Abstract
Background and objective
The incidence of diabetes co-morbidities could probably be better assessed by studying its associations with major corpulence parameters and glycaemic control indicators. We assessed the utility of body mass index (BMI), waist circumference (WC), and glycosylated haemoglobin (HbA1c) levels in metabolic control for type 2 diabetic patients.
Methods
Fasting and postprandial blood samples were collected from 238 type 2 diabetic patients aged 57.4±11.9 years. The sera were analysed for glucose, HbA1c, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and apolipoproteins (apoA-I and apoB). Ratios of lipids and apolipoproteins were calculated and their associations with BMI, WC, and HbA1c levels were analysed.
Results
Our investigation showed increases in most fasting and postprandial lipid parameters according to BMI and WC. In men, postprandial HDL-c and TG levels were significantly higher (p<0.05) in overweight and obese patients, respectively, as well as in patients with abdominal obesity. Contrariwise, postprandial TC levels were significantly higher (p<0.01) in overweight and abdominal obese women. However, elevations of apoA-I and apoB levels were according to BMI and WC in both genders. There was a strong influence of BMI, WC, and HbA1c levels on the apoB/apoA-I ratio compared to traditional fasting and postprandial lipid ratios in both men and women. The apoB/apoA-I ratio was more correlated with postprandial TC/HDL and LDL-c/HDL-c ratios in men and with postprandial TG/HDL-c in women.
Conclusion
The apoB/apoA-I ratio is helpful in assessing metabolic risk caused by overall obesity, abdominal obesity and impaired glycaemia in type 2 diabetic patients.
Keywords: type 2 diabetes, body mass index, waist circumference, HbA1c, metabolic control
Recent estimates state that the worldwide prevalence of type 2 diabetes is increasing and is likely to affect over 400 million people by 2030. This rising prevalence of type 2 diabetes is thoroughly linked to the upsurge in obesity. More than 90% of type 2 diabetes is attributable to excess weight (1).
Measured by body mass index (BMI), obesity is regarded as a powerful risk factor for type 2 diabetes and its co-morbidities and has increased clearly during the past decades. Estimates suggest that obesity will affect up to 1 billion people in 2030 (2). Although the BMI is one of the most commonly used indicators of overweight and obesity, it does not take into account the body fat pattern (3). Independently from overall obesity, assessment of abdominal obesity by waist circumference (WC) has been shown to be a strong risk factor for type 2 diabetes as well. These obesity indicators may vary according to some factors such as ethnicity, age, and sex (4, 5). Among Europeans and the white US population, general obesity is considered as a consistent predictor of diabetes. However, central obesity has been shown to be a better predictor in Asian populations (6, 7). In contrast, in 2004, Janssen et al. (8) showed that obesity-related health risk is explained by WC and not by BMI.
Regardless of BMI and WC as powerful indicators of obesity, assessment of glucose and lipid levels appears to identify diabetic individuals at increased risk for development of cardiovascular diseases (CVD). Glycosylated haemoglobin (HbA1c) is considered as a long-term marker for blood glucose fluctuations. Elevated HbA1c is an independent risk factor for coronary heart disease (CHD) in patients with or without diabetes (9). However, and contrary to apolipoproteins, studies suggest that lipid levels may not vary much between the fasting and the postprandial periods and that the risk of CHD and stroke is similarly increased for both postprandial and fasting lipid rates (10).
This study was conducted on patients with type 2 diabetes to evaluate, during both fasting and postprandial states, the impact of overweight/obesity (identified through BMI), abdominal fat accumulation (measured by WC), and HbA1c levels (in mmol/mol) on glucose and lipid blood parameters, and also on dyslipidaemia by studying the mutual association between traditional lipid ratios and apolipoproteins ratios.
Patients and methods
Study population
A total of 238 type 2 diabetic patients (86 males and 152 females) were enrolled in this study. Our investigation lasted 9 months, from March to December 2013, at the level of the Public Establishment of Local Health Centre (Diabetes Centre of Ex Gambetta and Mostefa Ben Brahim Polyclinic) in Sidi-Bel-Abbes city and Meslem Tayeb Hospital in Mascara city. These two cities are located in the north-western region of Algeria.
According to data available at the health departments of these two cities, the approximate number of patients with type 2 diabetes was about 22,000 (according to the census of December, 2012), of whom two-thirds were women and almost 18% were treated solely with oral anti-diabetic agents.
During periodic medical follow-up sessions and based on a careful analysis of their medical records, we randomly solicited type 2 diabetic patients who met the inclusion criteria, that is, aged between 19 and 75 years, diabetes duration of less than 15 years, not suffering from diabetes complications, and exclusively under oral anti-diabetic treatments. We used the simple random sampling method without replacement, in which each individual in the targeted population has the same probability of being selected.
The mean ages of male and female patients were 56.8±13.1 and 57.7±11.3 years, respectively. The average diabetes duration was 6.8±3.7 years (6.3±3.4 years for males and 7.1±3.9 years for females). The most common anti-diabetic agents prescribed for our patients were metformin alone (38.7%) or in combination with glimepiride (55.9%), followed by sulfonylureas (5.4%). Participants were asked to abstain from their oral anti-diabetic medications during the day of blood sampling. Patients treated with insulin or using lipid-lowering drugs during the study, as well as pregnant women, were excluded from this study. For data collection, we used a structured questionnaire to get necessary information about general habits and a ‘three-day food diary’ to assess the dietary intake of each patient. The diaries were analysed using the software program Nutri Survey for windows 2007, SEAMEO-TROPMED RCCN-University of Indonesia (results of food diaries will be published later). Furthermore, a signed informed consent was obtained from all patients and their treating physicians before starting the study protocol, considering the ethical approval No. 142 dated 13 February 2013 from ‘the Director of Health and Population of the Wilaya of Sidi-Bel-Abbes (Algeria) according to Article 25 of Decree No. 387 of 31 July 2006 about clinical trials’.
Anthropometric measurements
Body weight (in kilograms) was measured using an electronic balance (TS-2003A: 360 lb, Capacity: 180 kg, Graduations 0.1 kg) and height (in metres) was measured with a body meter (Seca 206, Germany; measuring range 0–220 cm, graduation length 1 mm). The BMI was calculated as weight (kg)/height2 (m2). Patients were instructed to be lightly dressed and to respect the appropriate position for height measurement (gathered feet, straight body, heels touching the wall, and staring out the horizon).
WC was measured respecting every single cm with a measuring tape (maximum 150 cm, graduation length 1 mm). The tape was gently tightened around the patient's abdomen roughly at the horizontal line just above the uppermost lateral border of the ilium, which corresponds with the line passing through the navel in men and a bit above in women.
Blood pressure measurement
OMRON M3 Digital Automatic Blood Pressure Monitor (Omron Healthcare, Ltd. Kyoto, Japan) was used for calculating morning blood pressure.
Blood sampling and assay methods
For fasting glucose and lipid profiles, venous blood samples were collected from each patient 12 h after an overnight fast. We did not get information about the kind of food taken before the 12 h of fasting. However, for the postprandial state, blood samples were drawn 2 h after a breakfast meal for glucose and 3–4 h for lipid parameters. The usual breakfast meal in 94% of our patients provides an average of 692.0±11.0 kcal [fat: 44.1±0.4 g (57%); protein: 7.6±0.1 g (6%); carbohydrates 49.4±0.7 g (38%)].
Enzymatic colorimetric methods (Spinreact Reagents, Spain) (11) were used to determine the fasting and the postprandial serum concentrations of glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), and direct low-density lipoprotein cholesterol (LDL-c). TC/HDL-c, TG/HDL-c, and LDL-c/HDL-c ratios were, respectively, calculated for fasting and postprandial states. The quantitative determination of apo A-I and apo B was performed using turbidimetric tests without undergoing a prior fasting period (Spinreact Reagents, Spain) (12), then the apo B/apoA-I ratio was calculated.
The HbA1c levels were determined by an ion exchange resin separation method. Patients were categorised into three groups: HbA1c<53.01 mmol/mol; HbA1c=53.01–80.34 mmol/mol; HbA1c>80.34 mmol/mol.
Statistical analysis
All data were processed and analysed by using SPSS 20.0 (Statistical Package for the Social Sciences, IBM Corporation; Chicago, IL, August 2011). Results are expressed as means ±standard deviations. Student's t-test was used for comparing means values and statistical significance was set at p=0.05. Relationships between apolipoprotein ratios and lipid ratios were studied using Pearson correlation tests and simple linear regression tests with a confidence interval of 95%.
Results
Table 1 displays the subjects’ characteristics. No difference between the two genders was observed with respect to age, diabetes duration, WC, blood pressure, duration of overweight and/or obesity, and HbA1c levels. A significant gender effect was observed for weight, height, and BMI (p<0.05). Furthermore, a highly significant effect of gender was noticed for fasting glycaemia but not for postprandial glucose levels.
Table 1.
Characteristics of the patients
| Variables | All patients | Males | Females | P value for statistical testa |
|---|---|---|---|---|
| n | 238 | 86 | 152 | – |
| Age (years), mean±S.D. | 57.36±11.93 | 56.84±13.11 | 57.66±11.25 | 0.615 |
| Duration of diabetes (years), mean±S.D. | 6.82±3.74 | 6.33±3.39 | 7.10±3.90 | 0.127 |
| Anthropometric characteristics, mean±S.D. | ||||
| Weight (kg) | 75.66±13.00 | 78.59±14.28 | 74.00±11.94 | 0.009 |
| Height (cm) | 164.69±8.55 | 171.39±7.01 | 160.89±6.85 | 0.000 |
| Waist circumference (cm) | 97.40±13.56 | 95.99±12.16 | 98.20±14.28 | 0.229 |
| BMI (kg/m2) | 27.89±4.58 | 26.73±4.64 | 28.55±4.44 | 0.003 |
| Overweight, obesity duration (years) | 9.66±6.29 | 10.25±6.40 | 9.31±6.25 | 0.463 |
| Blood pressure, mean±S.D. | ||||
| Systolic pressure (mmHg) | 12.89±1.52 | 12.87±1.58 | 12.90±1.49 | 0.875 |
| Diastolic pressure (mmHg) | 7.59±0.95 | 7.61±0.94 | 7.58±0.96 | 0.821 |
| HbA1c (mmol/mol), mean±S.D. | 59.45±10.05 | 58.91±10.38 | 59.78±9.83 | 0.280 |
| Fasting glycaemia (g/L), mean±S.D. | 1.60±0.61 | 1.75±0.62 | 1.51±0.59 | 0.004 |
| Postprandial glycaemia (g/L), mean±S.D. | 2.31±1.03 | 2.42±1.15 | 2.24±0.96 | 0.200 |
| Prevalence of weight categories, n (%) | ||||
| Normal weight, BMI<25 kg/m2 | 71 (29.83) | 37 (43.02) | 34 (22.36) | 0.053 |
| Overweight, BMI=25.0−29.9 kg/m2 | 92 (38.65) | 26 (30.23) | 66 (43.42) | 0.101 |
| Obesity, BMI≥30 kg/m2 | 75 (31.51) | 23 (26.74) | 52 (34.21) | 0.104 |
| Abdominal obesityb | 191 (80.25) | 50 (58.13) | 141 (92.76) | 0.028 |
| Educational status, n (%) | ||||
| Without education | 91 (38.23) | 18 (20.93) | 73 (48.02) | 0.050 |
| Primary school | 43 (18.06) | 18 (20.93) | 25 (16.44) | 0.182 |
| Middle school | 46 (19.32) | 25 (29.06) | 21 (13.81) | 0.118 |
| Secondary school | 42 (17.64) | 17 (19.76) | 25 (16.44) | 0.420 |
| Higher education | 16 (6.72) | 8 (9.30) | 8 (5.26) | 0.249 |
| Smoking, n (%) | ||||
| Never | 200 (84.03) | 48 (55.81) | 152 (100) | 0.024 |
| Former | 8 (3.36) | 8 (9.30) | – | – |
| Current | 30 (12.60) | 30 (34.88) | – | – |
| Practice of sports activity, n (%) | ||||
| Yes | 52 (21.84) | 31 (36.04) | 21 (13.81) | 0.361 |
| No | 186 (78.15) | 55 (63.95) | 131 (86.18) | 0.193 |
| Family disease history, n (%) | ||||
| Hypertension | 17 (7.14) | 5 (5.81) | 12 (7.89) | 0.371 |
| Overweight, obesity | 33 (13.86) | 16 (18.60) | 17 (1.18) | 0.331 |
| Diabetes | 73 (30.67) | 25 (29.06) | 48 (31.57) | 0.252 |
| Cardiovascular disease | 17 (7.14) | 4 (4.65) | 13 (8.55) | 0.358 |
| All | 61 (25.63) | 23 (26.74) | 38 (25.00) | 0.407 |
| No | 37 (15.54) | 13 (15.11) | 24 (15.78) | 0.161 |
| Menopausal status, n (%) | ||||
| Pre-menopause | – | – | 59 (38.81) | |
| Oestrogen use (yes) | – | – | 42 (27.63) | – |
| Oestrogen use (no) | – | – | 17 (11.18) | – |
| Post-menopause | – | – | 93 (61.18) | – |
Comparison between males and females; mean values were compared using Student's t-test. Percentages were compared with Chi-square test.
According to the International Diabetes Federation (IDF), waist circumference >80 cm for women and >94 cm for men.
The prevalence of overweight, obesity, and abdominal obesity was more pronounced in females than in males, whereas the prevalence of normal weight was higher in male patients (43.02%) as compared to female patients (22.36%).
Our results about risk factors and lifestyle showed that 34.9% of men were current cigarette smokers (we considered subjects who reported smoking cigarettes regularly during the year before the survey to be current smokers). Women had slightly lower educational level compared to men (48.02% of women had no formal education). Regarding the history of family diseases, diabetes was the most prevalent risk factor for both men (29.06%) and women (31.57%).
Based on the BMI, in male patients a moderate elevation of fasting glucose was noted in the normal weight group (BMI<25 kg/m2) followed by the obese group (BMI≥30 kg/m2) (Table 2). The postprandial glucose level was rather higher among the overweight group (2.59±1.44 g/L) and the normal weight group (2.48±1.17 g/L). However, in female patients, an elevation of fasting glucose levels was observed in overweight and obese patients (1.58±0.66 vs. 1.51±0.62 g/L, respectively). The topmost level of postprandial blood glucose was noted in the normal weight group. There was a highly significant difference between the fasting and the postprandial glucose levels. Between genders, a significant difference in fasting glycaemia was noted for the group of normal weight patients.
Table 2.
Influence of BMI and waist circumference on fasting blood parameters, postprandial blood parameters, and apolipoproteins
| Fasting state (g/L) | Postprandial state (g/L) | Apolipoproteins (g/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Variables | F Glucose | F TC | F HDL-c | F LDL-c | F TG | PP Glucose | PP TC | PP HDL-c | PP LDL-c | PP TG | apo A-I | apo B |
| Males (n=86) | ||||||||||||
| Based on BMI | ||||||||||||
| BMI<25 kg/m2 | 1.82±0.71 | 1.66±0.35 | 0.35±0.11 | 1.08±0.38 | 1.26±0.65 | 2.48±1.17*** | 1.68±0.43 | 0.34±0.12 | 1.07±0.36 | 1.49±0.99 | 1.15±0.44 | 0.89±0.23 |
| BMI=25.0−29.9 kg/m2 | 1.63±0.63 | 1.66±0.33 | 0.39±0.14 | 1.05±0.30 | 1.25±0.55 | 2.59±1.44*** | 1.76±0.50 | 0.32±0.08* | 1.16±0.48 | 1.59±1.16 | 1.19±0.34 | 0.84±0.32 |
| BMI≥30 kg/m2 | 1.79±0.45 | 1.77±0.42 | 0.37±0.12 | 1.07±0.35 | 1.37±0.64 | 2.15±0.64* | 1.77±0.46 | 0.38±0.16 | 1.07±0.34 | 1.61±0.79* | 1.29±0.52 | 0.91±0.33 |
| Based on WC | ||||||||||||
| WC≤94 cm | 1.83±0.66 | 1.63±0.34 | 0.34±0.11 | 1.07±0.38 | 1.16±0.54 | 2.47±1.10*** | 1.65±0.41 | 0.33±0.13 | 1.06±0.34 | 1.22±0.43 | 1.20±0.48 | 0.86±0.23 |
| WC>94 cm | 1.69±0.60 | 1.73±0.37 | 0.39±0.13 | 1.06±0.32 | 1.38±0.65 | 2.39±1.20*** | 1.78±0.48 | 0.35±0.12* | 1.12±0.43 | 1.79±1.20* | 1.20±0.40 | 0.89±0.32 |
| Females (n=152) | ||||||||||||
| Based on BMI | ||||||||||||
| BMI<25 kg/m2 | 1.38±0.36# | 1.72±0.31 | 0.39±0.13 | 1.16±0.29 | 1.44±0.82 | 2.30±0.95*** | 1.78±0.36 | 0.36±0.10 | 1.10±0.28 | 1.58±0.89 | 1.26±0.27 | 0.92±0.32 |
| BMI=25.0−29.9 kg/m2 | 1.58±0.66 | 1.66±0.36 | 0.40±0.11 | 1.04±0.33 | 1.45±0.72 | 2.27±0.95*** | 1.81±0.50** | 0.40±0.12# | 1.08±0.40 | 1.64±0.92 | 1.38±0.40# | 1.00±0.52 |
| BMI≥30 kg/m2 | 1.51±0.62 | 1.72±0.36 | 0.40±0.10 | 1.04±0.31 | 1.60±0.77 | 2.18±0.99*** | 1.82±0.39 | 0.39±0.11 | 1.07±0.30 | 1.79±0.63 | 1.32±0.35 | 1.03±0.46 |
| Based on WC | ||||||||||||
| WC≤80 cm | 1.34±0.39# | 1.88±0.43 | 0.38±0.17 | 1.43±0.16# | 1.45±0.36 | 2.34±0.33* | 1.64±0.43 | 0.28±0.10 | 0.96±0.28* | 1.99±1.13## | 1.04±0.23 | 0.82±0.21 |
| WC>80 cm | 1.52±0.60 | 1.68±0.34 | 0.40±0.10 | 1.04±0.31 | 1.50±0.78 | 2.24±0.95*** | 1.82±0.43*** | 0.40±0.11# | 1.09±0.34 | 1.65±0.80* | 1.35±0.36# | 1.00±0.47 |
BMI: body mass index; WC: waist circumference; F: fasting; PP: postprandial.
Asterisks: Significantly different from the identical fasting parameter at p<0.05
p<0.01.
p<0.001.
# and ##: significantly different from male patients at
p<0.05
p<0.01.
On the basis of WC, significant increase of the fasting glycaemia was noted in female patients suffering from abdominal obesity (1.52±0.60 g/L) compared to those with normal WC (1.34±0.39 g/L). In both genders, abdominal obesity does not seem to be a factor helping to increase the blood glucose during the postprandial state.
Comparing between the fasting and postprandial states and except for HDL-c and TG, the lipid values in male patients did not differ according to the BMI and/or WC (Table 2). Significantly lower HDL-c levels were obtained during the postprandial state for BMI=25.0–29.9 kg/m2 and abdominal obesity (WC>94 cm). However, TG levels showed significant increases during the postprandial state with BMI≥30 kg/m2 and abdominal obesity. In contrast, highly significant increases in TC were observed during the postprandial state in female patients with BMI=25.0–29.9 kg/m2 (p<0.01) and abdominal obesity (p<0.001). Similarly, in women suffering from abdominal obesity, postprandial TG levels (1.65±0.80 g/L) were significantly higher than fasting levels (1.50±0.78 g/L). The LDL-c level was lower during the postprandial state with WC≤80 cm.
Apolipoproteins (apo A1 and apo B) showed a considerable increase according to BMI and/or WC in both genders. However, a significant effect of gender differences was noted on apo A-I in patients with BMI=25.0–29.9 kg/m2 and abdominal obesity (p<0.05).
Table 3 shows the effect of BMI, WC, and HbA1c level on fasting lipid ratios, postprandial lipid ratios, and apolipoproteins ratios in type 2 diabetic patients. Among male patients with BMI=25–29.9 kg/m2, all lipid ratios (TC/HDL-c, TG/HDL-c and LDL-c/HDL-c) increased significantly during the postprandial state compared to the fasting state. When we compared lipid ratios between fasting and postprandial states according to WC values, postprandial TC/HDL-c and TG/HDL-c ratios increased noticeably in patients with abdominal obesity (WC>94 cm). However, elevated HbA1c levels (≥7% or≥53.01 mmol/mol) are more likely to cause an increase in postprandial ratios of TC/HDL-c and TG/HDL-c. In female patients, only the TG/HDL-c ratio showed a significant postprandial increase in obese women (BMI≥30 kg/m2). Based on WC, the postprandial TC/HDL-c and TG/HDL-c ratios were significantly higher than the fasting ratios (Table 3). The TC/HDL-c ratio was more influenced by a high level of HbA1c.
Table 3.
Variations of apolipoproteins ratio, and fasting and postprandial lipid ratios according to BMI, waist circumference, and HbA1c level
| Fasting state | Postprandial state | Apolipoproteins | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Variables | TC/HDL-c | TG/HDL-c | LDL-c/HDL-c | TC/HDL-c | TG/HDL-c | LDL-c/HDL-c | apo B/apo A-I |
| Males (n=86) | |||||||
| Based on BMI | |||||||
| BMI<25 kg/m2 | 4.95±1.31 | 3.95±2.61 | 3.19±1.25 | 5.24±1.30 | 4.94±3.63 | 3.32±1.12 | 0.81±0.24 |
| BMI=25.0–29.9 kg/m2 | 4.63±1.46 | 3.51±1.72 | 2.99±1.30 | 5.75±2.36* | 5.13±3.46* | 3.86±2.14* | 0.75±0.41 |
| BMI≥30 kg/m2 | 4.96±1.63 | 3.90±1.88 | 3.05±1.30 | 5.00±1.63 | 4.85±2.88 | 3.04±1.21 | 0.77±0.30 |
| Based on WC | |||||||
| WC≤94 cm | 5.05±1.30 | 3.81±2.46 | 3.32±1.32 | 5.24±1.36 | 4.17±2.48 | 3.37±1.21 | 0.77±0.25 |
| WC>94 cm | 4.71±1.52 | 3.81±1.96 | 2.93±1.21 | 5.40±2.02* | 5.56±3.78* | 3.44±1.74 | 0.79±0.35 |
| Based on HbA1c | |||||||
| HbA1c<53.01 mmol/mol | 5.09±1.57 | 3.57±1.74 | 3.41±1.41 | 5.18±1.23 | 4.76±3.16 | 3.35±1.16 | 0.80±0.19 |
| HbA1c=53.01–80.34 mmol/mol | 4.83±1.42 | 4.02±2.30 | 2.98±1.24 | 5.43±2.09* | 5.28±3.64* | 3.42±1.79 | 0.79±0.37 |
| HbA1c>80.34 mmol/mol | 4.14±0.73 | 3.38±2.84 | 2.62±0.55 | 5.29±1.28* | 3.86±1.96 | 3.55±1.08 | 0.68±0.26 |
| Females (n=152) | |||||||
| Based on BMI | |||||||
| BMI<25 kg/m2 | 4.89±2.00 | 4.22±2.88 | 3.35±1.65 | 5.31±1.88 | 5.08±4.12 | 3.29±1.26 | 0.75±0.29 |
| BMI=25 to 29.9 kg/m2 | 4.37±1.29 | 3.91±2.22 | 2.76±1.05 | 4.82±1.70# | 4.49±2.95 | 2.92±1.36# | 0.75±0.36 |
| BMI≥30 kg/m2 | 4.48±1.25 | 4.23±2.38 | 2.74±1.07 | 4.91±1.57 | 4.92±2.24* | 2.92±1.20 | 0.81±0.37 |
| Based on WC | |||||||
| WC≤80 cm | 5.76±2.70 | 4.38±1.83 | 4.41±2.02# | 6.57±2.75# | 8.49±6.35*## | 3.86±1.59 | 0.81±0.24 |
| WC>80 cm | 4.43±1.29 | 4.06±2.46 | 2.76±1.07 | 4.83±1.53*# | 4.48±2.43*# | 2.94±1.24# | 0.77±0.36 |
| Based on HbA1c level | |||||||
| HbA1c<53.01 mmol/mol | 4.78±1.68 | 4.52±2.59 | 3.02±1.41 | 5.17±1.96 | 5.62±4.07* | 3.04±1.34 | 0.81±0.41 |
| HbA1c=53.01–80.34 mmol/mol | 4.41±1.26 | 3.93±2.21 | 2.80±1.06 | 4.82±1.55* | 4.39±2.31 | 2.94±1.27 | 0.74±0.32 |
| HbA1c>80.34 mmol/mol | 4.41±1.88 | 3.68±3.02 | 2.96±1.62 | 5.16±1.65 | 4.38±2.74 | 3.28±1.27 | 0.81±0.30 |
BMI: body mass index; WC: waist circumference
Significantly different from the identical fasting lipid ratio at p<0.05.
# and ##: significantly different from male patients at
p<0.05
p<0.01.
Between genders, appreciable lowering of TC/HDL-c, TG/HDL-c, and LDL-c/HDL-c ratios was observed in female patients suffering from abdominal obesity; low TC/HDL-c and LDL-c/HDL-c ratios were also observed in overweight women (BMI=25.0–29.9 kg/m2). The apo B/apo A-I ratio did not show any significant difference between the two genders (Table 3).
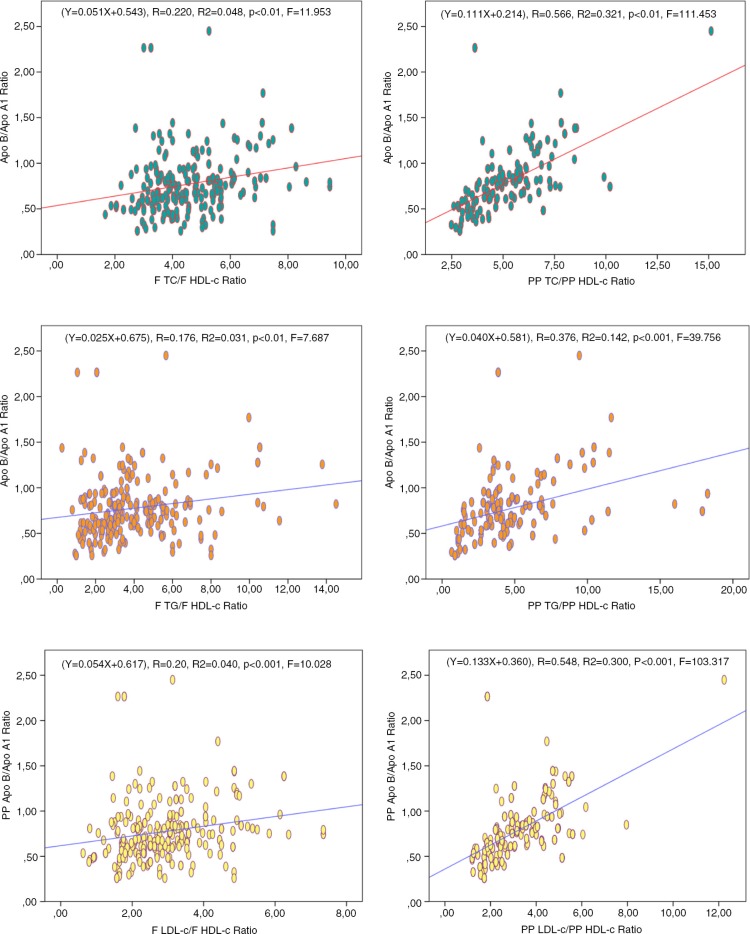
As illustrated in Fig. 1, the apo B/apo A-I ratio was significantly correlated with TC/HDL-c, TG/HDL-c, and LDL-c/HDL-c ratios both in fasting and in postprandial states. However, we noticed that the apo B/apo A-I ratio provides the strongest positive correlations with TC/HDL-c (p<0.01, r 2=0.321, F=111.453) and LDL-c/HDL-c (p<0.001, r 2=0.300, F=103.317) ratios during the postprandial period.
Fig. 1.
Mutual association between apolipoproteins ratio and lipid ratios during fasting and postprandial states.
As depicted in Table 4, we evaluated the risk of myocardial infarction in our diabetic patients in terms of increased apo B/apo A-I ratio. Tentative risk zones used in this table are based on cut-off values adapted from the Apolipoprotein-related Mortality Risk (AMORIS) (13) and INTERHEART (14) studies. For men, the proportion of patients who were likely to be exposed to a moderate or high risk was 32.6 and 26.7%, respectively. Contrariwise, for female patients, 37.5% were exposed to a higher risk of myocardial infarction.
Table 4.
Risk of myocardial infarction in terms of increased apo B/apo A-I ratio in the studied cases
| Low risk | Moderate risk | High risk | |
|---|---|---|---|
| Men apo B/apo A-I ratio | 0.40 − 0.69 | 0.70 − 0.89 | 0.90 − 1.10 |
| Prevalence, n (%) | 35 (40.69) | 28 (32.55) | 23 (26.74) |
| Women apo B/apo A-I ratio | 0.30 − 0.59 | 0.60 − 0.79 | 0.80 − 1.00 |
| Prevalence, n (%) | 45 (29.60) | 50 (32.89) | 57 (37.50) |
Adapted with thresholds of AMORIS and INTERHEART studies.
Discussion
Diabetes mellitus is one of the most prevalent chronic diseases in almost all countries. Between 2010 and 2030, the number of adults with diagnosed diabetes is expected to increase by 69% in developing countries and by 20% in developed countries (1). Concurrently, the prevalence of obesity is increasing dramatically all over the world (15). Obesity is medically defined as a state of increased adipose tissue of a magnitude sufficient to lead to worsening of all the elements of the metabolic syndrome, namely insulin resistance, hyperinsulinaemia, dyslipidaemia, and hypertension (2, 16). The classification system for obesity is based on BMI as the most frequently used diagnostic tool. However, the body fat distribution, particularly abdominal fat accumulation, exerts a strong influence on the development of glucose intolerance and type 2 diabetes co-morbidities. Several investigators have reported that individuals with similar BMI but with different WC show different metabolic risks of diabetes and CVD (17, 18). In the same context, as reported by Elley et al., HbA1c is considered as an important indicator of blood glucose control; an HbA1c ‘threshold’ of 53.01 mmol/mol is a significantly higher risk of macrovascular disease in diabetic patients. Every 1% higher HbA1c level above that threshold is associated with an independent increased CVD risk (19).
The purpose of the present study was to establish, during fasting and postprandial states in type 2 diabetes patients, the influence of overweight/obesity (defined by BMI), abdominal obesity (measured by WC), and HbA1c levels on blood glucose and lipids parameters and dyslipidaemia (defined by lipid and apolipoprotein ratios).
Preliminary results from this study indicate some unfavourable interactions of BMI and WC with the glucose and individual lipid profiles of diabetic patients during both fasting and postprandial states. Though the gender difference in our population has a limited effect on major metabolic changes, the increase in lipid and apolipoprotein levels is more pronounced with BMI and WC during the postprandial state. Our finding shows that apo B levels exceed the optimal target of ~0.9 g/L (13) and are strongly influenced by BMI and WC in both genders, thereby indicating a high cardiometabolic risk in our patients. However, in addition to contributions of obesity and body fat distribution to the development of pre-diabetes and type 2 diabetes co-morbidities, obesity promotes alterations in other intermediate risks in relation to dyslipidaemia, glucose intolerance, the inflammatory state, as additional unknown mechanisms may vary between the genders (20, 21). Gender differences regarding the effect of overweight/obesity on metabolic disorder is likely to result from the influence of several factors, such as delayed dietary fat clearance, which results from abdominal adipose tissue accumulation in men, along with a concomitant decrease in the postprandial metabolism of fatty acids and a decreased ability to store lipids in subcutaneous adipose tissue (22).
The risk of vascular disease is two- to four-fold greater in adults with diabetes than among non-diabetic individuals (23). However, association between diabetes and risk factors for CVD, such as dyslipidaemia (previously called hyperlipaemia), hypertension, and hyperinsulinaemia, bring out the necessity of an aggressive management (24). Therefore, tables of cardiovascular risk (e.g. the Framingham score or the EuroSCORE) (25, 26) and international recommendations (e.g. recommendations of the National Cholesterol Education Program – NCEP, 2001) (27), centred on the classic lipid profile, were developed to validate the effectiveness of lipid-lowering therapies. But important limitations of these parameters in cardiovascular risk prediction have been demonstrated (28). There is now more evidence from cohort and meta-analysis studies suggesting that lipid ratios (TC/HDL-c, TG/HDL-c, and LDL-c/HDL-c) have higher association with CVD than with individual lipids (29–32). On the contrary, prospective risk studies suggest that the use of apo B/apo A-I ratio may be a promising and convenient marker of risk of CVD [e.g. AMORIS study (13), INTERHEART study (14), EPIC-Norfolk study (33), and ULSAM study (34)].
The apo A-I, apo B, and apo B/apo A-I ratios have many advantages that surpass their use compared with normal lipid parameters and their ratios in predicting CVD. Apo B reflects the atherogenic side and apo A-I indicates the anti-atherogenic side. Hence, the apo B/apo A-I ratio reflects the risk of cardiovascular events (13). Another important feature is that apolipoproteins concentrations are not affected by meals and are slightly influenced by biological variables, unlike the ordinary lipid parameters, which fluctuate widely depending on food intake. Therefore, measurement of apolipoproteins does not require fasting blood samples (12, 13, 35, 36). In clinical practice, apolipoproteins A-I and B may be measured directly in plasma without noticeable interference with high triglyceride levels using internationally standardised methods that are accurate and precise (12, 37).
The results of this study clearly show, for both genders, that BMI, WC, and impaired glycaemia (defined by HbA1c) were proportionally related with the degree of dyslipidaemia during both fasting and postprandial states. During the postprandial stage, the apolipoproteins ratio (apo B/apo A-I) was more correlated with TC/HDL-c (r 2=0.321, p<0.01, F=111.543) and LDL-c/HDL-c (r 2=0.300, p<0.001, F=103.317) and lightly correlated with TG/HDL-c (r 2=0.142, p<0.001, F=39.756). Similar results have been presented in other studies (9, 10, 38–42).
Although the vast majority of patients with established type 2 diabetes should be considered at high short-term risk, the association of diabetes with other risk factors such as overall and/or visceral obesity and blood glucose alterations should lead to intensive monitoring and management of this situation. Our findings indicate that all TC/HDL-c and LDL-c/HDL-c ratios were beyond the values of the therapeutic target identified by most authors (4.0 for TC/HDL-c and 2.5 for LDL-c/HDL-c) (43, 44), which reflects a high risk of CVD in all patients. Values for the apo B/apo A-I ratio during therapy should preferably be reduced to <0.7 or even lower in patients with great risk (45). After adapting our data with the risk ranges of AMORIS and INTERHEART studies (13, 14), we found that women are more prone to cardiovascular risk (myocardial infarction) than men in terms of increased apo B/apo A1 ratio.
One limitation of the present study is the short postprandial time (3–4 h), because changes may affect lipid profiles beyond that time. The effect of age differences and genetic variations between the two genders is another limitation; variations in genes such as those encoding apolipoproteins A1 and B may directly affect postprandial lipaemia due to their involvement in lipid metabolism. Finally, information about what each participant had eaten for breakfast before blood sampling was based on self-declaration of the patients themselves.
In conclusion, most patients with type 2 diabetes, whether male or female, have dyslipidaemia to varying degrees. Dyslipidaemia, one of the CVD risk factors, becomes more severe with higher BMI and/or WC and with increased levels of HbA1c. BMI coupled with WC is more predictive of cardiovascular risk than each body parameter separately.
Compared with individual lipid parameters, the changes in lipid ratios could reflect impaired lipid metabolism at earlier stages. However, lipid ratios are often influenced by food intake, which could make interpretation of the results more difficult depending on the fasting or postprandial state. There are now a number of user-friendly reasons for replacing traditional lipids and their ratios with measures of apo B and apo A-I and their ratio in clinical practice. As apolipoproteins can be analysed in non-fasting samples, this is of great practical advantage for patients and doctors. In addition, it is very helpful to use a single ratio for risk prediction instead of referring to a larger number of lipid ratios.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 4.Resnick HE, Valsania P, Halter JB, Lin X. Differential effect of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21:1828–35. doi: 10.2337/diacare.21.11.1828. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Mitchell BD, Hazuda HP, Stern MP. Greater influence of central distribution of adipose tissue on incidence of non-insulin-dependent diabetes in women than men. Am J Clin Nutr. 1991;53:1312–17. doi: 10.1093/ajcn/53.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Chan JM, Rimm EB, Colditz CA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 7.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesityrelated health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 9.Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7:24–9. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 11.Bergmeyer HU. Methods of enzymatic analysis. New York: Verlag Chemie; 1974. [Google Scholar]
- 12.Tietz NW. Fundamentals of clinical chemistry. 3rd ed. Philadelphia, PA: W. B. Saunders; 1987. [Google Scholar]
- 13.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS Study): a prospective study. Lancet. 2001;358:2026–33. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Öunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 15.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:937–52. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metabol. 2004;89:2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 18.Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, et al. Body fat distribution and risk of coronary heart disease in men and women in the European prospective investigation into cancer and nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–43. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 19.Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with Type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–301. doi: 10.1111/j.1464-5491.2008.02581.x. [DOI] [PubMed] [Google Scholar]
- 20.Bastiena M, Poiriera P, Lemieuxa I, Despré JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Wormser D, Kaptoge S, Angelantonio ED, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, et al. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 1999;19:2448–55. doi: 10.1161/01.atv.19.10.2448. [DOI] [PubMed] [Google Scholar]
- 23.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–5. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg IJ. Diabetic dyslipidemia: causes and consequences. J Clin Endo Metab. 2001;8:965–71. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones DM, Wilson PWF, Larson MG, Beiser A, Leip EP, D'Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–4. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 27.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–14. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 28.Gotto AM, Jr., Whitney E, Stein EA, Shapiro DR, Clearfield M, Weis S, et al. Relation between baseline and ontreatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/Tex CAPS) Circulation. 2000;101:477–84. doi: 10.1161/01.cir.101.5.477. [DOI] [PubMed] [Google Scholar]
- 29.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–85. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 31.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian cardiovascular society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol. 2009;25:567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao W, Gong W, Wu1 N, Li Y, Ye K, Lu B, et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. 2014;13:2–6. doi: 10.1186/1476-511X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Steeg WA, Boekholdt SM, Stein EA, El-Harchaoui K, Stroes ESJ, Sandhu MS, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: a case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146:640–8. doi: 10.7326/0003-4819-146-9-200705010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Dunder K, Lind L, Zethelius B, Berglund L, Lithell H. Evaluation of a scoring scheme, including proinsulin and the apolipoprotein B/apolipoprotein A1 ratio, for the risk of acute coronary events in middle-aged men: Uppsala Longitudinal Study of Adult Men (ULSAM) Am Heart J. 2004;148:596–601. doi: 10.1016/j.ahj.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Tamang HK, Timilsina U, Singh KP, Shrestha S, Raman RK, Panta P, et al. Apo B/Apo A-I ratio is statistically a better predictor of cardiovascular disease (CVD) than conventional lipid profile: a study from Kathmandu Valley, Nepal. J Clin Diagn Res. 2014;8:34–6. doi: 10.7860/JCDR/2014/7588.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shilpasree AS, Sahukar S, Murthy J, Kumar K. A study of serum apolipoprotein A1, apolipoprotein B and lipid profile in stroke. J Clin Diagn Res. 2013;7:1303–6. doi: 10.7860/JCDR/2013/5269.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcovina S, Packard J. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–46. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 38.Hou X, Lu J, Weng J, Ji L, Shan Z, Liu J, et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS One. 2013;8:1. doi: 10.1371/journal.pone.0057319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taskinen MR, Barter PJ, Ehnholm C, Sullivan DR, Mann K, Simes J, et al. Ability of traditional lipid ratios and apolipoprotein ratios to predict cardiovascular risk in people with type 2 diabetes. Diabetologia. 2010;53:1846–55. doi: 10.1007/s00125-010-1806-9. [DOI] [PubMed] [Google Scholar]
- 40.Yan A, Liu Y, Huang H. Association of glycosylated hemoglobin level with lipid ratio and individual lipids in type 2 diabetic patients. Asian Pac J Trop Med. 2012;5:469–71. doi: 10.1016/S1995-7645(12)60080-7. [DOI] [PubMed] [Google Scholar]
- 41.Belfki H, Ben Ali S, Bougatef S, Ben Ahmed D, Haddad N, Jmal A, et al. The apolipoprotein B/apolipoprotein A 1 ratio in relation to metabolic syndrome and its components in a sample of the Tunisian population. Exp Mol Pathol. 2011;91:622–5. doi: 10.1016/j.yexmp.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Maciel PM, Reaven GM. Identification of cardiometabolic risk: visceral adiposity index versus triglyceride/HDL cholesterol ratio. Am J Med. 2014;127:152–7. doi: 10.1016/j.amjmed.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Leiter LA, Genest J, Harris SB, Lewis G, McPherson R, Steiner G, et al. Dyslipidemia in adults with diabetes. Can J Diabetes. 2006;30:230–40. [Google Scholar]
- 44.Pereira T. Dyslipidemia and cardiovascular risk: lipid ratios as risk factors for cardiovascular disease. In: Kelishadi R, editor. Dyslipidemia – from prevention to treatment. Rijeka: InTech; 2012. pp. 279–302. [Google Scholar]
- 45.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]