Abstract
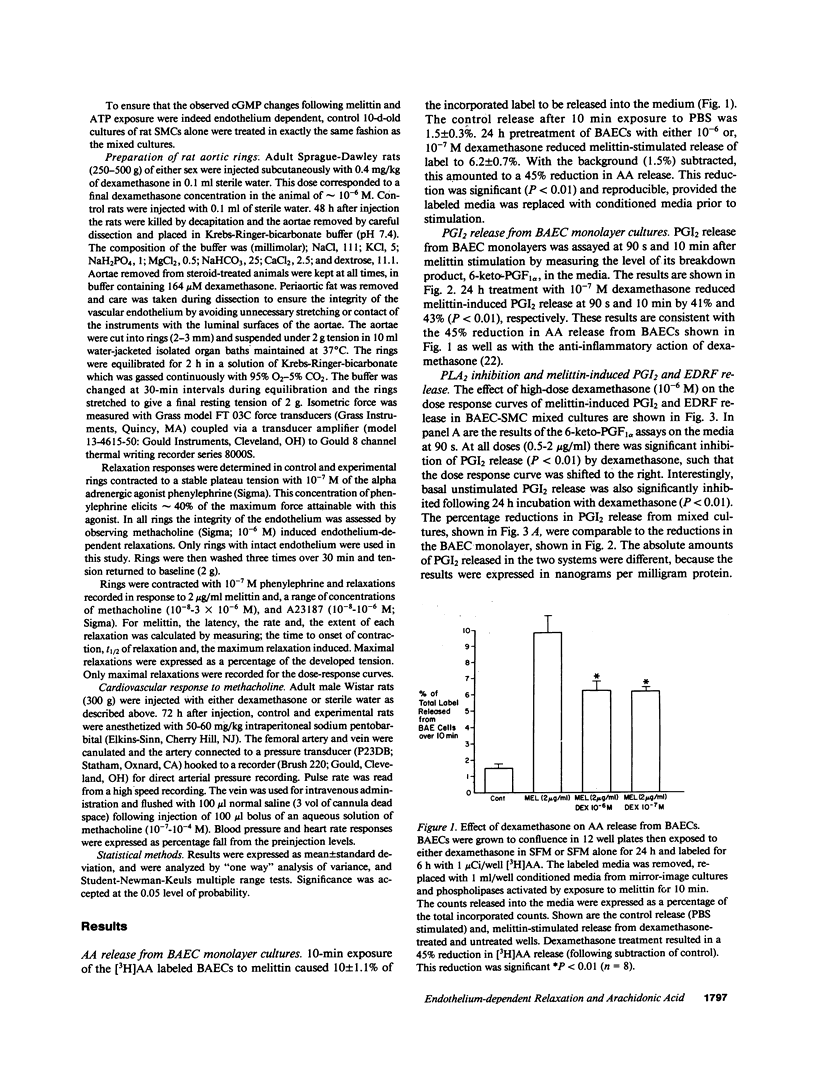
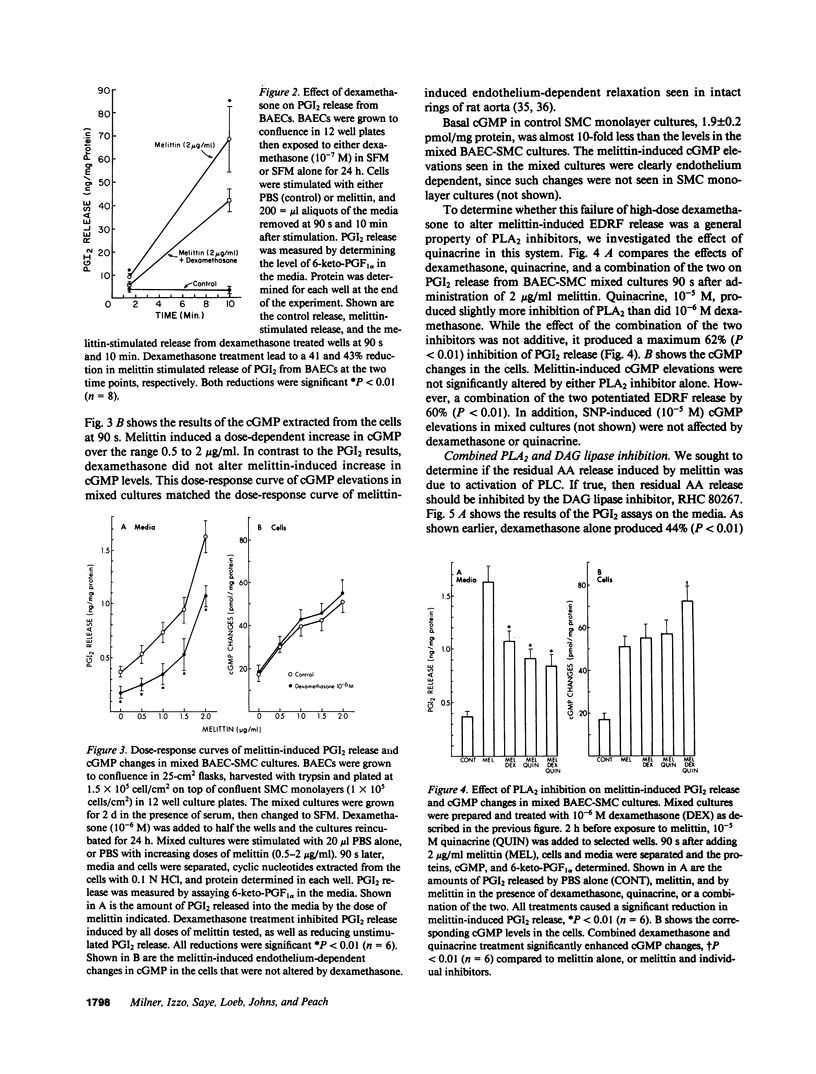
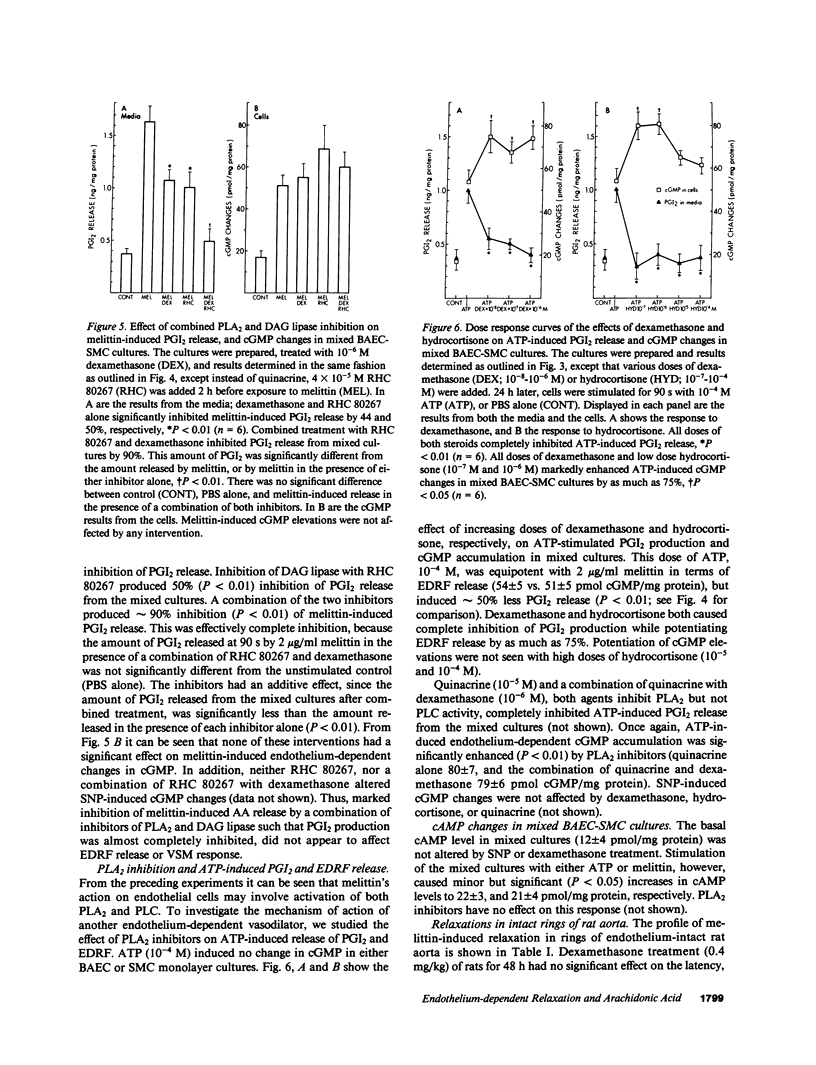
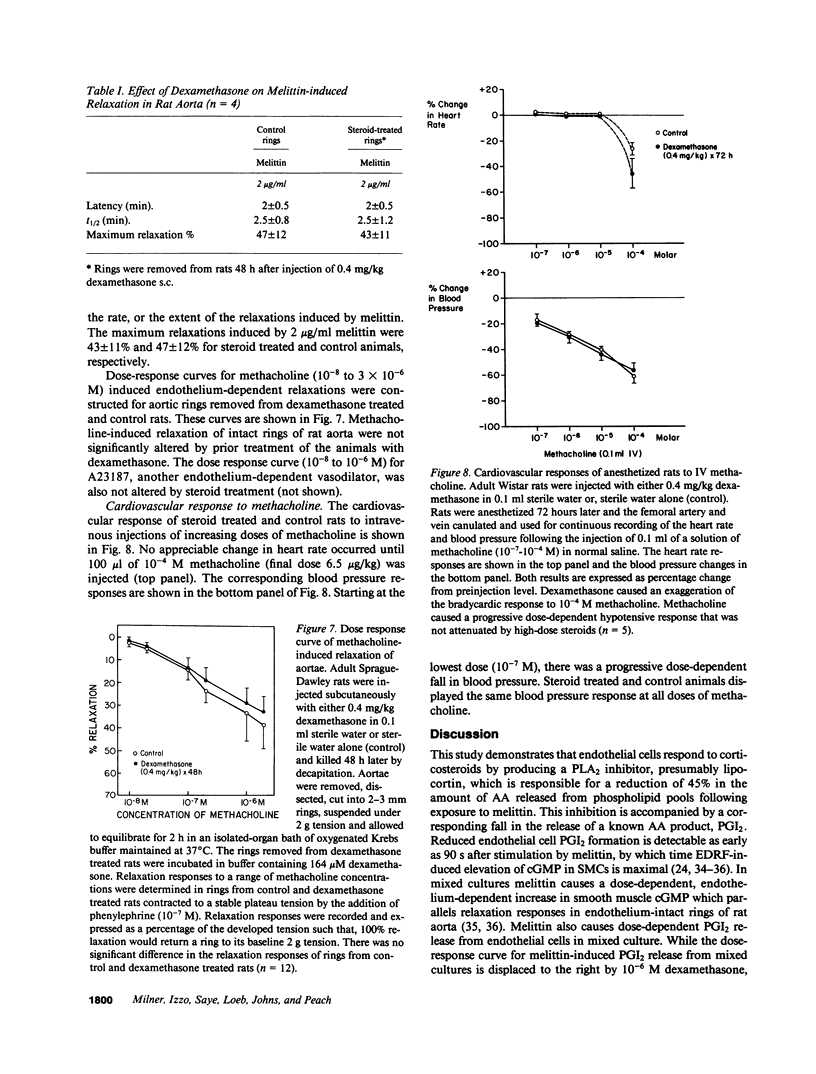
Endothelium-dependent relaxation is mediated by the release from vascular endothelium of an endothelium-derived relaxing factor (EDRF). It is not clear what role arachidonic acid has in this process. Inhibition of phospholipase A2, and diacylglycerol lipase in cultured bovine aortic endothelial cells caused a marked reduction in agonist-induced arachidonic acid release from membrane phospholipid pools, and complete inhibition of prostacyclin production. EDRF release, assayed by measuring endothelium-dependent cGMP changes in mixed endothelial-smooth muscle cell cultures, was not inhibited under these conditions. In fact, EDRF release in response to two agonists, melittin and ATP, was actually increased in cells treated with phospholipase A2 inhibitors. In addition, pretreatment of rats with high-dose dexamethasone, an inhibitor of PLA2, did not attenuate endothelium-dependent relaxation in intact aortic rings removed from the animals, or depressor responses in anesthetized animals induced by endothelium-dependent vasodilators. In summary, inhibition of arachidonic acid release from membrane phospholipid pools does not attenuate endothelium-dependent relaxation in rats, or the release and/or response to EDRF in cultured cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Pinto A., Mullane K. M., Levere R. D., Spokas E. Presence of cytochrome P-450-dependent monooxygenase in intimal cells of the hog aorta. Hypertension. 1985 Nov-Dec;7(6 Pt 1):899–904. doi: 10.1161/01.hyp.7.6.899. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S. The p35/p36 substrates of protein-tyrosine kinases as inhibitors of phospholipase A2. Cell. 1986 Jul 18;46(2):149–150. doi: 10.1016/0092-8674(86)90729-4. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix J. F., Colard O., Rothhut B., Russo-Marie F. Characterization and partial purification of 'renocortins': two polypeptides formed in renal cells causing the anti-phospholipase-like action of glucocorticoids. Br J Pharmacol. 1983 May;79(1):313–321. doi: 10.1111/j.1476-5381.1983.tb10526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Ryan U. S., Ryan J. W. Glucocorticoid modulation of prostacyclin production in cultured bovine pulmonary endothelial cells. J Pharmacol Exp Ther. 1985 Jun;233(3):650–655. [PubMed] [Google Scholar]
- Davies J. M., Williams K. I. Endothelial-dependent relaxant effects of vaso-active intestinal polypeptide and arachidonic acid in rat aortic strips. Prostaglandins. 1984 Feb;27(2):195–202. doi: 10.1016/0090-6980(84)90073-x. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. Endothelium-dependent vasodilation by melittin: are lipoxygenase products involved? Am J Physiol. 1985 Jul;249(1 Pt 2):H14–H19. doi: 10.1152/ajpheart.1985.249.1.H14. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Trogisch G., Busse R. Species-dependent differences in the nature of endothelium-derived vascular relaxing factor. Eur J Pharmacol. 1984 Nov 27;106(3):639–643. doi: 10.1016/0014-2999(84)90071-2. [DOI] [PubMed] [Google Scholar]
- Ganz P., Davies P. F., Leopold J. A., Gimbrone M. A., Jr, Alexander R. W. Short- and long-term interactions of endothelium and vascular smooth muscle in coculture: effects on cyclic GMP production. Proc Natl Acad Sci U S A. 1986 May;83(10):3552–3556. doi: 10.1073/pnas.83.10.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Hong S. L. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980 Jun 15;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of endothelial cell cyclic nucleotide metabolism by prostacyclin. J Clin Invest. 1981 Feb;67(2):540–546. doi: 10.1172/JCI110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Burke T. M., Wood K. S., Wolin M. S., Kadowitz P. J. Association between cyclic GMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery. J Pharmacol Exp Ther. 1984 Mar;228(3):682–690. [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Wolin M. S., McNamara D. B., Hyman A. L., Kadowitz P. J. Differences in responsiveness of intrapulmonary artery and vein to arachidonic acid: mechanism of arterial relaxation involves cyclic guanosine 3':5'-monophosphate and cyclic adenosine 3':5'-monophosphate. J Pharmacol Exp Ther. 1985 Jun;233(3):560–569. [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Silver M. J., Smith J. B., Macarak E. Bovine endothelial cells in culture produce thromboxane as well as prostacyclin. J Clin Invest. 1981 May;67(5):1292–1296. doi: 10.1172/JCI110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juchau M. R., Bond J. A., Benditt E. P. Aryl 4-monooxygenase and cytochrome P-450 in the aorta: possible role in atherosclerosis. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3723–3725. doi: 10.1073/pnas.73.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Loeb A. L., Johns R. A., Milner P., Peach M. J. Endothelium-derived relaxing factor in cultured cells. Hypertension. 1987 Jun;9(6 Pt 2):III186–III192. doi: 10.1161/01.hyp.9.6_pt_2.iii186. [DOI] [PubMed] [Google Scholar]
- Loeb A. L., Owens G. K., Peach M. J. Evidence for endothelium-derived relaxing factor in cultured cells. Hypertension. 1985 Sep-Oct;7(5):804–807. doi: 10.1161/01.hyp.7.5.804. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R., Winter I., Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987 Mar;9(3):295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Aiken J. W., Hemker D. P., Shebuski R. J., Gorman R. R. Prostacyclin stimulation of dog arterial cyclic AMP levels. Prostaglandins. 1979 Dec;18(6):915–925. doi: 10.1016/0090-6980(79)90128-x. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Singer H. A., Loeb A. L. Mechanisms of endothelium-dependent vascular smooth muscle relaxation. Biochem Pharmacol. 1985 Jun 1;34(11):1867–1874. doi: 10.1016/0006-2952(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Arachidonic acid-induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450-dependent pathway. J Pharmacol Exp Ther. 1987 Mar;240(3):856–863. [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Arachidonic acid-induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450-dependent pathway. J Pharmacol Exp Ther. 1987 Mar;240(3):856–863. [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Cytochrome P-450-dependent monooxygenase activity and endothelial-dependent relaxations induced by arachidonic acid. J Pharmacol Exp Ther. 1986 Feb;236(2):445–451. [PubMed] [Google Scholar]
- Proctor K. G., Falck J. R., Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987 Jan;60(1):50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Endothelium-dependent relaxation of rabbit aorta. I. Relaxation stimulated by arachidonic acid. J Pharmacol Exp Ther. 1983 Sep;226(3):790–795. [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Endothelium-dependent relaxation of rabbit aorta. II. Inhibition of relaxation stimulated by methacholine and A23187 with antagonists of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983 Sep;226(3):796–801. [PubMed] [Google Scholar]
- Singer H. A., Saye J. A., Peach M. J. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels. 1984;21(5):223–230. doi: 10.1159/000158515. [DOI] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]


