Abstract
Kir3 (or GIRK) channels have been known for nearly three decades to be activated by direct interactions with the βγ subunits of heterotrimeric G (Gαβγ) proteins in a membrane-delimited manner. Gα also interacts with GIRK channels and since PTX-sensitive Gα subunits show higher affinity of interaction they confer signaling specificity to G Protein-Coupled Receptors (GPCRs) that normally couple to these G protein subunits. In heterologous systems, overexpression of non PTX-sensitive Gα subunits scavenges the available Gβγ and biases GIRK activation through GPCRs that couple to these Gα subunits. Moreover, all Kir channels rely on their direct interactions with the phospholipid PIP2 to maintain their activity. Thus, signals that activate phospholipase C (e.g. through Gq signaling) to hydrolyze PIP2 result in inhibition of Kir channel activity. In this review, we illustrate with experiments performed in Xenopus oocytes that Kir channels can be used efficiently as reporters of GPCR function through Gi, Gs or Gq signaling. The membrane-delimited nature of this expression system makes it highly efficient for constructing dose-response curves yielding highly reproducible apparent affinities of different ligands for each GPCR tested.
G protein-coupled receptors (GPCRs) are proteins with an extracellular N terminus, a cytoplasmic C terminus, and a transmembrane domain composed of 7 helices connected by intracellular and extracellular loops (Ballesteros and Weinstein, 1994). GPCRs mediate most of their intracellular actions through signaling pathways that involve activation of G-proteins (Lefkowitz, 2007). In response to GPCR stimulation G-proteins signal to effector proteins, such as enzymes and ion channels. This results in rapid changes in the concentration of intracellular signaling molecules, such as cAMP, cGMP, inositol phosphates, diacylglycerol, arachidonic acid, and cytosolic ions. The GPCR superfamily includes receptors for diverse endogenous ligands in the form of hormones, neurotransmitters or neuromodulators. These include biogenic amines, peptides, amino acids, glycoproteins, prostanoids, phospholipids, fatty acids, nucleosides, nucleotides and Ca2+ ions. Sensory GPCRs bind diverse exogenous ligands, such as odorants, bitter and sweet tastants, pheromones, and photons of light. GPCR dysfunction results in human diseases, and many GPCRs are targets for pharmaceuticals and drugs of abuse. Approximately 80% of known hormones and neurotransmitters activate cellular signal transduction mechanisms by stimulating GPCRs (Birnbaumer et al., 1990). Furthermore, about half of the current drugs on the market target GPCRs generating tens of billions of dollars in revenues and representing a significant portion of the portfolio of many pharmaceutical companies. Due to their importance, GPCRs and their signaling have been studied extensively and breakthroughs in our understanding of how they work have received multiple Nobel prizes (Lin, 2013).
GPCRs associate with heterotrimeric G (Gαβγ) proteins to transduce ligand binding of the receptor to downstream effectors. Twenty different Gα, five different Gβ, and twelve different Gγ isoforms associate in distinct combinations with GPCRs (Milligan and Kostenis, 2006). The G-protein signaling cycle can be described in three major steps: 1) Binding of a ligand to the GPCR induces a conformational change to the receptor that is transduced to the Gα subunit, such that its affinity for intracellular GTP is greatly increased over the already bound GDP, and in a Mg2+-dependent manner GDP is exchanged with GTP. In this regard, the activated GPCR is acting as a guanine nucleotide exchange factor (GEF) to stimulate the exchange of nucleotides with the Gα subunit. 2) The Gα subunit uses the binding energy of GTP to produce a conformation favoring its dissociation from Gβγ and association with effector proteins. Similarly, the dissociated Gβγ can also interact with effectors. Thus, the dissociated G-protein subunits are activated to signal to downstream effectors. 3) The activation of the G-protein subunits ends by hydrolysis of GTP to GDP by the GTPase activity of the Gα subunit (either intrinsic GTPase or stimulated by specific interacting proteins – e.g. GTPase activating proteins or GAPs, such as RGS proteins), enabling re-association with Gβγ. Following re-association, the heterotrimeric G-protein can interact again with the GPCR and the activation cycle can proceed again. Three pathways comprise most of G-protein subunit signaling: Gs, Gi/o, and Gq. The Gs signaling pathway involves four Gα isoforms. Cholera Toxin (CTX) ADP-ribosylates Gαs subunits rendering them constitutively active. The Gi/o signaling pathway involves nine Gα isoforms. Pertussis Toxin (PTX) ADP-ribosylates the Gαi/o subunits functionally uncoupling them from their associated GPCRs. This renders the G-proteins unable to transduce ligand-induced GPCR conformational changes. The Gq pathway involves seven related Gα subunits for which no specific ADP-ribosylating toxin has been identified. RGS2 proteins have been found to specifically associate with Gαq proteins and can therefore be used as specific inhibitors of the Gq signaling pathway (Kehrl and Sinnarajah, 2002). Even though G-protein signaling has been intensely studied for several decades, many questions remain regarding how GPCR / G-protein subunit / effector protein interactions control the activity of effectors in a signaling-dependent manner.
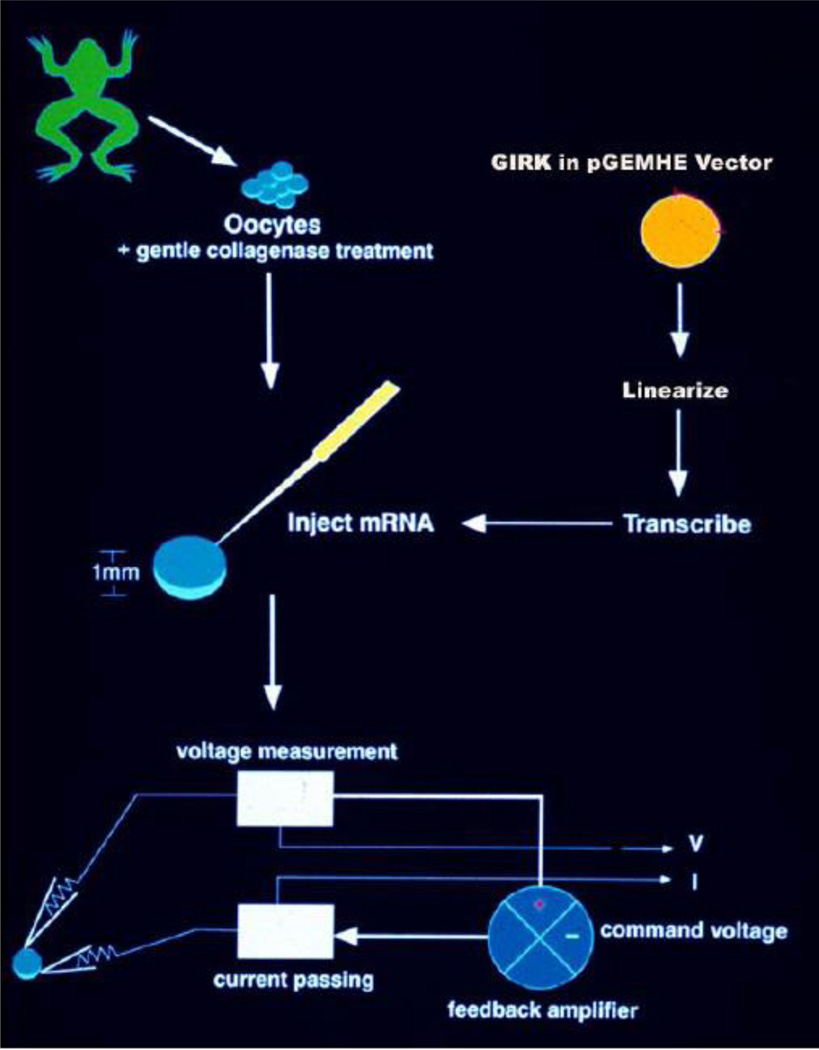
Oocytes from Xenopus laevis frogs have been widely used as a heterologous expression system for studying ion channels since the early 1980s when this system was initially developed by Miledi and coworkers (Barnard et al., 1982; Gundersen et al., 1984). Oocytes are most often injected with cRNA prepared in vitro, from a cDNA coding for the ion channel of choice, and the responses are analyzed electrophysiologically using the two-microelectrode, whole-cell voltage clamp (Miledi et al., 1982; Gundersen et al., 1984) (Fig. 1) or the patch-clamp technique (Hamill et al., 1981) using membrane patches (Methfessel et al., 1986). Current resulting from the injected RNA is typically much larger than that through endogenous channels, making the background from native oocyte currents insignificant. In some cases, the presence of an endogenous response can be utilized as a positive control for the second messenger system coupled to the initial response under study (e.g. see below the case of Gq signaling). The discovery of K+ channels sensitive to activation by G-proteins in the Xenopus system (Kubo et al., 1993; Dascal et al., 1993) demonstrated that heterologous expression of GPCRs could readily be coupled to co-expressed K+ channels through endogenous oocyte heterotrimeric G-proteins. Our goal is to review evidence that the oocyte expression system is a convenient and powerful system to study GPCR signaling using K+ channels as reporters of GPCR activity.
Figure 1. The Xenopus oocyte heterologous expression system.
Oocytes are isolated from Xenopus laevis frogs through a small abdominal incision and are subjected to mild collagenase treatment to isolate them from the surrounding follicular cells. In vitro transcribed cRNA from a frog vector (e.g. pGEM-HE) coding for the desired protein to be overexpressed (e.g. for GIRK) and flanked by RNA stabilizing elements found in the 5’ and 3’ untranslated regions of the Xenopus β-globin gene is injected into the isolated oocytes. Depending on the level of functional expression for each cRNA, two-electrode voltage clamp is utilized to record whole-cell currents, anywhere from 1 to several days following injection. Whole-cell currents in Xenopus oocytes were recorded by conventional two-electrode voltage-clamp (TEVC) as described previously (Chan et al., 1996). Recordings were performed with a GeneClamp500 amplifier (Axon Instruments) 3–5 days after cRNA injection. Electrodes were filled with 1.5% (wt/vol) agarose in 3 M KCl. Microelectrodes had a resistance of 0.3–1.0 MΩ. 96 mM [K]0 solutions were used such that EK = 0 mV. Two voltage protocols were routinely used in the experiments presented: one was a voltage ramp from −80 mV to +80 mV while before and after the ramp the membrane was held at 0 mV; the other was a voltage step protocol from 0 mV to –80 mV then to +80 mV and finally back to 0 mV. Current measurements were made at −80 mV and +80 mV to ensure the maintenance of rectification and time course data for inward currents were plotted.
Since their cloning, G-protein-sensitive inwardly rectifying K+ (GIRK or Kir3) channels have been extensively studied in the Xenopus oocyte system. They belong to the 15-member family of inwardly rectifying potassium (Kir) channels, named for their ability to conduct K+ ions better in the inward (Vm < EK) rather than the outward (Vm > EK) direction (Hibino et al., 2010). Four mammalian isoforms comprise the Kir3 subfamily: GIRK1-GIRK4 (Kir3.1-Kir3.4). GIRK1 channels function only as heteromers in association with one of the other subunits (GIRK2-GIRK4). These additional subunits can function both as homotetramers and heterotetramers in several tissues, including heart and brain.
Soejima and Noma (1984) first used the cell-attached mode of the patch-clamp technique to study GIRK channels in the heart. In the absence of acetylcholine (ACh) in the pipette (i.e. in the extracellular environment of the membrane patch) they recorded a low basal level of activity from rabbit atrial cells. This basal level of activity was not altered by applying ACh in the bath, suggesting that the channel could not be activated by generation of soluble second messengers. Yet, when they included ACh in the pipette, thus directly applying it to the extracellular side of the membrane, channel activity was dramatically stimulated. These experiments suggest that the activation of K+ currents by ACh (KACh) was confined to the membrane patch isolated by the pipette in the cell-attached recording requiring direct application of ACh on the extracellular side of the membrane. This conclusion was further confirmed two years later by demonstrating that G-protein signaling to KACh could occur in excised membrane patches from guinea pig atrial cells (Kurachi et al., 1986). In 1985, two laboratories demonstrated that G-proteins transduced the ACh signal to the K+ channels, using the whole-cell mode of the patch-clamp technique. Hille’s group showed that ACh stimulation of K+ currents was PTX sensitive. PTX ADP ribosylated the chick embryonic atrial Gαi subunits disrupting ACh signaling through the muscarinic type 2 receptor (Pfaffinger et al., 1985). Breitwieser and Szabo (1985) showed that inclusion of a non-hydrolyzable analog of GTP (GTPγS) in the patch pipette constitutively activated bullfrog atrial KACh (GTPγS diffuses into the cell from the patch pipette and activates the G-proteins and thus the channels, irreversibly). Two years later Logothetis and colleagues in the Clapham and Neer labs (1987) showed that perfusion of purified Gβγ subunits in the inside-out mode of the patch-clamp technique could activate chick atrial myocyte KACh independently of the endogenous G-proteins. KACh was the first effector shown to be activated directly by Gβγ and a model was proposed suggesting that following G-protein subunit activation, the Gβγ subunits stimulated KACh independently of the Gα subunits. The studies performed between 1984–1987 were all consistent with the conclusion that G-protein activation of KACh occurred in the plasma membrane and did not involve soluble second messengers.
Approximately a decade later in 1998, two reports from different laboratories showed stimulation of G-protein-sensitive channels was phosphatidylinositol bis-phosphate- (PIP2−) dependent (Huang et al., 1998; Sui et al., 1998). Additionally, intracellular Na+ ions, which use a G-protein independent mechanism to gate GIRK channels, also depended on PIP2. Unlike other Kir channels that exhibited PIP2 dependence for their activity, GIRK channels could not be activated by PIP2 alone but required the co-presence of Gβγ or Na+, both of which used independent mechanisms of enhancing the channel’s apparent affinity to PIP2 (Petit-Jacques et al., 1999). Kobrinsky and co-workers revealed the physiological importance of the PIP2 dependence of KACh (2000). Activation of the Gq pathway resulted in PIP2 hydrolysis and desensitization of KACh currents, a process that required re-synthesis of PIP2 for recovery from desensitization. Similar inhibition / desensitization of other PIP2-dependent currents has been demonstrated for Kir2 and other types of channels (e.g. Du et al., 2004; Rohács et al., 2005). Crystal structures of GIRK2 (Kir3.2) in complex with PIP2 as well as of the Kir2.2 channel (IRK2) have confirmed the Kir channel site of interactions with this signaling phospholipid and its critical position relative to the channel gates (Whorton and Mackinnon, 2011; Hansen et al., 2011). Additionally, the site of action of the Gβγ subunits has been identified (Mahajan et al., 2013; Whorton et al., 2013) along with a mechanism of how the PIP2-channel and the Gβγ-channel interaction sites are coupled (Meng et al., 2012; Mahajan et al., 2013).
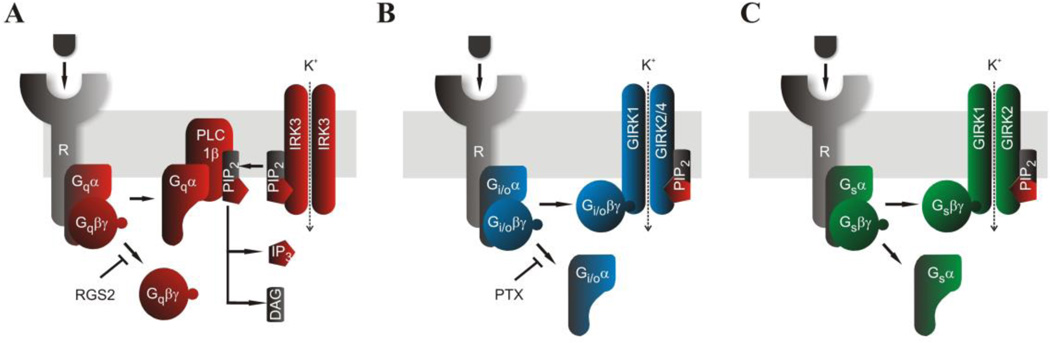
Figure 2 shows schematically the three major signaling pathways as they couple to Kir channels in the oocyte system. Co-expression of the GPCR of choice with the Kir channel reporter allows for membrane-delimited G-protein signaling, and its quantification can be achieved through measurement of ionic currents. Assessment of Gq signaling in oocytes involves co-expression of a Gq-coupled GPCR (GqPCR) with KIR2.3 as the channel reporter with ligand-induced hydrolysis of PIP2 resulting in current inhibition (Fig. 2A). KIR2.3 activity is not affected directly by G-protein subunits but is highly dependent on interactions with PIP2 to maintain its activity. Stimulation of the GqPCR by the appropriate ligand leads to activation of PLCβ1 and hydrolysis of PIP2 to inositol tris-phosphate (IP3) and diacylglycerol (DAG). The decrease in PIP2 concentration in the immediate vicinity of the channel causes diffusion of its bound PIP2 away from the channel binding site resulting in current inhibition. Co-expression of RGS2 along with the GqPCR and KIR2.3 inhibits ligand-induced current inhibition. The PIP2 hydrolysis products IP3 and DAG are ubiquitous signals. IP3 stimulates Ca2+ release from endoplasmic reticulum stores, while DAG stimulates PKC that phosphorylates numerous protein targets. A number of different types of experiments can ascertain if current inhibition involves PIP2 hydrolysis, is IP3-mediated, or PKC-dependent (patch-clamp experiments with excised membrane patches, experiments involving co-expression of IP3 phosphatase, or use of pharmacological blockers of PKC) (e.g. Keselman et al., 2007). Assessment of Gi/o signaling in oocytes involves co-expression of a Gi/o-coupled GPCR (Gi/oPCR) along with a GIRK channel reporter and ligand-induced current stimulation (Fig. 2B). Stimulation of the Gi/oPCR by the appropriate ligand leads to Gβγ-induced stimulation of GIRK currents (Chan et al., 1996). Co-expression of PTX along with the Gi/oPCR and GIRK channels decreases basal currents and abolishes agonist-induced current stimulation (Vivaudou et al., 1997). Assessment of Gs signaling in oocytes using ion channel activity as a reporter proved more challenging than Gq or Gi signaling. At the endogenous expression levels of G-protein subunits, GIRK channels exhibit specificity for Gi/o proteins and Gi/oPCRs as revealed by the PTX sensitivity of stimulated currents. Yet in heterologous expression systems, like the Xenopus oocytes, one could bias Gβγ signaling from Gαi/o to Gαs by overexpressing Gαs subunits. The β2-adrenergic receptor (β2-AR), a Gs-coupled GPCR (GsPCR), caused significant GIRK stimulation when it was overexpressed along with the GIRK channels and Gαs proteins (Lim et al., 1995). Under these conditions, isoproterenol- (ISO-) could induce large GIRK currents in Xenopus oocytes (Fig. 2C). Thus, oocyte overexpression of Gαs subunits biases signaling from the Gi/o to the Gs pathway.
Figure 2. Using ion channel activity to monitor Gq, Gi/o, and Gs signaling.
(A) Stimulation of a GqPCR , such as 2AR, activates phospholipase C (PLC) to hydrolyze plasma membrane phosphatidylinositol 4,5 bisphosphate (PIP2) resulting in inhibition of the KIR2.3 channel current. RGS2 is shown to inhibit Gq signaling. (B) Stimulation of a Gi/oPCR, such as mGluR2, activates G proteins that stimulate GIRK1/GIRK2 and GIRK1/GIRK4 currents via the Giβγ subunits. The active protomer of PTX is shown to inhibit Gi/o signaling. (C) Stimulation of a GsPCR, such as β2-AR, activates G proteins that stimulate GIRK1/GIRK2 currents via the Gsβγ subunits, only when Gαs is overexpressed.
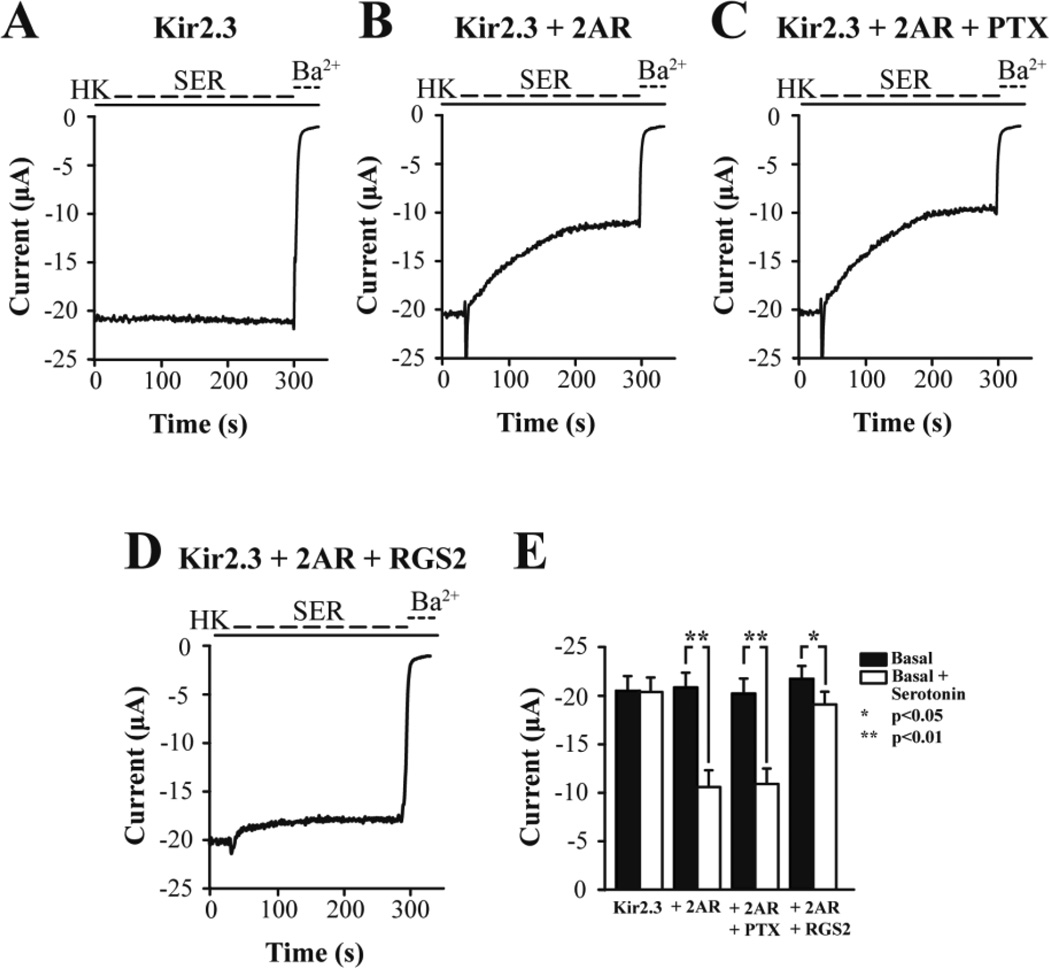
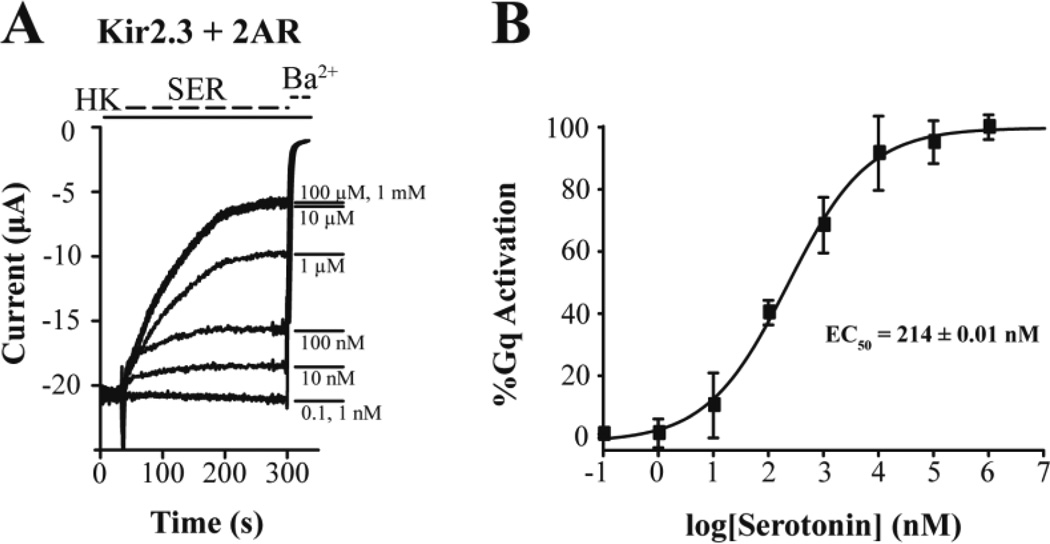
We employed the KIR2.3 channel as a reporter of GqPCR signaling and used two-electrode voltage-clamp to monitor currents. Oocytes only expressing KIR2.3 showed robust currents, ~20 µA (Fig. 3A). Subsequent serotonin perfusion did not alter the KIR2.3 current, suggesting the lack of endogenous oocyte serotonin receptors coupling to the Gq signaling pathway. Perfusion of Ba2+ ions blocks Kir currents, as evidenced by the effect of 3 mM BaCl2 on KIR2.3 currents. Yet, when the 5HT2AR (or 2AR), a GqPCR, was co-expressed with the KIR2.3 channel, 1 µM serotonin perfusion caused robust inhibition of K+ current (Fig. 3B). The spike observed at the onset of serotonin perfusion signifies activation of the oocyte endogenous Ca2+-activated Cl− current (ICl-Ca), and provides additional evidence of the serotonin-induced PIP2 hydrolysis and IP3-mediated rise in intracellular Ca2+. Expression of the active protomer of PTX showed no effect on the serotonin-induced inhibition of the KIR2.3 current, suggesting that this was a Gi/o-independent effect (Fig. 3C). In contrast, co-expression of the RGS2 protein together with the 2AR and KIR2.3 greatly attenuated the serotonin-induced current inhibition, suggesting that this process is Gq mediated (Fig. 3D). These results are summarized in Figure 3E. Since KIR2.3 current inhibition signified Gq activation, we proceeded to perform a serotonin dose-response experiment and determine the apparent affinity of 2AR for serotonin in the Xenopus oocyte expression system (Fig. 4A). The normalized Gq activation (relative to the maximal current inhibition – 100%) was plotted as a function of serotonin concentration and the points obtained were fitted by a curve according to the Hill equation, giving an EC50 = 214 nM (Fig. 4B).
Figure 3. Gq signaling activity of 2AR in response to serotonin.
Representative barium-sensitive traces of KIR2.3 currents obtained in response to 1 µM serotonin (SER) in oocytes expressing (A) KIR2.3 alone, (B) KIR2.3 together with the serotonin receptor 2AR, (C) KIR2.3, 2AR and PTX, and (D) KIR2.3, 2AR and RGS2. (E) Summary of basal and serotonin-induced levels of current for all groups.
Figure 4. Measurement of the apparent affinity of 2AR for serotonin using ion channel activity.
(A) Representative barium-sensitive traces of KIR2.3 currents obtained in oocytes expressing 2AR in response to increasing concentrations (0.1 nM to 1 mM) of serotonin (SER). (B) Dose-response curve showing Gq-mediated inhibition of KIR2.3 current elicited by serotonin in oocytes expressing 2AR. Response was normalized to the maximum value. (EC50 = 214 ± 0.01 nM).
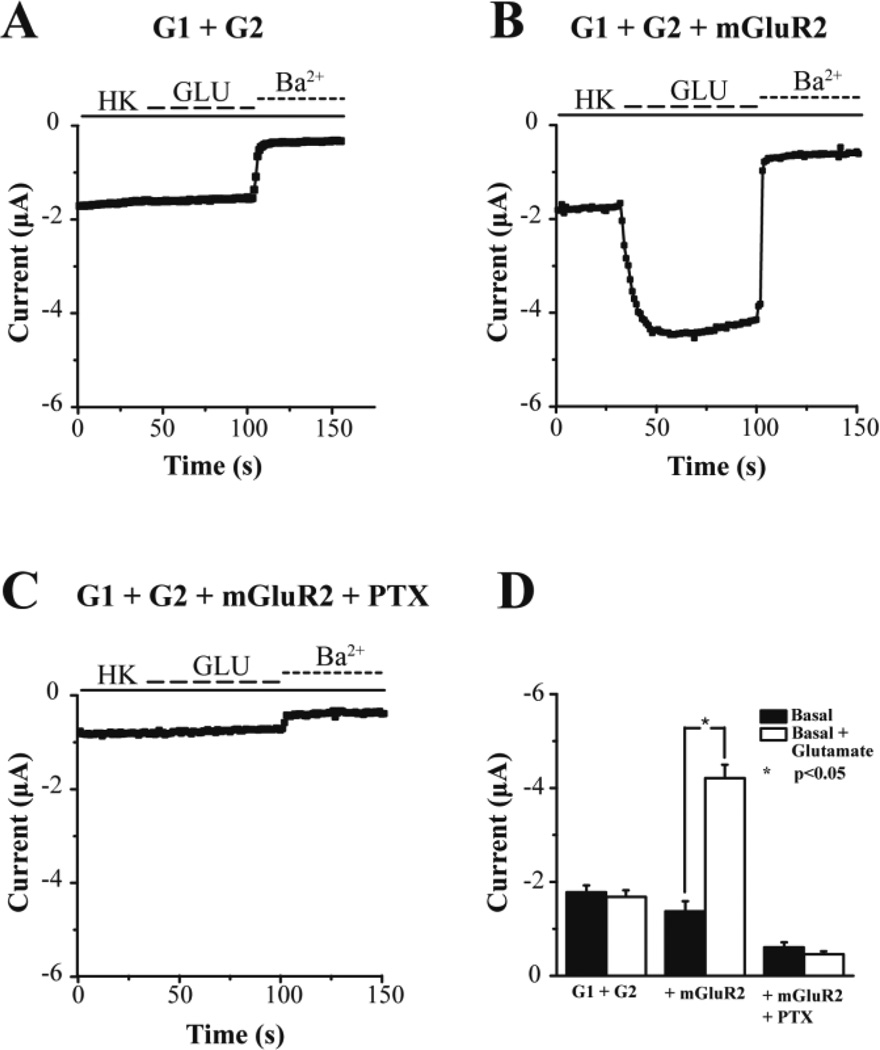
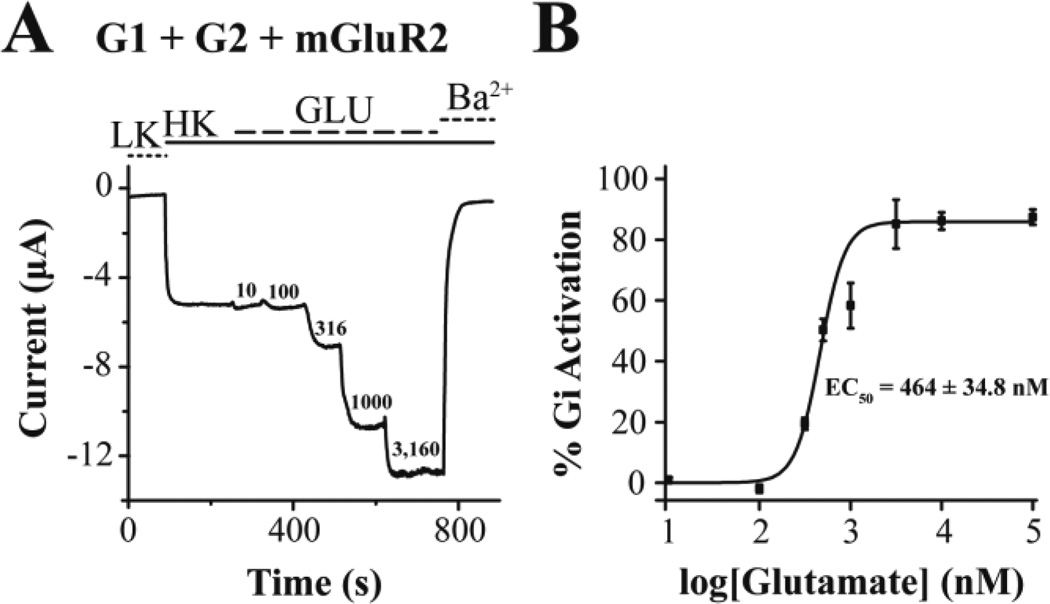
We next turned to expressing the neuronal GIRK1/GIRK2 (G1/G2) as a reporter of GiPCR signaling. Expression of G1/G2 alone in oocytes produced currents of ~2 µA (Fig. 5A). Glutamate perfusion showed no changes in the G1/G2 current while perfusion of BaCl2 blocked the G1/G2 currents, suggesting the lack of oocyte endogenous glutamate receptors coupling to the Gi/o signaling pathways. Yet, when the mGluR2, a GiPCR, was co-expressed with the G1/G2 channel, 10 µM glutamate perfusion caused robust stimulation of K+ current (Fig. 5B). When PTX was co-expressed with the mGluR2 and G1/G2 channel, the basal current was reduced and the glutamate-induced currents were abolished (Fig. 5C). These results are summarized in Figure 5D. We next performed a glutamate dose-response experiment and determined the apparent affinity of mGluR2 for glutamate in the Xenopus oocyte expression system, using G1/G2 heteromeric channels (Fig. 6A). The normalized Gi activation is plotted as a function of glutamate concentration and the points obtained were fitted by a curve according to the Hill equation, giving an EC50 = 0.5 µM (Fig. 6B).
Figure 5. Gi signaling activity of mGluR2 in response to glutamate.
Representative barium-sensitive traces obtained from oocytes expressing the channel GIRK1/GIRK2 (G1/G2) alone (A), the channel G1/G2 and metabotropic type 2 Glutamate receptor (mGluR2) (B), or the channel G1/G2, mGluR2 and PTX (C). Channel activity was monitored using the Two-Electrode Voltage-Clamp in the presence or absence of 10μM glutamate in the bath solution. (D) Summary bar graph of the basal and agonist-induced currents (mean ± SEM) monitored in each of the above cases. Glutamate can significantly potentiate the current amplitude in oocytes expressing G1/G2 and mGluR2, an effect that is abolished when PTX is co-injected to the oocytes.
Figure 6. Measurement of the apparent affinity of mGluR2 for glutamate using ion channel activity.
(A) Representative barium-sensitive trace from a single oocyte expressing the heterotetrameric channel GIRK1/GIRK2 (G1/G2) and mGluR2 depicting a dose dependent channel activity when increasing concentrations of glutamate were present in the bath solution. (B) Representative glutamate dose-response curve. Channel activity was measured in TEVC recordings in Xenopus oocytes expressing G1/G2 and mGluR2 and Gi activity was estimated as the Glutamate induced activity normalized with the barium-sensitive basal current. The % Gi responses (% GIRK activation) elicited by different concentrations of glutamate (EC50 = 464 ± 34.8 nM).
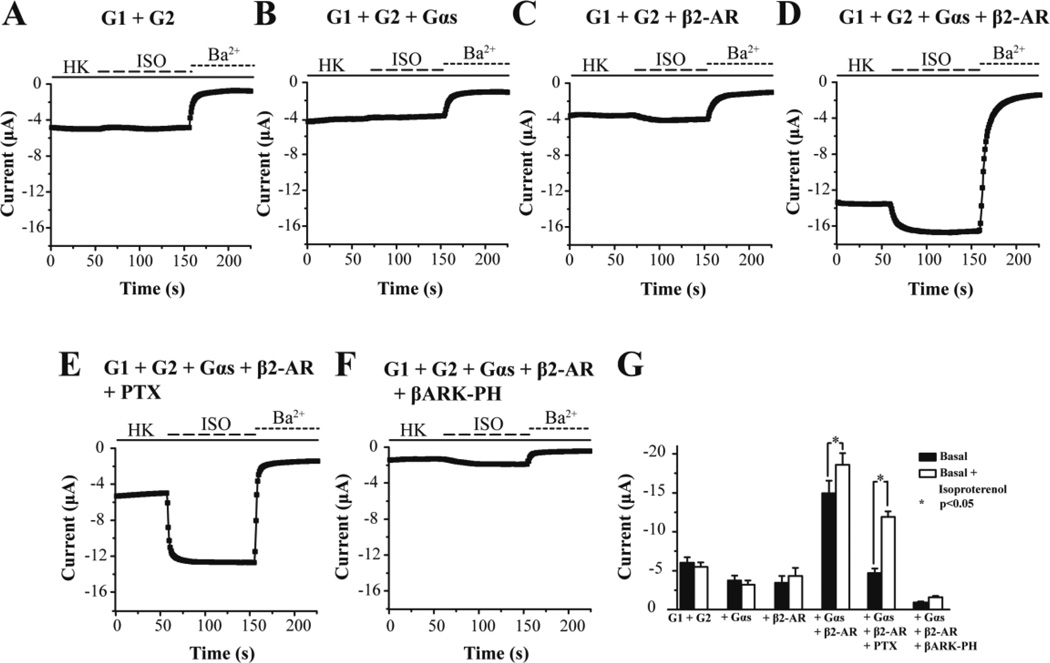
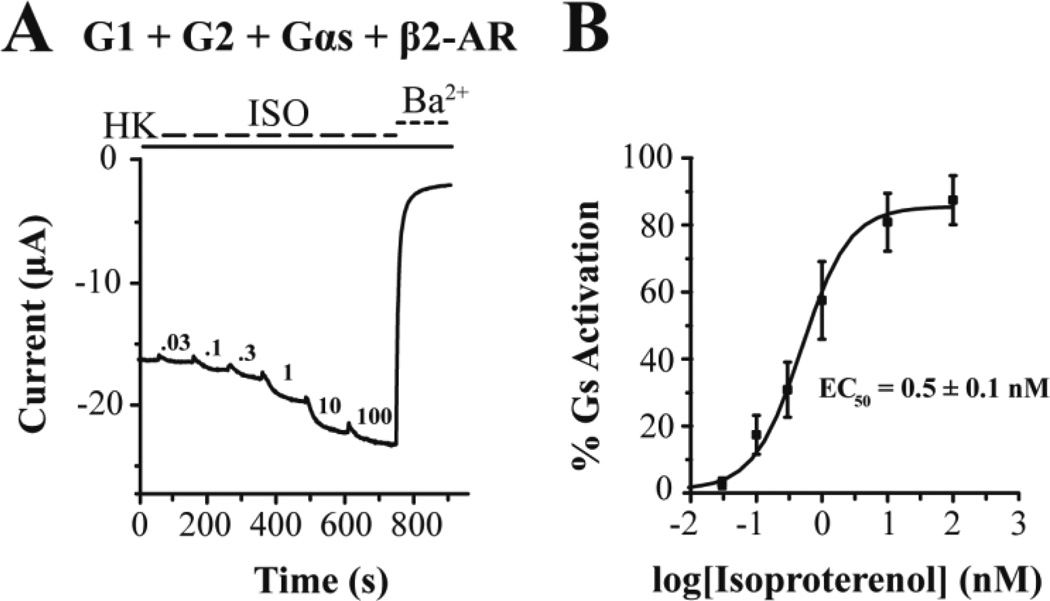
Finally we turned to using the G1/G2 channel as a reporter for GsPCR signaling, following the work of Lim and co-workers (1995), who showed that G-protein coupling to GIRK channels could be biased towards Gs signaling. G1/G2 currents were not affected by perfusion of ISO (Fig. 7A), suggesting the lack of oocyte endogenous receptors sensitive to this adrenergic receptor agonist. In the absence of the β2-AR, Gαs overexpression did not reveal ISO-induced currents (Fig. 7B). Co-expression of the β2-AR with G1/G2 gave very small or no ISO-induced currents, suggesting inefficient coupling of the oocyte endogenous Gβγ subunits coming from the GsPCR (Fig. 7C). Lim and colleagues had shown a differential dependence of agonist-independent (basal) versus ISO-dependent GIRK1 currents expressed in oocytes, presumably as heteromers with endogenous oocyte GIRKs (Lim et al., 1995; Hedin et al., 1996); 200 pg of injected Gαs cRNA gave saturating 15-fold enhancement of ISO-dependent current, while by 1 ng of injected Gαs cRNA the basal current was maximally decreased presumably by scavenging free Gβγ. Co-expression of the β2-AR, Gαs and the G1/G2 channel gave significant agonist-independent (or basal) and ISO-dependent currents (Fig. 7D). Co-expression of PTX abolished the increase in basal currents and revealed larger ISO-induced currents (Fig. 7E). The Lefkowitz group reported first that PKA-mediated phosphorylation of β2-AR allowed for a switch of the signaling specificity from Gs to Gi (Daaka et al., 1997). Whether the PTX-sensitive basal currents are the result of a similar PKA-dependent mechanism remains to be further investigated. Co-expression of the Gβγ scavenger, the PH domain of the β-AR kinase (βARK), greatly attenuated all currents (Fig. 7F), reaffirming that the ISO-induced currents were mediated by the Gβγ subunits. These results are summarized in Figure 7G. The normalized Gs activation is plotted as a function of ISO concentration (Fig. 8A) and the points obtained were fitted by a curve according to the Hill equation, giving an EC50 = 0.49 nM (Fig. 8B). This apparent affinity for ISO appears ~100-fold higher than that determined in other systems. A ten-fold reduction in the injected Gαs cRNA concentration (to 100 pg) yielded an EC50 = 100 nM (data not shown). Thus, high levels of Gαs greatly increase the efficiency of the β2-AR/Gαs coupling causing the enhanced apparent affinity.
Figure 7. Gs signaling activity of the β2-AR in response to isoproterenol.
Representative traces of GIRK1/GIRK2 (G1/G2) currents obtained at −80 mV in response to a high K+ solution (HK, solid line) and 100 nM isoproterenol (ISO, dashed line) in oocytes expressing (A) G1 + G2, (B) G1 + G2 + Gαs, (C) G1 + G2 + β2-AR, (D) G1 + G2 + β2-AR + Gαs, (E) G1 + G2 + Gαs + β2-AR, and (F) G1 + G2 + β2-AR + Gαs + βARK-PH. Barium (3 mM Ba2+, dotted line) inhibited G1/G2 and allowed for subtraction of G1/G2 independent currents. (G) Summary figure where each bar represents the mean of n=5–6 experiments with error bars depicting the standard error of the mean. Significance between “basal” and “basal + isoproterenol” was determined using paired t-tests p<0.05.
Figure 8. Measurement of the apparent affinity of β2-AR in response to isoproterenol.
(A) Representative trace of G1/G2 currents obtained at −80 mV in response to a high K+ solution (HK, solid line) and 0.03, 0.1, 0.3, 1, 10, and 100 nM isoproterenol (ISO, dashed line) in oocytes expressing G1 + G2 + β2-AR + Gαs. Barium (3 mM Ba2+, dotted line) inhibited G1/G2 and allowed for subtraction of G1/G2 independent currents. (B) Dose-response curve of isoproterenol-induced G1/G2 activation. Data were fit using the Hill equation with an EC50 = 0.5 ± 0.1 nM.
Since the GPCR coupling to the G-protein heterotrimer is controlled by the Gα subunits, one could also utilize this system to investigate which Gα subunit couples to a given GPCR to affect channel activity. In such experiments appropriate controls are required to ensure that the particular Gα subunit tested is able to associate and signal through the Gβγ subunits used.
We have previously used the Xenopus oocyte heterologous expression system to study heteromeric GPCR complexes between mGluR2 (a class C GPCR), and 2AR (a class A GPCR) (Fribourg et al., 2011). Glutamate signaling in oocytes expressing the mGluR2 alone (1 ng of cRNA) gave PTX-sensitive responses like those shown in Fig. 5. Serotonin signaling in oocytes expressing the 2AR alone (2 ng of cRNA) gave RGS2-sensitive responses like those shown in Fig. 3. Co-expression of mGluR2 (1 ng of cRNA) and 2AR (2 ng of cRNA) doubled the mGluR2 response, while it halved the 2AR response. Antipsychotic or hallucinogenic drugs showed clear cross signaling through the co-expressed mGluR2/2AR receptors only at the 1:2 cRNA ratio, respectively.
The great advantage of the oocyte system over mammalian cell lines as a heterologous expression system is that one can accurately inject different amounts of cRNA that are turned into protein by the translational machinery of the oocyte. Similar control of the amount of cDNA through transfection of mammalian cell lines is certainly much more challenging. Since the stoichiometry of certain heteromeric GPCR complexes is critical to their ability to cross signal, the oocyte system offers a convenient and powerful way to functionally study such complexes. Additionally, an advantage of the oocyte expression system over mammalian cell lines lies in that it allows for simultaneous expression of multiple cRNA species whose level of injection into the cell can be fully controlled. Since the stoichiometry of certain heteromeric GPCR complexes is critical to their ability to cross signal, the oocyte system offers a convenient and powerful way to functionally study such complexes. Furthermore, experiments like those shown in Fig. 7E, F, involving as many as five or more species of cRNA, can be routinely performed with high success in this system, unlike cDNA transfection of as many species in mammalian cell line expression systems.
Acknowledgements
The authors are grateful to Heikki Vaananen for building and maintaining the Xenopus laevis frog facility at VCU and for preparing viable oocytes that have been used to perform the experiments presented. Many thanks go to all former Logothetis lab members whose contributions over the years have allowed use of this heterologous expression system expertly in addressing mechanistic questions with success. The work has been supported by NIH grants to DEL R01 HL059949-16, R01 HL090882-4 and to MF T32 MH096678-03.
References
- 1.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1994;25:366–428. [Google Scholar]
- 2.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol. 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim. Biophys. Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- 4.Lin H-H. G-protein-Coupled Receptors and Their (Bio) Chemical Significance Win 2012 Nobel Prize in Chemistry. Biomed. J. 2013;36:118–124. doi: 10.4103/2319-4170.113233. [DOI] [PubMed] [Google Scholar]
- 5.Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br. J. Pharmacol. 2006;1:S46–S55. doi: 10.1038/sj.bjp.0706405. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehrl JH, Sinnarajah S. RGS2: a multifunctional regulator of G-protein signaling. Int. J. Biochem. Cell Biol. 2002 May;34(5):432–438. doi: 10.1016/s1357-2725(01)00141-8. Review. [DOI] [PubMed] [Google Scholar]
- 7.Barnard EA, Miledi R, Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc. R. Soc. London. 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 8.Gundersen CB, Miledi R, Parker I. Messenger RNA from human brain induces drug- and voltage- operated channels in Xenopus oocytes. Nature. 1984;308:421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- 9.Miledi R, Parker I, Sumikawa K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982;1:1307–1312. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 11.Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflügers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 12.Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- 13.Dascal N, Lim NF, Schreibmayer W, Wang W, Davidson N, Lester HA. Expression of an atrial G-protein-activated potassium channel in Xenopus oocytes. Proc Natl Acad Sci U S A. 1993;90:6596–6600. doi: 10.1073/pnas.90.14.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soejima M, Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 15.Kurachi Y, Nakajima T, Sugimoto T. Acetylcholine activation of K+ channels in cell-free membrane of atrial cells. Am. J. Physiol. 1986;251:H681–H684. doi: 10.1152/ajpheart.1986.251.3.H681. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 17.Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- 18.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 19.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 20.Sui J-L, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of Phosphatidylinositol phosphates. PNAS. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petit-Jacques J, Sui J-L, Logothetis DE. Synergistic activation of GIRK channels by Na+, Mg2+ and Gβγ subunits. J. Gen. Physiol. 1999;114:673–684. doi: 10.1085/jgp.114.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nature Cell Biology. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- 23.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with PIP2 determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 24.Rohács T, Lopes CMB, Michailidis I, Logothetis DE. PtdIns(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nature Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 25.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen SB, Tao X, MacKinnon R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature. 2011;477:495–498. doi: 10.1038/nature10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan R, Ha J, Zhang M, Kawano T, Kozasa T, Logothetis DE. A Computational Model Predicts That Gβγ Acts at a Cleft Between Channel Subunits to Activate GIRK1 Channels. Sci. Signal. 2013;6:ra69. doi: 10.1126/scisignal.2004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature. 2013;498:190–197. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng XY, Zhang HX, Logothetis DE, Cui M. The molecular mechanism by which PIP2 opens the intracellular G-loop gate of a Kir3.1 channel. Biophys. J. 2012;102:2049–2059. doi: 10.1016/j.bpj.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keselman I, Fribourg M, Felsenfeld DP, Logothetis DE. Mechanism of PLC-mediated Kir3 current inhibition Channels. 2007;1:113–123. doi: 10.4161/chan.4321. [DOI] [PubMed] [Google Scholar]
- 31.Chan KW, Langan MN, Sui J, Kozak JA, Pabon A, Ladias JAA, Logothetis DE. A recombinant inwardly-rectifying potassium channel coupled to GTP-binding proteins. J. Gen. Physiol. 1996;107:381–397. doi: 10.1085/jgp.107.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J. Biol. Chem. 1997 Dec 12;272(50):31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- 33.Lim NF, Dascal N, Labarca C, Davidson N, Lester HA. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβγ subunits in Xenopus oocytes. J. Gen. Physiol. 1995;105:421–439. doi: 10.1085/jgp.105.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- 35.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of thecoupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–89. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 36.Fribourg M, Moreno JL, Terrell Holloway T, Provasi D, Mahajan R, Park G, Lia Baki L, Adney SK, Hatcher C, Ruta JD, Albizu L, Li Z, Shim J, Fabiato A, MacKerell AD, Brezina V, Sealfon SC, Filizola M, González-Maeso J, Logothetis DE. Decoding the signalling of a GPCR heteromeric complex reveals the mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]