Abstract
Hemorrhagic metastatic brain tumors of hepatocellular carcinoma (HCC) are rare and have been mostly presented as intracranial hemorrhage (ICH). A 51-year-old male patient presented with sudden altered level of consciousness. He suffered from HCC since 2010 and transarterial chemoembolization was performed three times for HCC. The brain computed tomography (CT) scans revealed subdural hematoma (SDH) in the right fronto-temporal area and 6.0×3.5 cm sized ICH in the right parieto-occipital lobe. Brain angiographic CT scans demonstrated that the hemorrhagic lesions did not include any enhancing lesions and vascular abnormalities. We undertook a decompressive craniectomy and evacuation of the acute SDH and ICH. During evacuation of ICH, the yellowish mass was observed in the cortical surface of the right occipital lobe. Pathological examination displayed the findings of metastatic brain tumor from HCC. Metastatic brain tumors should be considered in the differential diagnosis as a cause of spontaneous SDH with ICH.
Keywords: Neoplasm metastasis; Carcinoma, hepatocellular; Intracranial hemorrhages; Hematoma, subdural
INTRODUCTION
Intracranial hemorrhage (ICH) from distant metastasis of primary cancer is relatively rare and its incidence accounts for 0.9-11% [1]. According to the literature, the incidence of ICH due to the hepatocellular carcinoma (HCC) is 1.3-54.8% [2,3]. Brain metastasis from HCC is frequently associated with ICH, but spontaneous epidural hematoma (EDH) from metastasis of HCC is very rare and it has been reported only 8 cases in the literature [4,5,6,7,8,9,10,11]. Intracranial subdural hematoma (SDH) from HCC has been reported only a case in Japan [12]. We describe a case of concomitant intracranial acute SDH and ICH originating from metastatic HCC and review of the literatures.
CASE REPORT
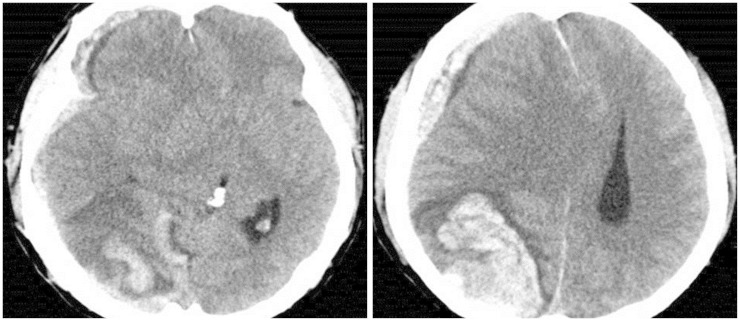
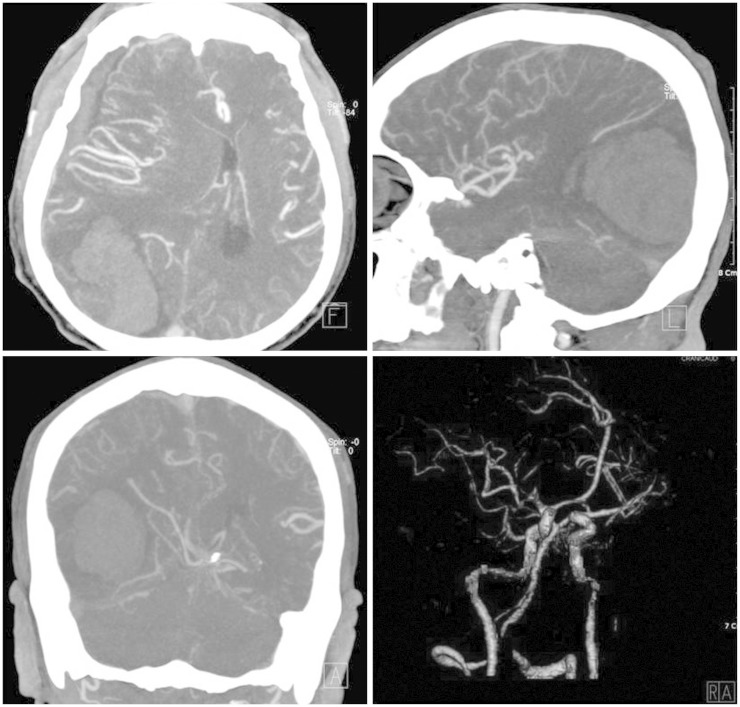
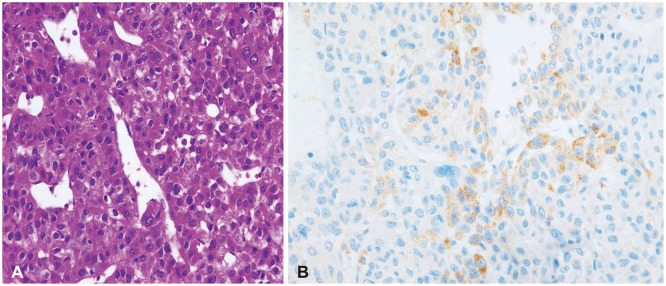
A 51-year-old male patient visited our hospital presented with sudden altered consciousness. He had been well previously and no history of head trauma. He suffered from HCC since June 2010 and transcatheter arterial chemoembolization (TACE) was performed three times for HCC in past history. The patient was not followed-up after receiving TACE. Neurological examination revealed quadriparesis, semicomatose mentality, and both pupils were unequal without light response. Whole blood analysis revealed a leukocyte count of 7,700/mm3, hemoglobin 10.6 g/dL, platelet count 6,200 mm3, serum albumin 2.5 g/dL, total bilirubin 5.9 mg/dL, and international normalized ratio 2.0. Anti-human immunodeficiency virus antibodies and anti-hepatitis C virus antibodies were negative except hepatitis B surface antigen. Other laboratory data showed abnormal findings of coagulation, such as prolonged prothrombin time and partial thromboplastin time, and slightly elevated fibrinogen level. The score of the liver disease was 10 points and Child-Pugh class was C. He took brain computed tomography (CT) scans. The brain CT scans revealed SDH in the right fronto-temporal area with midline shift and 6.0×3.5 cm sized ICH in the right parieto-occipital lobe, and no evidence of head injury (Fig. 1). Brain angiographic CT scans disclosed no enhancing lesions or vascular abnormalities in hemorrhagic lesions (Fig. 2). We underwent a decompressive craniectomy and evacuation of the acute SDH and ICH. There was no evidence of trauma on the scalp, subgaleal space, and skull bone. During evacuation of ICH, the yellowish-brown and firm mass was observed in the cortical surface of the right occipital lobe (Fig. 3). The tumor was totally removed and meticulous hemostasis was performed. Pathological examination displayed the findings of metastatic brain tumor from HCC. The specimen of tumor showed the pleomorphism and trabecular pattern, cells with abundant cytoplasm, prominent nucleoli, and numerous mitotic figures (Fig. 4A). Immunohistochemical staining of hepatocyte antigen was also positive (Fig. 4B). Postoperatively, his clinical status was not improved, and disseminated intravascular coagulation developed concurrently and the patient died on the 8th postoperative day.
Fig. 1. Computed tomographic scan of brain demonstrating subdural hemorrhage in the right fronto-temporal area and 6.0×3.5 cm sized intracerebral hemorrhage in the right parieto-occipital lobe.
Fig. 2. Brain angiographic computed tomographic scans showing no enhanced lesion or vascular abnormality in the hemorrhagic area.
Fig. 3. Gross photography of tissue for biopsy was yellowish and brown.
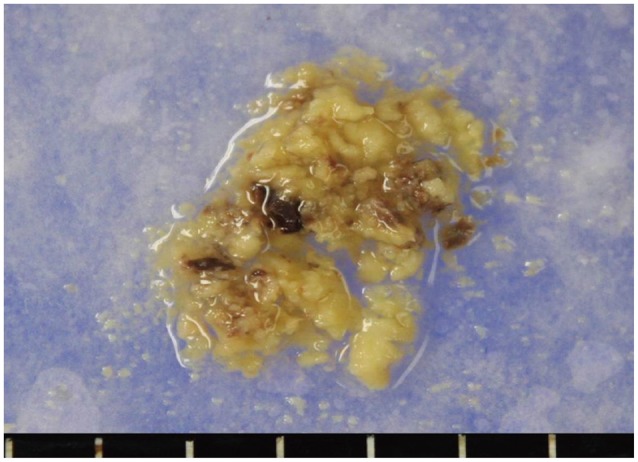
Fig. 4. Photomicrographs of the surgical specimen. A: The result of hematoxylin and eosin staining shows pleomorphism, trabecular pattern of cells with abundant cytoplasm and prominent nucleoli. Numerous mitotic figures are also observed (×400). B: The cells were diffusely reactive in the immunohistochemical staining to hepatocyte antigen (×400).
DISCUSSION
Hepatocellular cellular carcinoma is one of the most common cancers in Asia and Africa, with high prevalence of chronic viral hepatitis B and C infection. Brain metastases are the most common intracranial neoplasm in adults, and represent an important cause of morbidity and mortality. The number of brain metastases that are detected antemortem is increasing due to improvements in modern neuroimaging modalities that can detect small metastases in asymptomatic patients. The prognosis for these patients is very poor, but efforts to improve survival are currently under active investigation. Choi et al. [2] reported that the treatment modality, number of brain lesions, and Child Pugh classification were statistically significant prognostic factors for survival. In our case, the tumor was single lesion and mass was totally removed. So the significant prognostic factor of this patient was Child Pugh C. The noticeable findings of brain metastasis from HCC is that it has a tendency of bleeding, and it can rebleed after treatment [13].
The incidence of HCC metastasis has been reported to be less than 5% with the most common sites being the regional lymph nodes, lung, peritoneum and adrenal glands, however, brain metastases are rare [3,14]. Friedman [15] and Nakagawa [9] reported that the incidence of brain metastasis from HCC is 0.3% to 1.7%. Choi et al. [2] reported that 62 (0.9%) among 6,919 patients with HCC had a diagnosis of brain metastasis in Korea, and various types of ICH were also observed in 54.8% of HCC brain metastasis.
Hepatocellular carcinoma initially invades the hepatic and portal veins, and then extensively involves the regional lymph nodes. Pulmonary vascular metastasis develops after venous invasion and may be followed by widespread hematogenous metastasis to the brain or bone [16,17,18]. In HCC and other carcinomas, seeding of the brain, the meninges, or the cranium is usually developed in the distribution of the middle cerebral artery [19].
The widely accepted theory is that impairment of the blood perfusion is developed by tumor embolism in the dural vein, thereby causing the dilation and breakdown of capillary vessels, resulting in SDH [20]. Prominent neovascularity, sinusoidal vessels, and concomitant liver dysfunction with coagulopathy may predispose to hemorrhage in metastatic HCC [21]. But the combination of metastasis in dural vessels with a coagulation defect can lead to a large SDH [22]. Only 8 cases of acute and chronic EDH by HCC brain metastasis have been reported in the literature [4,5,6,7,8,9,10,11]. HCC associated with SDH have been reported only 1 case in Japan [1]. Tanaka et al. [12] reported the case of nontraumatic SDH secondary to dural metastasis of HCC in 2004. To our best knowledge, concomitant ICH and SDH like our case had not been reported.
In our case, the location of SDH was obviously far away from ICH. But it can't be ignored that ICH has been penetrated the pia mater resulting SDH. Gross invasion of the dura was not observed in our case. However, we can't exclude microscopic dural invasion because we did not obtain dural biopsy.
In conclusion, we report an uncommon case of concomitant SDH and ICH complicated by brain metastasis of HCC, and discuss the pertinent literatures. Metastatic brain tumors should be considered in the differential diagnosis as a cause of spontaneous SDH in patients with HCC.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Wakisaka S, Tashiro M, Nakano S, Kita T, Kisanuki H, Kinoshita K. Intracranial and orbital metastasis of hepatocellular carcinoma: report of two cases. Neurosurgery. 1990;26:863–866. doi: 10.1097/00006123-199005000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 3.Kuga Y, Waga S, Itoh H. Intracranial hemorrhage due to brain metastasis from hepatocellular carcinoma--case report. Neurol Med Chir (Tokyo) 1990;30:768–771. doi: 10.2176/nmc.30.768. [DOI] [PubMed] [Google Scholar]
- 4.Endo M, Hamano M, Watanabe K, Wakai S. [Combined chronic subdural and acute epidural hematoma secondary to metastatic hepatocellular cancer: case report] No Shinkei Geka. 1999;27:331–334. [PubMed] [Google Scholar]
- 5.Hayashi K, Matsuo T, Kurihara M, Daikoku M, Kitange G, Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma: a case report. Surg Neurol. 2000;53:379–382. doi: 10.1016/s0090-3019(00)00208-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim BG, Yoon SM, Bae HG, Yun IG. Spontaneous intracranial epidural hematoma originating from dural metastasis of hepatocellular carcinoma. J Korean Neurosurg Soc. 2010;48:166–169. doi: 10.3340/jkns.2010.48.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KW, Chung DS, Huh PW, Hong YK, Rha HK, Kang JK. Intracranial metastasis of hepatocellular carcinoma associated with epidural hematoma: a case report. J Korean Neurosurg Soc. 1996;25:1738–1742. [Google Scholar]
- 8.McIver JI, Scheithauer BW, Rydberg CH, Atkinson JL. Metastatic hepatocellular carcinoma presenting as epidural hematoma: case report. Neurosurgery. 2001;49:447–449. doi: 10.1097/00006123-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Yoshino E, Suzuki K, Tatebe A, Andachi H. Spontaneous epidural hematoma from a hepatocellular carcinoma metastasis to the skull--case report. Neurol Med Chir (Tokyo) 1992;32:300–302. doi: 10.2176/nmc.32.300. [DOI] [PubMed] [Google Scholar]
- 10.Nakao N, Kubo K, Moriwaki H. Cranial metastasis of hepatocellular carcinoma associated with chronic epidural hematoma--case report. Neurol Med Chir (Tokyo) 1992;32:100–103. doi: 10.2176/nmc.32.100. [DOI] [PubMed] [Google Scholar]
- 11.Woo KM, Kim BC, Cho KT, Kim EJ. Spontaneous epidural hematoma from skull base metastasis of hepatocellular carcinoma. J Korean Neurosurg Soc. 2010;47:461–463. doi: 10.3340/jkns.2010.47.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Kawaguchi J, Hayashi H, et al. Nontraumatic subdural hematoma secondary to dural metastasis of hepatocellular carcinoma: case report and review of the literature. Jpn J Stroke. 2004;26:382–386. [Google Scholar]
- 13.Han JH, Kim DG, Park JC, Chung HT, Paek SH, Chung YS. Little response of cerebral metastasis from hepatocellular carcinoma to any treatments. J Korean Neurosurg Soc. 2010;47:325–331. doi: 10.3340/jkns.2010.47.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto T, Dohmen T, Miura K, et al. Skull metastasis from hepatocellular carcinoma with chronic hepatitis B. World J Gastrointest Oncol. 2010;2:165–168. doi: 10.4251/wjgo.v2.i3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman HD. Hepatocellular carcinoma with central nervous system metastasis: a case report and literature review. Med Pediatr Oncol. 1991;19:139–144. doi: 10.1002/mpo.2950190215. [DOI] [PubMed] [Google Scholar]
- 16.Lee JP, Lee ST. Hepatocellular carcinoma presenting as intracranial metastasis. Surg Neurol. 1988;30:316–320. doi: 10.1016/0090-3019(88)90306-0. [DOI] [PubMed] [Google Scholar]
- 17.Loo KT, Tsui WM, Chung KH, Ho LC, Tang SK, Tse CH. Hepatocellular carcinoma metastasizing to the brain and orbit: report of three cases. Pathology. 1994;26:119–122. doi: 10.1080/00313029400169321. [DOI] [PubMed] [Google Scholar]
- 18.Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982;10:437–444. doi: 10.1227/00006123-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Yang WT, Yeo W, Leung SF, Chan YL, Johnson PJ, Metreweli C. MRI and CT of metastatic hepatocellular carcinoma causing spinal cord compression. Clin Radiol. 1997;52:755–760. doi: 10.1016/s0009-9260(97)80154-7. [DOI] [PubMed] [Google Scholar]
- 20.Russell DS, Cairns H. Subdural false membrane or haematoma (pachymeningitis interna haemorrhagica) in carcinomatosis and sarcomatosis of the dura mater. Brain. 1934;57:32–48. [Google Scholar]
- 21.Cho DC, Yi HJ, Ko Y, Oh SJ, Lee SR, Paik SS. Recurrent metastatic hepatocellular carcinoma presenting as consecutive "mirror image" intracerebral haematomas. J Clin Neurosci. 2005;12:699–702. doi: 10.1016/j.jocn.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Ambiavagar PC, Sher J. Subdural hematoma secondary to metastatic neoplasm: report of two cases and a review of the literature. Cancer. 1978;42:2015–2018. doi: 10.1002/1097-0142(197810)42:4<2015::aid-cncr2820420450>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]