a “wound” was made by scratching the CRC cell monolayer4, 19. After 48 hours in the presence of 25ng/ml EGF CRC cells migrated into the gap to fill the “wound”. As shown in Figure 3H, STIM1 knockdown remarkably inhibited EGF-mediated wound-closing in SW480 cells. Taken together, our data indicated that STIM1 overexpression might promote CRC metastasis by enhancing CRC cell motility and facilitating dissemination.
Figure 3. The role of STIM1 in colorectal cancer cell motility.
A, STIM1 overexpression (STIM1 OE) via transfection with plncx2-STIM1 increased thapsigargin (2μM) induced SOCE. plncx2 was used as a vector control in this experiments. B, Quantification of thapsigargin-induced SOCE peak value (ΔR) in A. C, The effects of ectopic STIM1 overexpression on DLD-1 cell migration. DLD-1 cells expressing ectopic STIM1 or control vector were plated onto the upper chamber of the Boyden cell migration chamber and allowed to migrate for 18 hours. D, Western blotting showing the knockdown of STIM1 expression levels in three CRC cell lines with stably expressed STIM1 shRNAs. Combination of STIM1 sh1 and STIM1 sh2 decreased STIM1 protein levesl by about 70%, and STIM1 sh3 decreased STIM1 expression by over 90%. E, STIM1 depletion with STIM1 shRNA inhibited thapsigargin induced SOCE in DLD-1 cells. F, B, Quantification of thapsigargin-induced SOCE peak value (ΔR) in E. G, The effect of STIM1 knockdown on the migration of three CRC cell lines determined by transwell migration assay. DLD-1, HCT-116 and SW480 cells expressing control or STIM1 shRNAs were allowed to migrate for 18–20 hours in Boyden chamber assay. H The effects of STIM1 knockdown on EGF (25ng/ml) -induced migration in SW480 cells. *, ** and *** indicate p<0.05, 0.01, and 0.001 respectively. NS indicates not statistically different. Two-tailed p values were calculated by Student’s t-test.
STIM1 promotes CRC cell migration by inducing COX-2 expression
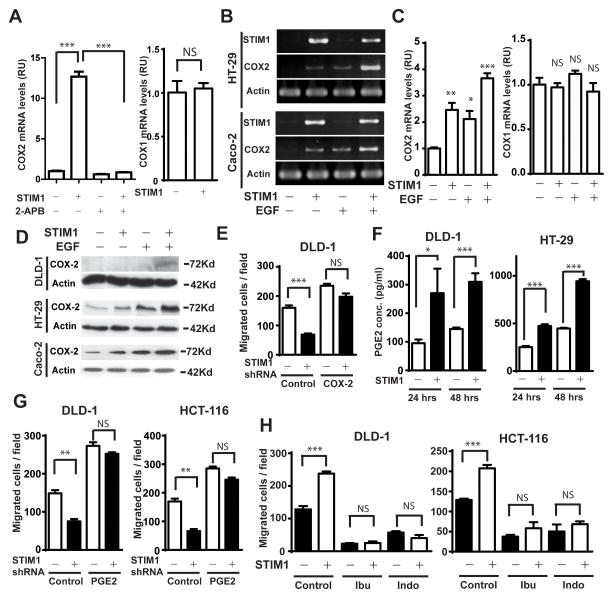
There is increasing evidence that COX-2, a pro-inflammatory key enzyme in the synthesis of prostaglandins, plays important roles in CRC progression20. We recently reported that histamine and EGF induced the expression of COX-2 by activating NFAT and NFκB through store-operated Ca2+ signaling21, 22. We hypothesized that hyperactive SOCE, as a result of STIM1 overexpression, would increase the levels of COX-2 in CRC. To examine this hypothesis we used qRT-PCR to determine effects of ectopic STIM1 on COX-2 expression in DLD-1 cells. As shown in Figure 4A, ectopically-expressed STIM1 increased COX-2 levels remarkably, without affecting the expression levels of COX-1 (Figure 4A). Treatment of DLD-1 cells with SOCE inhibitor 2-APB inhibited COX-2 expression by about 40%, and more importantly, almost completely abrogated STIM1-mediated COX-2 expression (Figure 4A). Next, we used semi-quantitative PCR to determine the effects of STIM1 overexpression on EGF-induced COX-2 expression in two different CRC cell lines. As shown in Figure 4B, ectopic expression of STIM1 in HT29 and Caco-2 cells not only dramatically increased the basal levels of COX-2 mRNA, but also significantly enhanced EGF-induced increase in COX-2 expression. The STIM1-mediated overexpression of COX-2 in DLD-1, HT29 and Caco-2 cells were further confirmed with quantitative real time PCR and western blot (Figure 4C and 4D). The expression of COX-1 in HT29 cells were not affected by either STIM1 or EGF treatment (Figure 4C).
Figure 4. The role of COX-2 in STIM1-mediated CRC cell migration.
A, Ectopic expression of STIM1 increased the levels of COX-2 in DLD-1 cells, which was abrogated in the presence of SOCE inhibitor 2-APB (100μM). As a control, the expression levels of COX-1 was not affected. B, Semi quantitative PCR data showing that ectopic STIM1 increased basal and EGF (25ng/ml)-induced COX-2 expression in HT29 and Caco-2 cells. C, The effects of ectopically expressed STIM1 on basal and EGF-induced COX-2 expression in HT-29 cells was determined through quantitative real-time PCR. The expression levels of COX-1 in these cells were used as a control. D, Ectopic STIM1 increased basal and EGF-induced COX-2 protein overexpression in DLD-1, HT-29 and Caco-2 cells was determined through western blot. E, Ectopically expressed COX-2 rescued cell migration in STIM1 knockdown DLD-1 cells. F, The effect of STIM1 overexpression on PGE2 synthesis in DLD-1 and HT-29 cells. Cells were cultured in serum free medium for indicated time and PGE2 levels in the medium were determined by using a ELISA kit (R&D system, CAT# KGE004B). G, Exogenous PGE2 (5 μM) rescued cell migration in STIM1 knockdown DLD-1 and HCT-116 cells. H, Inhibition of COX-2 with ibuprofen (100 μM) and indomethacin (20 μM) inhibited both the basal and STIM1-mediated migration of DLD-1 and HCT-116 cells. Cells were allowed to migrate for 18 hours in Boyden chamber assay. *, **, and *** indicate p<0.05, 0.01 and 0.001, respectively. NS indicates not statistically different. Two-tailed p values were calculated by Student’s t-test.
To examine the roles of STIM1-mediated COX-2 overexpression in CRC cell migration, we ectopically expressed COX-2 in control and STIM1 knockdown DLD-1 cells. We observed increased cell migration in DLD-1 cells after ectopic expression of COX-2 (Figure 4E). Importantly, COX-2 overexpression was able to rescue the inhibition of DLD-1 cell migration by STIM1 knockdown. PGE2 levels in DLD-1 and HT-29 conditioned medium increased by 2–3 fold after STIM1 overexpression (Figure 4F), suggesting that STIM1-mediated COX-2 overexpression would lead to increased production of prostaglandins by CRC cells. Moreover, exogenously supplemented PGE2 was able to stimulate cell migration and rescued STIM1 knockdown effects in DLD-1 and HCT-116 cells (Figure 4G). We further used ibuprofen and indomethecin, two commonly used non-steroid anti-inflammatory drugs, to inhibit COX-2 activity in colorectal cancer cells. As shown in Figure 4H, treatment with ibuprofen and indomethecin inhibited the migration of DLD-1 cells and HCT-116 cells by about 70%. Strikingly, the ectopic STIM1-promoted DLD-1 cell migration was almost completely abolished in the presence of ibuprofen and indomethecin. Taken together, our data suggested that elevated levels STIM1 induced COX-2 expression and prostaglandin synthesis, which was critical for CRC cell motility.
Discussion
Despite significant progress in our understanding of cancer dissemination in the last decade, the roles of Ca2+ in cancer metastasis remained poorly understood3. Recent studies from our group and others have suggested the importance of SOCE in the progression of various cancers2, 3, 11–13, 21. Our previous studies indicated that store-operated Ca2+ entry channels could be potential cancer therapeutic targets for breast cancer2. Indeed, several groups already showed that blockage of SOCE inhibited the migration, invasion and proliferation of cancer cells 10, 11, 23, 24. It is not clear, however, whether SOCE is dysregulated in tumors, or how dysregulated SOCE contribute to tumor progression. Here we provide evidence that STIM1, an essential component in SOCE, is overexpressed in CRC and elevated levels of STIM1 promotes CRC progression and CRC cell migration. STIM1 overexpression in CRC patients significantly correlates with CRC invasion, tumor size, lymph node metastasis and the serum levels of CEA. Although the prognositic value of pre-operative CEA levels in CRC patient is still a matter of debate, there is convincing evidence supporting the correlation between CEA values and CRC metastasis at the time of presentation. Orai1 expression also was upregulated in more than 80% of CRC surgical samples, further supporting the hyperactivation of SOCE pathway during CRC progression. In contrast, the expression of TRPV6 in CRC patient specimens had no correlation with clinicopathological parameters (supplementary Figure 3). Our data suggested that hyperactive SOCE promoted CRC progression and metastasis.
STIM2 is a STIM1 homolog that involves in stabilizing cytosolic and ER Ca2+ levels through feedback mechanisms8. It has been recently reported that STIM2 was overexpressed in CRC patients and yet STIM2 overexpression puzzlingly suppressed CRC tumor formation 25. It was also previously suggested that STIM1/STIM2 expression ratio served as a potential prognostic marker for breast cancer26. However, we found no significant correlation between STIM2 expression levels and CRC progression (supplementary Fig 3). The role of STIM2 in cancer progression is an understudied area and future investigation is warranted.
COX-2 is overexpressed in about 90% of colorectal carcinomas and 50% of adenomas, and is associated with poor prognosis among CRC patients20. We recently reported that SOCE was essential for EGF and histamine-induced COX-2 expression21, 22. In the current study, we further demonstrated that activation of SOCE through ectopic STIM1 dramatically increased both basal COX-2 and EGF-induced COX-2 expression in multiple CRC cell lines, including DLD-1, a CRC cell line with very low basal COX-2 levels.
Importantly, inhibition of COX-2 with ibuprofen and indomethacin, two commonly used NSAIDs, not only inhibited CRC cell migration, but also abolished elevated mobility in STIM1 overexpressing CRC cells. NSAIDs are drugs most commonly to relieve pain, fever and inflammation. Recent clinical studies provided interesting data supporting the roles of NSAIDs and other anti-inflammatory drugs in the prevention of CRC20. Our data suggested that NSAIDs and selective COX-2 inhibitors might also be beneficial to block CRC metastasis, especially in patients with hyperactive SOCE activity. It was recently reported that the inhibition of SOCE by curcumin might contribute to its anti-inflammatory effect27. It remained to be determined whether curcumin might be useful in inhibiting STIM1-mediated COX-2 overexpression.
Interestingly, NSAIDs also inhibited the migration of DLD-1, a CRC cell line with very low COX-2 basal levels. The NSAID inhibitory effects could be in part attributed to the inhibition of COX-1, which plays a housekeeping role in prostaglandin production. It is also possible the NSAID effects is partially due to their off-target inhibition of SOCE, as recently reported by Munoz and colleagues28. Nonetheless, ectopic COX-2 and PGE2 were sufficient to rescue the STIM1 knockdown effect, suggesting that STIM1-mediated CRC cell migration was at least partially due to its regulation of COX-2 expression and prostaglandin synthesis. The role of COX-2, however, may not be limited to CRC cell migration. It is worth noting that although STIM1 knockdown had no effect on CRC cell proliferation in vitro, STIM1 overexpressing tumors tend to be bigger. It would be intriguing to determine whether STIM1 regulates CRC growth through COX-2 and prostaglandin-mediated angiogenesis and inflammatory cell recruitment.
It is intriguing to note that STIM1 was originally reported to be a putative tumor suppressor gene candidate based on its localization on human chromosome region 11p15.5. While the loss of 11p13-11p15.5 has been associated with several malignancies, evidence supporting the tumor suppressor role of STIM1 is scarce. It would be interesting to determine whether STIM1 may alternatively be a tumor suppressor in a different context. It is also worth noting that the proto-oncogene HRAS is also localized to 11p15.5.
Recent reports from other groups and ours suggested that the SOCE pathway is frequently dysregulated to promote cancer cell migration, invasion, metastasis and tumor growth in various cancers 2, 10–13, 21–23, 26, 29–31. The association of STIM1 with CRC lymph node metastasis, pre-operative CEA levels and cell migration suggested that STIM1 dysregulation in CRC may promote CRC distant metastasis and correction of dysregulated SOCE may improve disease free and overall survival in CRC patients. With that said, cancer metastasis is a complicated, multi step event and cell migration is only part of the metastatic process. It would be important to determine the association between STIM1 and CRC prognosis in large cohort of CRC patients, and to establish the causal role of STIM1 in CRC metastasis using xenograft or genetically engineered CRC mouse models in future research endeavors.
Materials and methods
Immunohistochemistry staining
CRC tissue microarray (TMA) with TNM staging information, tumor grade and tumor stage was purchased from Biomax (Rockville, MD). TMA slides were baked in a 60°C oven for 60 min, deparafinized and hydrated to dH2O. Epitope was retrieved by incubating with 0.21% Citrate Buffer (pH 6) in a 99°C water bath for 30 min. After epitope retrieval, slides were washed in running water for 2–5 min, incubated with 3% H2O2 for 5 min and washed with dH2O and PBS. After incubating with normal serum (1:20, diluted in 2% BSA/PBS) 10 min at room temperature in a humidity chamber, slides were incubated with 150 μl monoclonal anti-STIM1 antibody (1:300) (Thermo Scientific) at 4°C in a humidity chamber overnight. Slides will then be washed with PBS (3 times, 10 min each) and incubated with peroxidase conjugated anti-mouse secondary antibody (1:500) at room temperature for 60 min. After 3 wash with PBS (10 min each), slides were transfer to a bath of diaminobenzidine (DAB) for 5–15 min, washed in running water for one minute, and then counter stain using Harris modified hemotoxylin (Fisher). Slides were then dehydrated and mounted. The results of STIM1 immunohistochemical staining was estimated by two investigators without knowledge of patient’s clinical information. The staining intensity of STIM1 was graded on a scale from 0 to 3 (0 for no staining, 1 for weak immunoreactivity, 2 for moderate immunoreactivity, and 3 for strong immunoreactivity). The percentage of immunoreactivity was scored on a scale from 0 to 3 (0 for no positive cells, 1 for <25% of cells positive, 2 for 25%–50% of cells positive, and 3 for >50% of cells positive). The staining intensity score and the percentage of immunoreactivity score were then multiplied to obtain a composite score (CS). Depending on the CS, STIM1 expression was classified as high expression (CS>6) and low expression(CS≤6).
Cell migration assay
Transwell cell migration assay was carried out as previously described2, 32, 33. Cells (1×105) suspended in starvation medium were added to the upper chamber of an insert (6.5 mm diameter, 8-μm pore size, Becton Dickenson), and the insert was placed in a 24-well dish containing starvation medium with or without 10% FBS. Migration assays were carried out for 24 hours. Cells were fixed with 3.7% formaldehyde, stained with crystal violet staining solution, and cells on the upper side of the insert were removed with a cotton swab. Three randomly selected fields (10 × objectives) on the lower side of the insert were photographed, and the migrated cells were counted. The migration was expressed as the average number of migrated cells in a field.
Wound healing assay
Cells were seeded in wells of Culture-Insert (Ibidi GmbH, Martinsried, Germany) for 24 h. The Culture-Inserts were removed to reveal the wound gap. The baseline and the wound closure after treatment were photographed using a digital camera attached to an inverted microscope (Nikon Eclipse Ti, Nikon, Japan) with a 10x objective34.
Patients and tissue specimens
Enrolled in this study were 38 CRC patients (21 males and 17 females; Mean age, 62.13±15.75 years) treated at the Department of Surgery at Kaohsiung Medical University Hospital. These patients have AJCC/UICC(American Joint Commission on Cancer/International Union Against Cancer) stage I – III CRC. Patients with other malignant diseases in their medical history were excluded. Clinical stage and pathological features of primary tumors were defined according to criteria of the AJCC/UICC 35. Written informed consent was obtained from all subjects and/or guardians for use of their tissue samples. Sample acquisition and subsequent use were approved by the institutional review board at the Kaohsiung Medical University Hospital. Complete medical history, physical examination, and laboratory studies, including assessing serum carcinoembryonic antigen (CEA) levels were reviewed. Computed tomography (CT) or magnetic resonance imaging (MRI) of abdomen, abdominal ultrasonography, and chest radiography, bone scans, and colonoscopy were performed before surgical intervention. Tumor grading and normalcy of adjacent tissues were evaluated by pathologists. The relevant data were listed in Table 1. Samples were further used for real-time PCR, ELISA and immunofluorescence analysis.
STIM1 ELISA
To determine whether STIM1 protein levels were elevated in CRC tissues, normal and tumor tissues were collected from a cohort of 20 patients (a different cohort from the 38 patients used in the qPCR study) with colon cancer in KMUH. Frozen tissue was prepared to determine STIM1 expression by STIM1 ELISA kit (Cusabio Biotech, PR China). Briefly, 200 μg of tissue lysate was added into microplate and was incubated with pre-coated STIM1 specific antibody at 4°C overnight. The wells were then emptied and rinsed five times with washing buffer. Plate absorbance was measured at a wavelength of 450 nm.
Reverse transcription-PCR, RT-PCR
Tumor tissues and paired adjacent normal tissues were collected from CRC patients. Total mRNA from these samples was extracted and STIM1 expression levels in these samples were evaluated using STIM1 primers: forward primer AGA AAC ACA CTC TTT GGC ACC, reverse primer AAT GCT GCT GTC ACC TCG; COX-2: forward primer AGA CAGCGTAAACTGCGCCTTT, reverse primer CAGCAATTTGCCTGGTGAATGATTC. Other primers used: STIM2: forward primer TGA TCT TCA GTC CTG CAA GC, reverse primer ACA AAG GTC ATG TGG GAT GC; Orai1: forward primer TTC CTA GCT GAG GTG GTG CT, reverse primer CGA TAA AGA TCA GGC CGA AG; COX-1: forward primer CCT CAT GTT TGC CTT CTT TGC, reverse primer GGC GGG TAC ATT TCT CCA TC; and β-actin: forward primer ATC TCC TTC TGC ATC CTG TCG GCA AT, reverse primer CAT GGA GTC CTG GCA TCC ACG AAA C. The SYBR Green PCR master mix reagent (Applied Biosystems, Foster City, CA) was used to amplify the cDNA and the products were detected by Applied Biosystems 7500.
Detection of serum CEA
A 3-ml sample of peripheral blood was obtained from enrolled patients less than one week prior to operation. Serum CEA levels were determined by means of an enzyme immunoassay test kit (DPC Diagnostic Product Co., Los Angeles, CA) with the upper limit of 5 ng/ml defined as normal according to the manufacturers of the kits that were used.
Cell culture
HT-29, DLD-1, HCT116, Caco-2 and SW480 cells were bought from ATCC. Cells were cultured (37°C, 5% CO2) in F-12K Nutrient Mixture, Kaighn’s Modification (GIBCO) with 10% fetal bovine serum, 10mM HEPES (J.T. Baker), 10mM TES (Sigma), 50μg/mL L-Ascorbic Acid (J.T. Baker), ITS supplement (Insulin: Transferrin: Sodium Selenite 0.01 mg/ml: 0.01 mg/ml: 10 ng/ml, from Sigma), 10μg/mL ECGS (Sigma) and 10% penicillin-streptomycin. For Ca2+ imaging experiments, cells were prepared onto glass coverslips and used 24–48 h after plating.
STIM1 Plasmids transfection
Cells were seeded in 6-cm plastic dishes one day before transfecting plasmid. STIM1 plasmids (Gifts from Prof. Baba (RIKEN, Japan)) or control plasmids were transfected into cells by using lipofectamine 2000 (Invitrogen). Following transfection, the cells were cultured for 24 hours and then prepared for EGF stimulation. For stable expression of STIM1 CRC cells were infected with retroviral vectors. Retroviral particles were prepared as previously described36.
WST-1 cell proliferation assay
Cells were harvested and then incubated with WST-1 reagent (Roche) at 37°C for 5 min. Absorbance was measured at 450nm with background subtraction at 600 nm.
RNA interference
Stable knockdown of STIM1 was performed using pSUPER.Retro.puro vector (Oligoengine) encoding small hairpin RNA. STIM1 sh1 and sh2 has been previously described2. STIM1 sh3 is targeting the following sequence: 5′-AGAAGGAGCTAGAATCTCAC-3′. A vector encoding non-targeting control shRNA was previously described37. Transient knockdown of STIM1 in CRC cancer cells were achieved through transfection of siRNA into cells using lipofectamine 2000 (Invitrogen, Inc). The siRNA for STIM1 or control siRNA was purchased from Invitrogen, Inc. Cells were seeded in 6 well plastic plates 1 day before applying siRNA.
Calcium imaging
The intracellular Ca2+ responses were induced by application of thapsigargin (TG) (Sigma Aldrich), according to methods previously described38. Before the experiments, cells were stained with 1 μM Fura2-AM (Molecular Probes) at 37°C for 20 min and then washed with BSS buffer (5.4 mM KCl, 5.5 mM D-glucose, 1 mM MgSO4, 130 mM NaCl, 20 mM Hepes pH 7.4, and 2 mM CaCl2). SOCE response were estimated based on the ratio of fluorescence intensities emitted upon excitation with consecutive 3-s pulses of 340 nm/380 nm light at a resolution of 1376 × 1038 pixels using an Olympus Cell^R IX81 fluorescence microscope (Olympus) equipped with an MT 20 illumination system (Olympus) and UPLanApo 10× objective lens.
Western blotting
Total cell lysates (60 μg) were analyzed by SDS-PAGE on a 12.5% gel. After electro-blotting to nitrocellulose membrane, membranes were blocked with non-fat dry milk for 2 hours at room temperature. Membranes were washed with PBST three times and then incubated with primary antibodies for 1 hour at room temperature. Antibodies were obtained from the following sources: STIM1 from cell signaling; beta-actin from cell signaling. Anti-STIM1 antibody was used at 1:3000 dilution. Anti- beta-actin was used at 1:8000 dilution. Then, membranes were washed with PBST three times and incubated with 1:8000 dilution of peroxidase-linked anti-mouse IgG (Amersham Biosciences) for 1 hour at room temperature. After washing with PBST, the bands were detected by an ECL-plus western blotting detection system (Amersham Biosciences).
Statistical analysis
JMP 9.0 software for windows (SAS Institute, Cary, North Carolina) was used for the statistical analysis (univariate analysis, regression analysis, multivariate analysis). The difference between STIM1 expression level and clinical pathology features were performed by chi-square test. The correction of age, gender and location were performed by multiple linear regression. In addition, the multivariate analysis was conducted to examine the associations between STIM1 expression level and multi factors. Regression analysis was performed to adjust the effect of age, gender, and tumor location. Student’s t-test was used, as indicated in the figure legends, when the data are normally distributed. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Minjung Kim for assistance with IHC staining. This study was partly supported by funding from excellence for cancer research center grant, Department of Health, Executive Yuan, Taiwan, ROC (DOH100-TD-C-111-002) W.C. Chang, a grant (NSC 98-2320-B-037-028-MY2) from the National Science Council, Taiwan, ROC to W.C. Chang, and an NIH grant R01CA175741 to Shengyu Yang.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nature reviews Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Huang XY. Ca2+ Influx through L-type Ca2+ Channels Controls the Trailing Tail Contraction in Growth Factor-induced Fibroblast Cell Migration. J Biol Chem. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature reviews Molecular cell biology. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current biology: CB. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou MF, Kuo HC, Li JH, Wang YS, Chang CC, Chen KC, et al. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim Biophys Acta. 2011;1810:1278–1284. doi: 10.1016/j.bbagen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, et al. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochemical and biophysical research communications. 2011;411:786–791. doi: 10.1016/j.bbrc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Jans R, Mottram L, Johnson DL, Brown AM, Sikkink S, Ross K, et al. Lysophosphatidic acid promotes cell migration through STIM1- and Orai1-mediated Ca2+(i) mobilization and NFAT2 activation. J Invest Dermatol. 2013;133:793–802. doi: 10.1038/jid.2012.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. Faseb J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 18.Wolmark N, Fisher B, Wieand HS, Henry RS, Lerner H, Legault-Poisson S, et al. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg. 1984;199:375–382. doi: 10.1097/00000658-198404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarrigle D, Shan D, Yang S, Huang XY. Role of tyrosine kinase Csk in G protein-coupled receptor- and receptor tyrosine kinase-induced fibroblast cell migration. The Journal of biological chemistry. 2006;281:10583–10588. doi: 10.1074/jbc.M513002200. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Dubois RN. The Role of Anti-Inflammatory Drugs in Colorectal Cancer. Annu Rev Med. 2012 doi: 10.1146/annurev-med-112211-154330. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Chen BK, Wang YS, Tsai YT, Chen WC, Chang WC, et al. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell Signal. 2012;24:162–169. doi: 10.1016/j.cellsig.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Chai CY, Chen WC, Hou MF, Wang YS, Chiu YC, et al. Histamine regulates cyclooxygenase 2 gene activation through Orai1-mediated NFkappaB activation in lung cancer cells. Cell Calcium. 2011;50:27–35. doi: 10.1016/j.ceca.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida J, Iwabuchi K, Matsui T, Ishibashi T, Masuoka T, Nishio M. Knockdown of stromal interaction molecule 1 (STIM1) suppresses store-operated calcium entry, cell proliferation and tumorigenicity in human epidermoid carcinoma A431 cells. Biochemical pharmacology. 2012;84:1592–1603. doi: 10.1016/j.bcp.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Aytes A, Mollevi DG, Martinez-Iniesta M, Nadal M, Vidal A, Morales A, et al. Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog. 2012;51:746–753. doi: 10.1002/mc.20843. [DOI] [PubMed] [Google Scholar]
- 26.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Molecular cancer therapeutics. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 27.Shin DH, Seo EY, Pang B, Nam JH, Kim HS, Kim WK, et al. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. Journal of pharmacological sciences. 2011;115:144–154. doi: 10.1254/jphs.10209FP. [DOI] [PubMed] [Google Scholar]
- 28.Munoz E, Valero RA, Quintana A, Hoth M, Nunez L, Villalobos C. Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria. J Biol Chem. 2011;286:16186–16196. doi: 10.1074/jbc.M110.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry PA, Birnie R, Droop AP, Maitland NJ, Collins AT. The calcium sensor STIM1 is regulated by androgens in prostate stromal cells. Prostate. 2011;71:1646–1655. doi: 10.1002/pros.21384. [DOI] [PubMed] [Google Scholar]
- 30.Ryu S, McDonnell K, Choi H, Gao D, Hahn M, Joshi N, et al. Suppression of miRNA-708 by Polycomb Group Promotes Metastases by Calcium-Induced Cell Migration. Cancer Cell. 2013;23:63–76. doi: 10.1016/j.ccr.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Chantome A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Gueguinou M, et al. Pivotal Role of the Lipid Raft SK3-Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013;73:4852–4861. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, He H, Xiong Y, Lu S, Shen J, Cheng A, et al. Fascin protein is critical for transforming growth factor beta protein-induced invasion and filopodia formation in spindle-shaped tumor cells. The Journal of biological chemistry. 2011;286:38865–38875. doi: 10.1074/jbc.M111.270413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Yang S, Jakoncic J, Zhang JJ, Huang XY. Migrastatin analogues target fascin to block tumour metastasis. Nature. 2010;464:1062–1066. doi: 10.1038/nature08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang IH, Tsai YT, Chiu SJ, Liu LT, Lee HH, Hou MF, et al. Involvement of STIM1 and Orai1 in EGF-mediated cell growth in retinal pigment epithelial cells. Journal of biomedical science. 2013;20:41. doi: 10.1186/1423-0127-20-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-Legal MF, Shen B. Engineered production of iso-migrastatin in heterologous Streptomyces hosts. Bioorg Med Chem. 2008 doi: 10.1016/j.bmc.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Zhang JJ, Huang XY. Mouse models for tumor metastasis. Methods Mol Biol. 2012;928:221–228. doi: 10.1007/978-1-62703-008-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, He H, Pillai S, Xiong Y, Challa S, Xu L, et al. GATA3 transcription factor abrogates Smad4-mediated fascin overexpression, invadopodium formation and breast cancer cell invasion. J Biol Chem. 2013 doi: 10.1074/jbc.M113.506535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu WL, Tsai MH, Lin MW, Chiu YC, Lu JH, Chang CH, et al. Differential effects of arsenic on calcium signaling in primary keratinocytes and malignant (HSC-1) cells. Cell calcium. 2012;52:161–169. doi: 10.1016/j.ceca.2012.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.