Abstract
Purpose: Screening interactions of a resolvin E1 analog (RX-10045) with efflux transporters (P-glycoprotein [P-gp], multidrug resistance-associated protein [MRP2], and breast cancer-resistant protein [BCRP]).
Methods: Madin-Darby canine kidney cells transfected with P-gp, MRP2, and BCRP genes were selected for this study. [3H]-Digoxin, [3H]-vinblastine and [3H]-abacavir were selected as model substrates for P-gp, MRP2, and BCRP. Uptake and permeability studies across cell monolayer in both apical to basal (AP–BL) and BL–AP of these substrates were conducted in the presence of specific efflux pump inhibitors and RX-10045. Cell viability studies were conducted with increasing concentrations of RX-10045.
Results: Uptake studies showed a higher accumulation in the presence of inhibitors (GF120918 and ketoconazole for P-gp; MK571 for MRP2; and β-estradiol for BCRP) as well as RX-10045. Similarly, dose-dependent inhibition studies demonstrated higher accumulation of various substrates ([3H]-digoxin, [3H]-vinblastine, and [3H]-abacavir) in the presence of RX-10045. IC50 values of dose-dependent inhibition of RX-10045 for P-gp, MRP2, and BCRP were 239±11.2, 291±79.2, and 300±42 μM, respectively. Cell viability assay indicated no apparent toxicity up to 350 μM concentration. Enhanced permeability for model substrates was observed in the presence of RX-10045. Uptake studies in human corneal epithelial cells suggest that RX-10045 is a strong inhibitor of organic cation transporter-1 (OCT-1).
Conclusions: In summary, the resolvin analog (RX-10045) was identified as a substrate/inhibitor for efflux transporters (MRP2 and BCRP). Also, RX-10045 appears to be a strong inhibitor/substrate of OCT-1. Novel formulation strategies such as nanoparticles, nanomicelles, and liposomes for circumventing efflux barriers and delivering higher drug concentrations leading to a higher therapeutic efficacy may be employed.
Introduction
Resolvins are small endogenous mediator molecules that are enzymatically biosynthesized from omega-3 polyunsaturated fatty acids, that is, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).1 Other methods of biosynthesis include aspirin-triggered and/or non-aspirin-dependent pathways.2 Cyclooxygenase-2 (COX-2)-dependent reactions in the presence of aspirin and microbial P450-initiated pathways are reported to facilitate production of resolvins.2 These compounds belong to a family of potent lipid mediators that causes reversal of the inflammatory response back to a noninflamed state.3 Biosynthesized resolvins act by shielding tissues from leukocyte-mediated injuries, dampening leukocyte response/trafficking to the site of inflammation, and counter regulating proinflammatory gene expressions, thus reducing tissue inflammation.2 Resolvins are currently being studied to ameliorate the ocular pathological conditions such as dry eye,4 retinal diseases,5 and uveitis.6 This class of drugs opens up an entirely novel approach to treat inflammatory ocular conditions. Resolvin E1 analog (RX-10045) (Fig. 1) is a synthetic active pharmaceutical ingredient and an analog/derivative of naturally occurring resolvin E1 (RvE1). In vitro studies demonstrated potent anti-inflammatory and cell survival benefits with RX-10045.7 This novel molecule is highly effective against dry eye and goblet cell loss thereby accelerating tear production. Also, this compound can reduce corneal inflammation, epithelial damage, and accelerate corneal tissue repair. In addition, RX-10045 can inhibit the release of several key proinflammatory mediators from corneal epithelial cells (Pan Z, et al. Association for Research in Vision and Ophthalmology. http://www.iovs.org/content/49/5/2223.full.pdf 2008; E-125). This drug is originally formulated as an aqueous solution using propylene glycol as a solubilizing agent and tested for the treatment of dry eye with topical drop application. The drug was shown to be highly efficacious in murine models of dry eye syndrome. However, in Phase II clinical trials, RX-10045, although safe and well tolerated, produced equivocal efficacy results.7 A possible explanation is that disposition across the human cornea and conjunctiva may be limited due to efflux transporters expressed on the ocular surface.
FIG. 1.
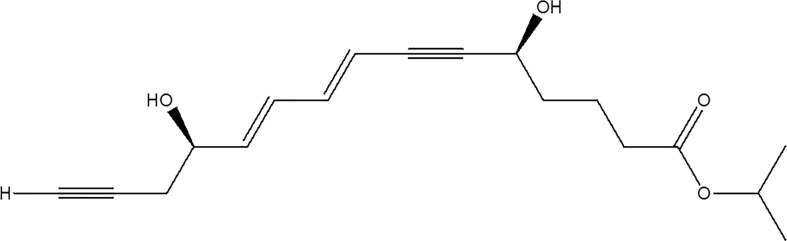
Structure of resolvin E1 analog (RX-10045).
ABC transporters such as multidrug resistance gene products (P-glycoprotein [P-gp]), multidrug resistance-associated protein (MRP), and breast cancer-resistant protein (BCRP) are expressed on the corneal epithelial cell membrane, which can lower drug permeability and alter drug absorption.8,9 The efflux transporters are plasma membrane proteins expressed highly in both prokaryotes and eukaryotes. P-gp, a 170 kDa transmembrane protein, is localized mostly on the apical surface of epithelial and endothelial cells. Expression and localization of P-gp on corneal cell in rabbits and human have been previously reported.10,11 In vivo studies in rabbits demonstrated active P-gp efflux-lowering erythromycin permeability across rabbit cornea.12 P-gp is considered as a biological barrier due to its ability to extrude toxins and xenobiotics into the extracellular environment.13 ABCC/MRP is a large branch of the ABC family consisting of 13 different proteins, which are similar to the MDR1 gene product in terms of function and localization. MRPs are classified as short and long transporters based on their structure. MRP2 is a 190–205 kDa transmembrane protein. Our laboratory has reported localization of MRP2 and its role in drug efflux on human corneal epithelial cells (HCECs) and rabbit cornea.14 These transporters are actively involved in effluxing xenobiotics thereby reducing intracellular drug bioavailability. Another efflux protein/transporter that reduces cellular bioavailability is BCRP. It is also an ABC efflux transporter conferring to multidrug resistance (MDR). The transporter is a half protein consisting of 6 transmembrane domains (TMD) compared to 12 TMD and 17 TMD for P-gp and MRP, respectively. It is a 75 kDa protein designated as ABCG2. The expression and functional role of BCRP in drug efflux have also been reported on HCECs.15 These efflux pumps eventually cause suboptimal levels of RX-10045 in target.
The interaction of RX-10045 with efflux transporters expressed on the ocular surface may lower permeability across the corneal epithelium. A better understanding of the interactions between the efflux transporter and RX-10045 may lead to attain therapeutic levels. Therefore, the objective of this study is to assess the affinity of RX-10045 toward efflux transporters in polarized Madin-Darby canine kidney (MDCK) cells, that is, MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP.
Methods
RX-10045 was prepared by PPD (Lot No. SYN-72799-05-11). Ketoconazole, a P-gp inhibitor, was acquired from Sigma Chemical Co. MK571, a specific inhibitor of MRP, was obtained from Biomol International (Plymouth Meeting). GF120918, a P-gp and BCRP inhibitor, was a generous gift from GlaxoSmithKline Ltd. Beta-estradiol was purchased from Sigma-Aldrich. [3H]-Digoxin (specific activity 35.4 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences, [3H]-abacavir (specific activity 0.2 Ci/mmol) and [3H]-vinblastine (specific activity 3.6 Ci/mmol) were obtained from Moravek chemical company. [3H]-Digoxin, [3H]-abacavir, and [3H]-vinblastine were used at 0.5, 0.5, and 0.25 μCi/mL, respectively. A stock solution of the RX-10045 (1 mg/mL) was freshly prepared before an experiment. Briefly, RX-10045 (1 mg) was accurately measured and dissolved in 50 μL dimethyl sulfoxide (DMSO) (Thermo Scientific) and the volume was made up with distilled deionized water. Furthermore, aliquots of this stock solution were diluted in Dulbecco's phosphate-buffered saline (DPBS, composition: 130 mM NaCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 0.5 mM MgSO4, 1 mM CaCl2, 0.03 mM KCl, and 5 mM glucose and pH 7.4) to achieve desired concentrations. Stock solutions of ketoconazole (10 mg/mL), GF120918 (1 mg/mL), beta-estradiol (1 mg/mL), and MK571 (25 mg/mL) were prepared in DMSO and aliquots were diluted in DPBS to achieve the desired concentration. Culture flasks (75 cm2 growth area) were procured from MidSci. Twelve-well culture plates were purchased from Midwest Scientific. Twelve-well transwell plates (Corning Incorporated) with an insert membrane growth area of 1 cm2 were selected for transport studies.
Cell culture
MDCK cells transfected with the human MDR1 gene (MDCKII-MDR1), human MRP2 gene (MDCKII-MRP2), and human BCRP gene (MDCKII-BCRP) were generously provided by Drs. A. Schinkel and P. Borst (The Netherlands Cancer Institute, Amsterdam, Netherlands). Dulbecco's modified Eagle's medium (DMEM) was procured from Caisson Laboratories, Inc. Tryple Express, nonessential amino acids, and fetal bovine serum (FBS) were procured from Invitrogen. Penicillin, streptomycin, sodium bicarbonate, lactalbumin, HEPES, amphotericin B, and polymyxin B sulfate were supplied by Sigma-Aldrich. MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells were cultured as described previously.16 Cells were maintained in DMEM supplemented with 10% calf serum, 100 IU/mL penicillin, 100 mg/mL streptomycin, and 20 mM HEPES, pH 7.4. Cells were plated at a density of 250,000/cm2 in 12-well tissue culture-treated plastic plates. Cells were incubated at 37°C in an atmosphere of 5% CO2 and 95% humidity in air and were allowed to grow for 5–8 days. Similarly, the human corneal epithelial cell line was procured as a generous gift from Dr. Araki-Sasaki (Kinki Central Hospital, Japan). HCECs are SV-40 virus-transfected human immortalized corneal cells. These cells were cultured and maintained as reported previously.17 HCECs were cultured in DMEM/F-12 media supplemented with 15% FBS (heat inactivated), penicillin, streptomycin, 22 mM sodium bicarbonate, 15 mM HEPES, 10 ng/mL of human epidermal growth factor, and 5 mg/mL insulin. The cells were grown at 37°C in a cell culture incubator, which maintained 5% CO2 and 95% relative humidity.
Uptake experiments
Uptake studies were conducted according to previously published protocols.18 Briefly, cells (MDCKII-MDR1, MDCKII-MRP2, MDCKII-BCRP, and HCEC) were seeded in a 12-well plate. After 5–7 days of seeding, the medium was aspirated and cells were rinsed 3 times with DPBS and then equilibrated with DPBS for 30 min at 37°C. Initial screening for uptake studies were conducted across MDCKII-MDR1, MDCKII-BCRP, and MDCKII-MRP2 cells with a known concentration of RX-10045 (50–600 μM) solution containing [3H]-digoxin (0.5 μCi/mL) for P-gp, [3H]-abacavir (0.5 μCi/mL) for BCRP, and [3H]-vinblastine (0.25 μCi/mL) for MRP2. In these studies, efflux pump inhibitors such as GF120918 and ketoconazole for P-gp, beta-estradiol for BCRP, and MK571 for MRP2 were selected. Cells were treated with a drug solution. The experiment was conducted at 37°C for 30 min. At the end of an experiment, the drug solution was aspirated. To terminate the uptake process, cells were rinsed 3 times with ice-cold stop solution (210 mM KCl, 20 mM HEPES [pH 7.4]). To determine the drug content, cell lysis was performed by adding 1 mL of lysis solution (0.3 M NaOH, 0.1% Triton X-100). Cells were left undisturbed overnight at room temperature. Samples (500 μL) were transferred to scintillation vials containing 3 mL scintillation cocktail. The solutions were mixed and radioactivity measured with a scintillation counter (Model LS 6500; Beckman Instruments, Inc.). To normalize the uptake data, cell protein content was measured by the Bradford assay. A protein calibration curve was prepared with bovine serum albumin as standard and was used to determine the protein content in the samples. The amount of radioactive substrate accumulated inside the cell was determined using the following equation:
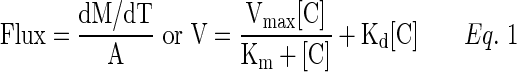 |
Concentrations of donor and samples are represented as Csample and Cdonor. As represented in Eq. (1), V is the total rate of uptake, Vmax is the maximum uptake for a carrier-mediated process, Kd is the rate constant, and Km is the concentration at half saturation.
Following a similar procedure, dose-dependent inhibition studies were performed. [3H]-Digoxin, [3H]–abacavir, and [3H]-vinblastine were spiked with various concentrations of RX-10045 ranging from 25 to 800 μM. Studies were performed as described before and data were obtained to determine the half-maximal inhibitory concentration (IC50) according to a previously published method.8,19 IC50 was calculated according to Eq. (2).
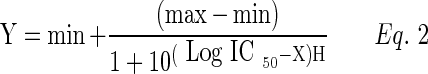 |
Y represents cellular accumulation of radioactive substrate; X denotes the logarithm of the RX-10045 concentration. Data were fitted to Eq. (2) with a transformed nonlinear regression analysis program (GraphPad Prism version 4.0) to calculate IC50. H is the Hill constant.
Permeability studies
Transport studies were performed on MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells grown on transwell inserts. Cells were seeded on transwells at a density of 3 million cells/plate and allowed to grow for 5–7 days. Each day the culture medium was removed and replaced with a fresh medium. Apparent drug permeability from apical to basolateral (AP-BL) and vice versa (BL-AP) was examined indirectly by calculating the amount of radioactive substrate ([3H]-digoxin for MDCKII-MDR1, [3H]-abacavir for MDCKII-BCRP, and [3H]-vinblastine for MDCKII-MRP2) transported into the receiver chamber. The donor chamber contained a test solution, whereas the receiver chamber contained DPBS. The concentration of RX-10045 to be employed for permeability studies was identified and selected from the earlier dose-dependent inhibition (IC50 value) studies. At predetermined time points (0, 15, 30, 45, 60, 90, 120, 150, and 180 min), samples were withdrawn from the receiver chamber and replaced with same volumes of DPBS to a maintain sink condition. Furthermore, the concentration of radioactive substrates transported across the cell monolayer was determined by measuring radioactivity as described earlier. To determine apparent permeability and efflux ratio, the cumulative amount of radioactive substrate transported was plotted with time according to Eqs. (3–5).
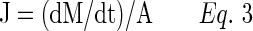 |
J represents flux, (dM/dt) represents the amount of radioactive substrate transported across the cell monolayer over a specific interval of time, and A represents the cross-section area of transwell.
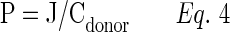 |
P represents apparent radioactive permeability and Cdonor represents concentration of radioactive substrate in the donor chamber.
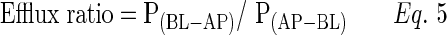 |
The drug efflux ratio may be calculated by the ratio of apparent permeability from BL to AP and AP to BL directions.
Cytotoxicity studies
To evaluate the cytotoxicity of RX-10045, the CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay Kit (Promega) was utilized. In vitro cytotoxicity assays were conducted across MDCKII-MDR1, MDCKII-BCRP, MDCKII-MRP2, and HCECs. In brief, cells were seeded at a density of 10,000 cells per well in a 96-well plate. Since the permeability studies were conducted for 3 h, cells were treated with 100 μL of freshly prepared RX-10045 solutions and exposed for 3 h, and cells treated with the culture medium (containing no drug) served as a negative control. Ten percent Triton X-100 served as a positive control. After a 3-h incubation, 20 μL of aqueous MTS and PMS reagent (prepared following the manufacture's protocol) was added to each well. The mixture was further incubated for 2.5 h allowing tetrazolium compound ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron coupling phenazine methosulfate [PMS]) reaction to take place. The amount of formazan formed was measured with a 96-well microtiter plate reader (SpectraFluor Plus; Tecan). Absorbance is measured at 490 nm, which is directly proportional to the number of living cells in culture.
Statistical analysis
All uptake and permeability experiments were conducted at least in quadruplicate and results are expressed as mean±SD. For IC50, data are expressed as the mean±SE. A difference between mean values was considered significant at P≤0.05. Fisher's least-significance difference method was applied to discriminate among the means.
Results
Uptake studies
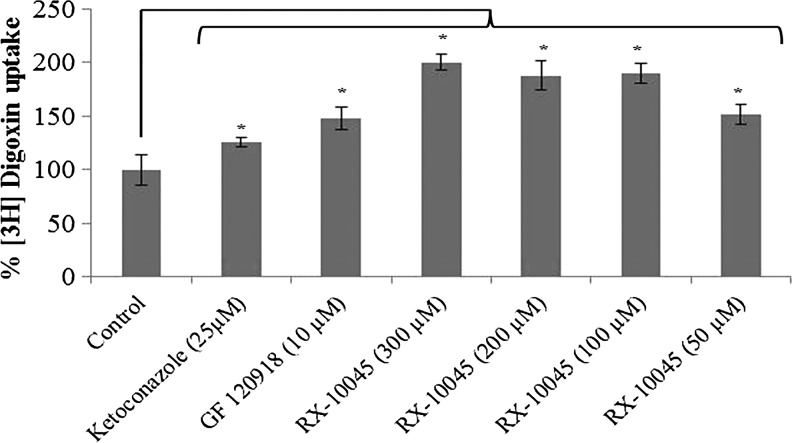
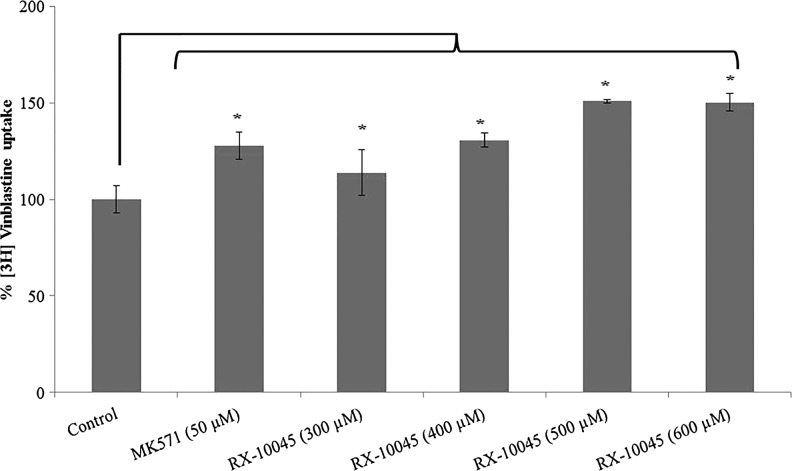
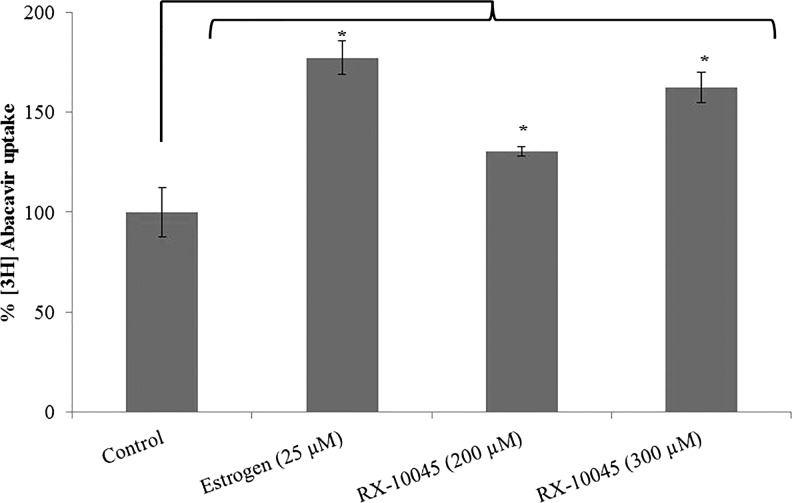
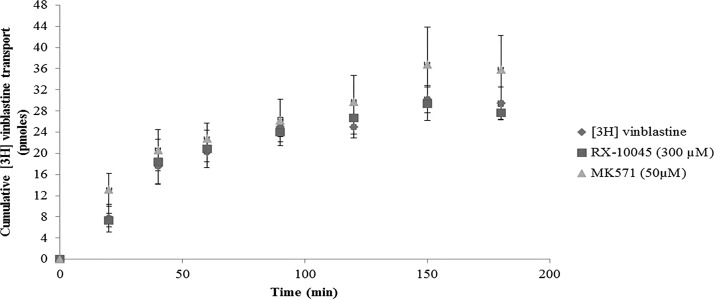
Uptake of [3H]-digoxin was conducted in MDCKII-MDR1 cells in the presence of GF120918 and ketoconazole, P-gp inhibitors. Intracellular accumulation of [3H]-digoxin in MDCKII-MDR1 cells in the presence of GF120918 and ketoconazole was higher by 1.25 and 1.47 times, respectively. We conducted initial screening uptake experiments for RX-10045 from 50 to 600 μM in MDCK cells transfected with MDR1, MRP, and BCRP genes. Since RX-10045 at lower concentrations did not inhibit any substrates of efflux transporter, the concentration was increased up to 600 μM, to determine if any interaction occurs. In the presence of RX-10045 (300 μM), [3H]-digoxin accumulation was ∼2 times higher than the control ([3H]-digoxin) (Fig. 2). Following a similar protocol, uptake of [3H]-vinblastine was performed on MDCKII-MRP2 cells. Cellular accumulation of [3H]-vinblastine in the presence of MK571 was increased by ∼1.28 times. RX-10045 (400 μM) also elevated the uptake of [3H]-vinblastine to 1.31 times relative to control (Fig. 3). In another set of experiments, similar results were observed in MDCKII-BCRP cells. Cellular accumulation of [3H]-abacavir increased by 1.77-fold in the presence of estradiol-β (25 μM) and 1.62-fold in the presence of RX-10045 (300 μM) (Fig. 4). These results indicate that intracellular accumulation of efflux transporter substrates increased significantly in the presence of RX-10045 relative to control.
FIG. 2.
Uptake of [3H]-digoxin in the presence of inhibitors (GF120918 and ketoconazole) and RX-10045 in MDCKII-MDR1 cells. [3H]-digoxin served as control. Data are shown as mean±SEM (n=4). *P<0.05 versus control (paired t-test). MDCK, Madin-Darby canine kidney; MDR, multidrug resistance; SEM, standard error of mean.
FIG. 3.
Uptake of [3H]-vinblastine in the presence of inhibitor (MK571) and RX-10045 in MDCKII-MRP2 cells, [3H]-vinblastine serves as control. Data are shown as mean±SEM (n=4). *P<0.05 versus control (paired t-test). MRP, multidrug resistance-associated protein.
FIG. 4.
Uptake of [3H]-abacavir in the presence of inhibitor (estrogen) and RX-10045 in MDCKII-BCRP cells. [3H]-abacavir served as control. Data are shown as mean±SEM (n=4). *P<0.05 versus control (paired t-test). BCRP, breast cancer-resistant protein.
Similarly, uptake studies were conducted in HCECs in the presence of MDCKII-MDR1, MDCKII-BCRP, and MDCKII-MRP inhibitors. Results demonstrated that there was no significant difference in digoxin uptake in the presence of RX-10045. Uptake of [3H]-vinblastine was significantly enhanced by 3.43 times in the presence of RX-10045 relative to [3H]-vinblastine alone. However, uptake of [3H]-abacavir was inhibited in the presence of RX-10045. Cellular accumulation of [3H]-abacavir was 3.4 times lower than the control.
Dose-dependent inhibition studies
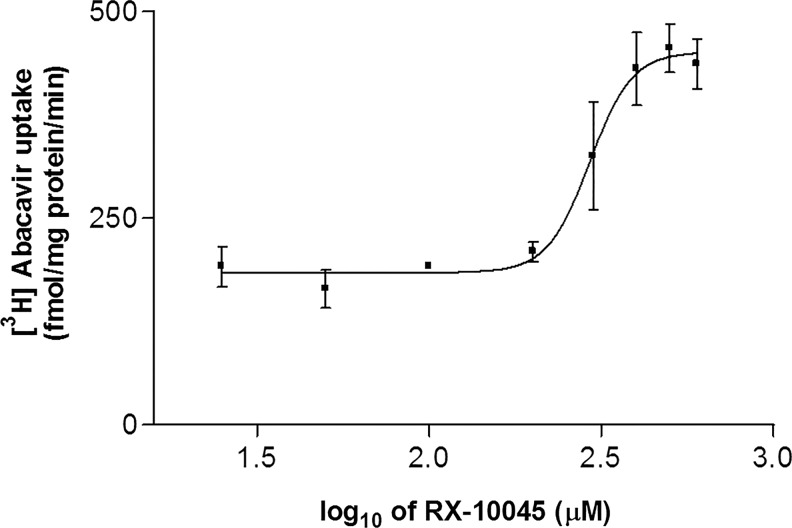
Half-maximal inhibitory concentrations of RX-10045 were calculated by an indirect method using different efflux transporters substrates such as [3H]-digoxin for MDCKII-MDR1, [3H]-vinblastine for MDCKII-MRP2, and [3H]-abacavir for MDCKII-BCRP. IC50 values for RX-10045 were calculated to be 239±11.2 μM in MDCKII-MDR1, 291±79.2 μM in MDCKII-MRP2, and 300±42 μM in MDCKII-BCRP from dose–response curves, respectively. Figure 5 shows half-maximal inhibitory concentration in MDCKII-BCRP cells. For further studies such as permeability and cytotoxicity studies, half-minimal inhibitory concentrations were selected and used.
FIG. 5.
Half-maximal inhibitory concentration (IC50) of RX-10045 in MDCKII-BCRP cells.
Permeability studies
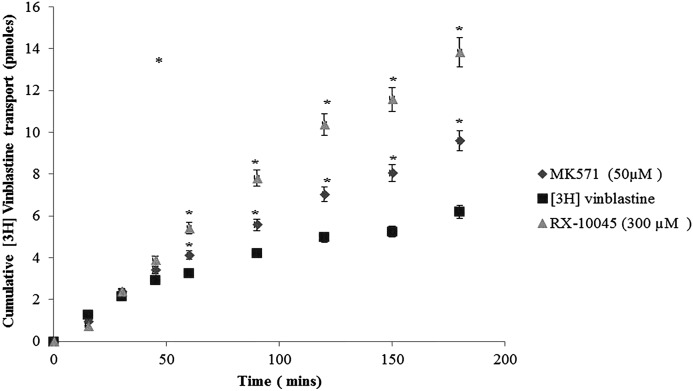
Transport experiments were conducted across 3 (MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP) cell lines in both AP-BL and BL-AP directions. These cells express efflux transporters on the apical side only. Apparent permeability of [3H]-digoxin in the AP-BL direction was not statistically significant in the presence of RX-10045 relative to control [3H]-digoxin 5.77±0.379×10−6 cm/min. Similarly, transport of [3H]-vinblastine across MDCKII-MRP2 cells was assessed in both AP-BL and BL-AP directions. Apparent permeability from the AP-BL direction for [3H]-vinblastine rises in the presence of MK571 (1.66 times) and in the presence of RX-10045 (2.53 times), whereas the apparent permeability of control [3H]-vinblastine from AP-BL was calculated to be 0.093±0.79×10−6 cm/s (Fig. 6) and from BL-AP was 0.440±0.0827 (Fig. 7). In another set of experiments, transport of [3H]-abacavir across MDCKII-BCRP cells was assessed in both AP-BL and BL-AP directions. Apparent AP-BL permeability of [3H]-abacavir in the presence of estradiol (inhibitor) and RX-10045 was enhanced by 2.36 and 2.42 times, respectively. Apparent permeability of [3H]-abacavir control was calculated to be 3.29±1.26×10−6 cm/s. Cumulative transport of [3H]-digoxin, [3H]-vinblastine, and [3H]-abacavir in the BL-AP direction in the presence of inhibitor or RX-10045 did not show a significant difference.
FIG. 6.
Apical to basal apparent permeability of [3H]-vinblastine in the presence of inhibitor (MK571) and RX-10045 [3H]-vinblastine served as control. Data are shown as mean±SD (n=4). *P<0.05 versus control (paired t-test). SD, standard deviation.
FIG. 7.
Basal to apical apparent permeability of [3H]-vinblastine in the presence of inhibitor (MK571) and RX-10045 with [3H]-vinblastine served as control. Data are shown as mean±SD (n=4).
Cytotoxicity studies
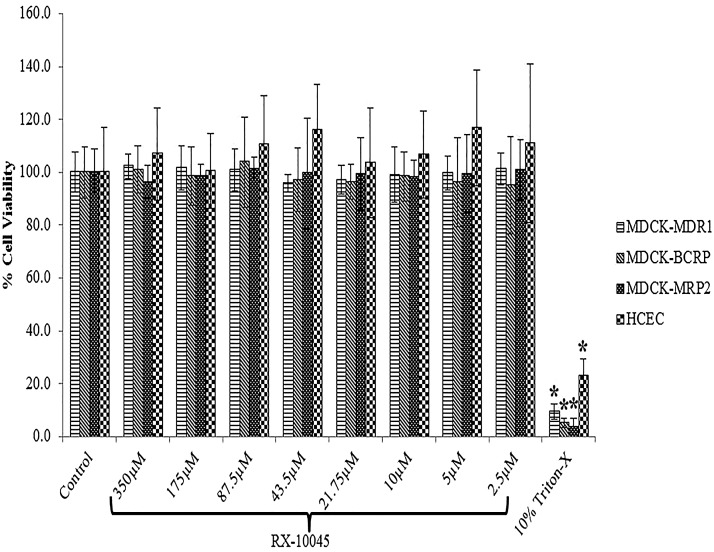
Cytotoxicity of RX-10045 was evaluated in 4 cell lines (MDCKII-MDR1, MDCKII-MRP2, MDCKII-BCRP and HCEC) over 3 h with different concentrations of RX-10045 ranging from 1 to 350 μM. We conducted initial screening uptake experiments from 50 to 600 μM in MDCK cells transfected with MDR1, MRP, and BCRP genes. Since RX-10045 at lower concentrations did not inhibit any substrate of efflux transporter, we increased the concentration up to 600 μM to determine if any interaction occurs. However, uptake studies of RX-10045 were conducted at less than 350 μM concentration. Moreover, the IC50 value of RX-10045 for all the cell lines was found to be less than 350 μM. Therefore, we conducted cytotoxicity studies up to 350 μM. Cells were examined for their viability following the manufacturer's protocol. Cells were added with a dye solution containing MTS. Metabolically active cells in the presence of dehydrogenase enzymes reduce MTS to a soluble formazan product. The amount of formazan formed is directly proportional to the number of live cells in culture. Percent cell viability was observed to be the same relative to negative control (culture medium without drug). The concentration range in the current study for RX-10045 appeared to have no effect on cell viability. In this study, 10% Triton X-100 served as a positive control, which significantly reduced cell viability in the range of 10%–25% for different cell lines (Fig. 8).
FIG. 8.
Cell viability studies for MDCK-MDR1, MDCK-MRP2, MDCK-BCRP and human corneal epithelial cells (HCECs) with increasing concentrations of RX-10045. The cell culture medium serves as negative control and Triton X-100 represents positive control. Data are shown as mean±SEM (n=8). *P<0.05 versus control (paired t-test).
Discussion
RX-10045 is a resolvin E1 analog evaluated for the treatment of dry eye, ocular inflammation, and ocular fibrosis. The interactions of resolvins with efflux transporters have not been previously investigated. This study therefore examined the interactions between RX-10045 and the efflux transporters P-gp, MRP2, and BCRP, whose expression and functional activity on the corneal epithelium were previously reported.10–12,14,15 To evaluate drug interactions, 3 MDCKII cell lines that are transfected with MDR1, MRP2, and BCRP human genes were selected for the current study. The rationale behind using MDCKII-transfected cell lines, which overexpress efflux transporters Pgp, MRP, and BCRP, was to delineate the individual efflux transporter-mediated interactions. These cell lines help in identification of the individual role of efflux transporters in reducing intracellular drug concentrations and permeability. Moreover, the results may help to modulate ocular drug delivery strategies for delivery of therapeutic amounts of RX-10045 to ocular tissues.
Intracellular accumulation of [3H]-digoxin was significantly elevated in the presence of P-gp inhibitors (GF 120918 and ketoconazole) and RX-10045 relative to [3H]-digoxin only.11,20,21 This result indicates that RX-10045 may interact with P-gp similar to other P-gp inhibitors. This interaction results in higher cellular accumulation of [3H]-digoxin. This observation suggests that RX-10045 may be a substrate for P-gp. Another set of experiments conducted on MDCKII–MRP2 cells with [3H]-vinblastine revealed higher accumulation in cells treated with inhibitor (MK571) and RX-10045. This suggests that RX-10045 may also interact with MRP2 as a competitive inhibitor, resulting in higher [3H]-vinblastine uptake. Similarly, we conducted studies with MDCKII–BCRP cells with [3H]-abacavir. Higher accumulation of [3H]-abacavir in the presence of beta-estradiol and RX-10045 was observed relative to control suggesting that this resolvin analog may be a substrate or inhibitor of BCRP. A significant rise in [3H]-digoxin, [3H]–vinblastine, and [3H]-abacavir uptake in the presence of GF120918, ketoconazole, MK571, and beta-estradiol indicates that P-gp, MRP2, and BCRP are highly expressed on MDCKII-transfected cells and are functionally active. These results are consistent with our earlier results.8,15,22 Uptake experiments indicate that RX-10045 may be a substrate or inhibitor for all 3 efflux transporters (P-gp, MRP2, and BCRP). Dose-dependent inhibition (IC50) in uptake studies suggests affinity of RX-10045 toward P-gp, BCRP, and MRP2. RX-10045 inhibited MDCKII-MRP2- and MDCKII-BCRP-mediated efflux in a dose-dependent manner.
Efflux transporters such as P-gp, MRP2, and BCRP are involved in the extrusion of a variety of therapeutic agents. Since the efflux transporters (P-gp, BCRP, and MRP2) are localized on the apical surface, [3H]-digoxin, [3H]-vinblastine, and [3H]-abacavir alone exhibited significantly lower transport in the AP-BL direction relative to the BL-AP direction (Table 1). The efflux ratio (ratio of BL-AP vs. AP-BL) is considered to be an indicator to identify efflux transporter (P-gp, MRP2 and BCRP) substrates.23 When the ratio of apparent permeability (BL-AP/AP-BL) approaches 1.0, then transport is equal in both directions. Efflux ratios of [3H]-digoxin, [3H]-vinblastine, and [3H]-abacavir were found to be 2.07, 4.75, and 10.35 in MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells, respectively. There was no significant difference observed in the permeability of [3H]-digoxin in the presence of RX-10045 indicating noninteraction with P-gp compared to other transmembrane efflux proteins (MRP2 and BCRP). A significant reduction in the efflux ratio was evident in the presence of MK-571 and beta-estradiol, confirming inhibition of MRP2 and BCRP functional activities (Table 1). Furthermore, the presence of RX-10045 affected AP-BL permeability of [3H]-vinblastine and [3H]-abacavir. The efflux ratio is reduced in MDCKII-BCRP and MDCKII-MRP2 cells, but not much in MDCK-MDR1 cells (Table 1). This significant reduction in the efflux ratio further confirms substrate specificity of RX-10045 toward MRP2 and BCRP.
Table 1.
Apparent Permeability of [3H]-Digoxin, [3H]-Vinblastine, and [3H]-Abacavir in the Presence of Inhibitors and RX-10045
| Apical to basolateral (×10−6) | Basolateral to apical (×10−6) | Efflux ratio | |
|---|---|---|---|
| MDCKII-MDR1 | |||
| [3H]-Digoxin | 5.77±0.379 | 11.96±2.59 | 2.07 |
| GF (5 μM) | 6.07±0.390 | 10.95±1.57 | 1.80 |
| RX-10045 (350 μM) | 5.86±0.298 | 11.13±0.95 | 1.90 |
| MDCKII-MRP2 | |||
| [3H]-Vinblastine | 0.093±0.028 | 0.440±0.0827 | 4.75 |
| MK571 (50 μM) | 0.154±0.050 | 0.389±0.005 | 2.53a |
| RX-10045 (300 μM) | 0.235±0.079 | 0.430±0.190 | 1.83a |
| MDCKII-BCRP | |||
| [3H]-Abacavir | 3.29±1.26 | 34.09±2.73 | 10.35 |
| Estradiol-2 (25 μM) | 7.77±0.883 | 27.61±3.24 | 3.56a |
| RX-10045 (350 μM) | 7.95±1.38 | 31.96±0.62 | 4.02a |
Data are shown as mean±SD (n=4).
P<0.05 versus control (paired t-test).
BCRP, breast cancer-resistant protein; MDCK, Madin-Darby canine kidney; MDR, multidrug resistance; MRP, multidrug resistance-associated protein; SD, standard deviation.
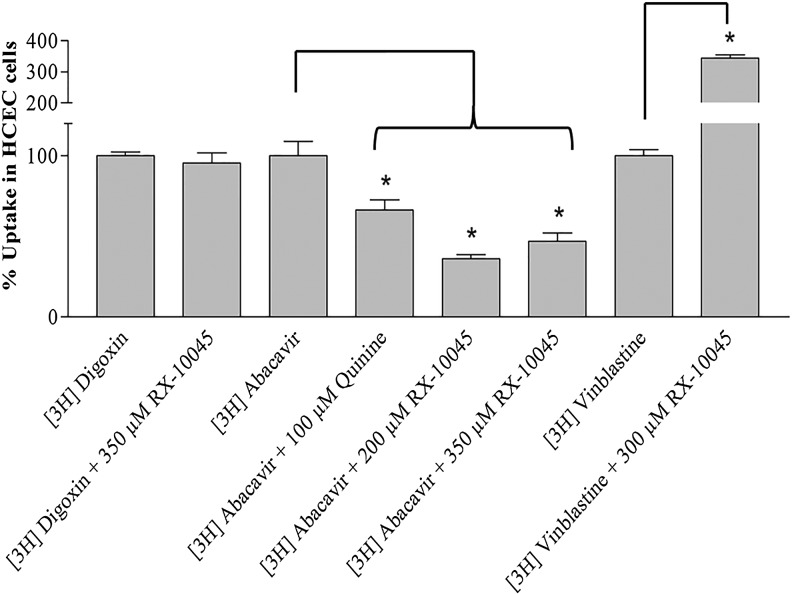
Uptake studies in HCECs demonstrated no effect of MDR1 (P-gp) on digoxin efflux in the presence of RX-10045. Results were comparable to MDCKII-MDR1 cells. However, the uptake of MRP2 substrate ([3H]-vinblastine) was significantly improved. We observed that the uptake of [3H]-vinblastine was ∼5 times higher in HCECs relative to MDCK-MRP2 cells possibly due to differences in isoform expression in HCEC/MDCK-MRP2 cells. Tang F. Horie and colleagues reported that different isoforms of MRP2 are overexpressed in MDCK-MRP2 (150 kDa) and Caco2 cells (190 kDa) with a different kinetic profile.24 Therefore, these isoforms also make the difference in substrate specificity, that is, percent [3H]-vinblastine uptake resulting in higher cellular accumulation. While the uptake of [3H]-abacavir along with RX-10045 was significantly reduced demonstrating reversal in drug accumulation relative to control [3H]-abacavir alone (Fig. 9). This surprising result prompted us to investigate the cause of inhibition of [3H]-abacavir uptake in the presence of RX-10045. Abacavir is a known substrate for an influx transporter, organic cation transporter-1 (OCT-1), and HCECs highly express OCT-1.25,26 Therefore, the increase in HCEC uptake of [3H]-abacavir may be due to uptake by the OCT-1. The presence of RX-10045 inhibited the [3H]-abacavir uptake because this resolvin can cause inhibition of OCT-1. To determine its inhibitory effect, inhibition of [3H]-abacavir uptake experiments were performed in the presence of 100 μM quinine (a known OCT-1 inhibitor). Uptake of [3H]-abacavir was significantly reduced in the presence of quinine and RX-10045 suggesting that RX-10045 is a strong inhibitor of OCT-1 (Fig. 9). Further studies are needed to establish RX-10045 as a inhibitor/substrate for OCT-1.
FIG. 9.
Uptake studies in HCECs for [3H]-digoxin, [3H]-abacavir, and [3H]-vinblastine in the presence of RX-10045 and quinine (OCT-1 inhibitor) at IC50 concentrations. Data are shown as mean±SEM (n=4). *P<0.05 versus control (paired t-test). OCT-1, organic cation transporter-1.
Conclusions
The delivery of drug into ocular cells may be inhibited due to the presence of membrane-bound functional efflux transporters. For the first time, we have demonstrated a resolvin E1 analog (RX-10045) to be a substrate/inhibitor for efflux transporters (MRP2 and BCRP). It is observed that the permeability of [3H]-digoxin was not affected in the presence of RX-10045 indicating that there may be negligible interaction with P-gp. It remains to be determined whether this is a class property of the resolvins; however, this report suggests that efflux transporters may impede the translocation of RX-10045 across target cell membranes. A significantly reduced uptake of [3H]-abacavir in the presence of RX-10045, compared to quinine (a known inhibitor of OCT-1), clearly suggests that RX-10045 is a strong inhibitor/substrate of OCT-1. Knowledge of resolvin interaction with efflux transporters can allow pharmaceutical scientists to develop new strategies and formulations27,28 to circumvent efflux barrier and deliver therapeutic concentrations of resolvin into target ocular tissues.
Acknowledgments
This study has been supported by NIH grants R01EY09171-16 and R01EY010659-14. We would like to thank Auven Therapeutics for supplying resolvin E1 analog (RX-10045).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Serhan C.N., et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196:1025–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arita M., Clish C.B., and Serhan C.N.The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 338:149–157, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Serhan C.N.Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25:101–137, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Li N., et al. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 26:431–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian H., et al. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest. Ophthalmol. Vis. Sci. 50:3613–3620, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Settimio R., et al. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 318621:2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resolvyx Announces Positive Data from Phase 2 Clinical Trial of the Resolvin RX-10045 in Patients with Dry Eye Syndrome. 2013. Available at http://www.resolvyx.com/news-pubs/releases/082409.asp
- 8.Kwatra D., et al. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. Int. J. Pharm. 395:114–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal D., et al. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 88:959–971, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vellonen K.S., et al. Effluxing ABC transporters in human corneal epithelium. J. Pharm. Sci. 99:1087–1098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S., et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 44:2909–2918, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dey S., Gunda S., and Mitra A.K.Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J. Pharmacol. Exp. Ther. 311:246–255, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Katragadda S., et al. Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin. Drug Deliv. 2:683–705, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Karla P.K., et al. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 336:12–21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karla P.K., et al. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr. Eye Res. 34:1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hariharan S., et al. Interaction of ocular hypotensive agents (PGF2 alpha analogs-bimatoprost, latanoprost, and travoprost) with MDR efflux pumps on the rabbit cornea. J. Ocul. Pharmacol. Ther. 25:487–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khurana V., et al. Functional characterization and molecular identification of vitamin C transporter (SVCT2) in human corneal epithelial (HCEC) and retinal pigment epithelial (D407) cells. Curr. Eye Res. 11:1–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hariharan S., et al. Enhanced corneal absorption of erythromycin by modulating P-glycoprotein and MRP mediated efflux with corticosteroids. Pharm. Res. 26:1270–1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y., and Prusoff W.H.Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099–3108, 1973 [DOI] [PubMed] [Google Scholar]
- 20.Sikri V., et al. Cotransport of macrolide and fluoroquinolones, a beneficial interaction reversing P-glycoprotein efflux. Am. J. Ther. 11:433–442, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., and Morris M.E.Effect of the flavonoids biochanin A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm. Res. 20:1184–1191, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S., Pal D., and Mitra A.K.Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int. J. Pharm. 339:139–147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polli J.W., et al. Rational use of in vitro P-glycoprotein assays in drug discovery. J. Pharmacol. Exp. Ther. 299:620–628, 2001 [PubMed] [Google Scholar]
- 24.Tang F., Horie K., and Borchardt R.T.Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharm. Res. 19:773–779, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Minuesa G., et al. Transport of lamivudine [(-)-beta-L-2′,3′-dideoxy-3′-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J. Pharmacol. Exp. Ther. 329:252–261, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Xiang C.D., et al. Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug. Metab. Dispos. 37:992–998, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Cholkar K., et al. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholkar K., et al. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech. 15:1454–1467, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]