Abstract
Tearing of the rotator cuff tendon in the shoulder is a significant clinical problem, with large/full-thickness tears present in ∼22% of the general population and recurrent tear rates postarthroscopic repair being quoted as high as 94%. Tissue-engineered biomaterials are increasingly being investigated as a means to augment rotator cuff repairs, with the aim of inducing host cell responses to increase tendon tissue regeneration. Silk-derived materials are of particular interest due to the high availability, mechanical strength, and biocompatibility of silks. In this study, Spidrex®, a novel knitted, non-mulberry silk fibroin scaffold was evaluated in vitro for its potential to improve tendon regeneration. Spidrex was compared with a knitted Bombyx mori silk scaffold, a 3D collagen gel and Fiberwire® suture material. Primary human and rat tenocytes successfully adhered to Spidrex and significantly increased in number over a 14 day period (p<0.05), as demonstrated by fluorescent calcein-AM staining and alamarBlue® assays. A similar growth pattern was observed with human tenocytes cultured on the B. mori scaffold. Morphologically, human tenocytes elongated along the silk fibers of Spidrex, assuming a tenocytic cell shape, and were less circular with a higher aspect ratio compared with human tenocytes cultured on the B. mori silk scaffold and within the collagen gel (p<0.05). Gene expression analysis by real-time PCR showed that rat tenocytes cultured on Spidrex had increased expression of tenocyte-related genes such as fibromodullin, scleraxis, and tenomodulin (p<0.05). Expression of genes that indicate transdifferentiation toward a chondrocytic or osteoblastic lineage were significantly lower in tenocytes cultured on Spidrex in comparison to the collagen gel (p<0.05). Immunogenicity assessment by the maturation of and cytokine release from primary human dendritic cells demonstrated that Spidrex enhanced dendritic cell maturation in a similar manner to the clinically used suture material Fiberwire, and significantly upregulated the release of proinflammatory cytokines (p<0.05). This suggests that Spidrex may induce an early immune response postimplantation. While further work is required to determine what effect this immune response has on the tendon healing process, our in vitro data suggests that Spidrex may have the cytocompatibility and bioactivity required to support tendon regeneration in vivo.
Introduction
Tendons are skeletal tissue structures that connect muscle to bone, transmitting tensile forces from muscles to generate joint movement.1 Tendon injuries are an increasingly common clinical problem occurring in otherwise healthy, active people and are primarily caused by overuse or trauma.2,3 Tears of the rotator cuff tendon in the shoulder are a particular problem, with full-thickness tears present in ∼22% of the general population4–6 and over 75,000 patients per annum requiring surgical intervention in the United States alone.7 In addition to the high general incidence, recurrent tear rates postsurgery are quoted as being as high as 94%.8–11 These injuries significantly affect the patient quality of life and are a substantial financial burden to the healthcare economy.7,12
Current rotator cuff repairs aim to reattach the tendon to the humeral head, debride a partial tear, or suture a full-thickness tear using open or arthroscopic surgeries.13 However, given the high recurrent tear rates, it has been suggested that augmentation with a tissue-engineered biomaterial may improve current techniques.2,10,14–16 The tissue-engineered biomaterial aims to promote full tendon regeneration and recovery by providing temporary mechanical support to the injured tendon, and enhancing tendon cell growth and activity.12,17–20
There are a number of Food and Drug Administration (FDA)-approved biomaterial scaffolds available for use in rotator cuff repair,21 and while some of these scaffolds appear to improve clinical outcomes, the results are generally inconclusive due to the lack of appropriate controls.22–24 This leads to the consensus view that currently available scaffolds, while promising, fail to meet clinical needs.15–17,21 Therefore, further development of new scaffold materials, and their evaluation in vitro, in vivo, and subsequently in clinic, are essential for advancing the use of biomaterials in tendon repair.
Silk is an FDA-approved natural material currently used in the biomedical industry as a suture in lip, eye, and skin repair.25 It is progressively being evaluated for further biomedical applications, largely due to its favorable biocompatibility and mechanical properties.25–28 Silk is produced by a number of different species of silkworms divided into two categories: mulberry, produced by the domesticated Bombyx mori silkworm; and non-mulberry, produced by a number of wild species of silkworm. Although the chemical composition of silk is thought to vary to match the functional requirement of each species, it largely consists of two main structural proteins, fibroin and sericin.29–31
There is much ongoing research into the effects of various combinations of silk source, processing techniques, structure, and topography, with each combination significantly altering the silk properties and its biocompatibility.26 However, it is generally believed that the glue-like sericin component is immunogenic, and once the silk has been degummed to remove the sericin, the remaining silk fibroin is highly biocompatible.32–34
Studies evaluating silk as a biomaterial for tendon regeneration have largely focused on the mulberry B. mori silk or composites of B. mori silk with either synthetic polymers or collagen.28,29,35–38 However, given that non-mulberry silks contain the cell binding RGD tripeptide motif,39–41 and have been shown to support fibroblast-like and bone marrow-derived mesenchymal stem cell growth in vitro,42,43 non-mulberry silk-derived scaffolds hold much promise as biomaterials for enhancing tendon tissue regeneration.44
In this study, in vitro assays were used to assess the cytocompatibility and immunogenicity of a novel knitted, non-mulberry silk fibroin scaffold designed for use in tendon tissue regeneration.
Materials and Methods
Reagents and ethical approval
The culture media, fetal bovine serum (FBS) and antibiotics used in tissue culture were from Invitrogen (Life Technologies Australia Pty Ltd.). Primary human tenocytes were isolated from patient biopsies collected with approval by the Northern Regional Ethics Committee and all patients provided written informed consent. Primary rat tenocytes were isolated in accordance with the Animal Ethics Committee of The University of Auckland, New Zealand. Peripheral blood mononuclear cells (PBMCs) were purified from healthy human volunteer blood, collected according to a protocol approved by the University of Auckland Human Participants Ethics Committee, New Zealand.
Tenocyte cell culture
Primary rat tenocytes were isolated from tendon fascicles teased from tails of mature female Wistar rats. Cultures of primary human tenocytes were prepared from bicep tendons obtained from patients undergoing orthopedic surgery. Tendon was roughly cut into <1 cm pieces and digested in 0.5 mg/mL dispase and 0.5 mg/mL collagenase (both from Sigma-Aldrich) in Dulbecco's modified Eagle's medium-F12 (DMEM-F12) with 10% FBS at 37°C for up to 18 h until all extracellular matrix had been digested. The cell suspension was then passed through a cell strainer, washed, and resuspended in enzyme-free media. Cells were cultured in DMEM-F12 with 10% FBS in 75-cm2 flasks (Corning, Inc.) and incubated at 37°C with 5% CO2 until confluent.
Scaffold preparation
Spidrex® and B. mori silk scaffolds were received from Oxford Biomaterials Ltd. The luminal Spidrex fibers were prepared from commercially sourced non-mulberry silk. The silk was treated using a proprietary process involving an enzymatic degumming step to remove the sericin. The remaining fibroin fibers were knitted into a tubular structure using a small circular knitting machine of 12 or 32 needles (gauge 20). The knitted tubes were subsequently cut into ∼1 cm2 sections, sterilized by autoclaving, and soaked in DMEM-F12 for 2 h before experimentation.
Fiberwire® (Arthrex), a polyester jacket suture material with a polyethylene core, currently used in rotator cuff repair surgeries, was received presterilized and similarly soaked in DMEM-F12 before experimentation.
Collagen gels were prepared by neutralizing high concentration rat collagen type I (BD Biosciences) with 1M sodium hydroxide (0.023×collagen volume) and diluting to a final collagen concentration of 2 mg/mL with or without 4×105cells/mL suspended in DMEM-F12 with 5% FBS. Fifty microliters gels were set at 37°C with 5% CO2 for 1 h before addition of fresh DMEM-F12 with 5% FBS.
Scanning electron microscopy
Samples were fixed with 10% neutral buffered formalin at 4°C for 6 days and subsequently washed with phosphate buffered saline (PBS). Samples were passed through a series of graded alcohol concentrations to dehydrate the scaffolds before undergoing critical point drying. The dried samples were coated with 20 nm platinum at 20 mA using a Quorum Q150RS sputter coater (Quorum Technologies Ltd.), mounted on stubs and imaged at 15 kV with a Philips XL30S FEG (field emission gun) SEM (scanning electron microscopy) (Philips).
Qualitative analysis of scaffold cytocompatibility
Primary human or primary rat tenocytes were seeded onto presoaked 1 cm2 sections of scaffold in a 24-well plate for a period of 1, 7, and 14 days in DMEM-F12 with 5% FBS at 2.5×104 cells/scaffold. Media were changed after 4 days. At the conclusion of the culture period, cell-seeded scaffolds were washed in PBS. Cells were then stained for 10 min at 37°C with 2 μM calcein-AM (Invitrogen) in PBS, washed in fresh PBS, and viewed immediately using fluorescent microscopy. Calcein-AM is converted to a green fluorescent product within live cells.
Quantitative analysis of scaffold cytocompatibility
Primary human or primary rat tenocytes were seeded onto presoaked 1 cm2 sections of scaffold in a 24-well plate, as described for the qualitative cytocompatibility assay for a period of 1, 7, and 14 days. At each time point 5% alamarBlue® (Invitrogen) (final concentration in well) was added for 4 h at 37°C. At the end of this incubation, 200 μL of the alamarBlue-conditioned medium was transferred from each well to a 96-well plate (Greiner Bio-One) and fluorescence (excitation 540 nm; emission 630) read using a Synergy 2 multidetection microplate reader (BioTek Instruments, Inc.).
Assessment of tenocyte morphology
Primary human tenocytes were seeded onto presoaked 1 cm2 sections of scaffold in a 24-well plate at 2.5×104 cells/scaffold, and stained with calcein-AM, as described for the qualitative cytocompatibility assay. Images (1.31×0.97 mm) were captured using fluorescent microscopy and analyzed using the ImageJ software (http://rsb.info.nih.gov/ij/). At least two images were analyzed per scaffold, per time point.
Images were converted to grayscale, and a threshold was set to remove background noise and isolate individual cells. Images were then converted to binary and analyzed for circularity (whereby circularity=4×π×area/perimeter2, with a shape index of 1 representing a circle) and aspect ratio (diameter of the longest axis/diameter of the shortest axis) using built-in functions of the ImageJ software. Observations were made by two independent scorers (D.M. and A.C.), who were blinded to each other's results. The coefficient of variation between scorers measurements of circularity and aspect ratio were 5.8% and 9.8%, respectively.
Real-time PCR analysis
RNA for real-time PCR was prepared from primary rat tenocytes cultured on presoaked 1 cm2 sections of scaffold in a 24-well plate for 1, 7, and 14 days. Cells were lysed from materials using β-mercaptoethanol in RLT buffer (Qiagen Pty Ltd.) and incubated at 55°C with 0.2 mg/mL proteinase K (Invitrogen) for 15 min. 80% v/v ethanol was added and RNA extracted using the RNeasy mini kit (Qiagen Pty Ltd.). Genomic DNA was removed from all RNA preparations with the RNase-free DNase set (Qiagen Pty Ltd.). The quantity and purity of the RNA were measured using a NanoDrop Lite spectrophotometer (Thermo Scientific), with a 260/280 absorbance value of >1.8 being considered acceptable. Five hundred nanograms RNA was used to make cDNA. cDNA was synthesized with Superscript III (Life Technologies) and used for multiplex real-time PCR in the ABI PRISM® 7900HT Sequence Detection System (Life Technologies). Primers and probe sets were purchased as TaqMan® Gene Expression Assays (Cat. # 4331182; Life Technologies). All probes used to detect target genes were labeled with FAM™ and the 18S rRNA endogenous control probe was labeled with VIC®. The ΔΔCt method was used to calculate the relative levels of expression compared with a control sample from day 1.45
Genes assayed were biglycan (Rn01529734_g1) and fibromodulin (Rn00589918_m1), extracellular matrix proteins important in maintaining a tendon stem cell niche46; collagen type I (Rn00673737_m1), the main structural collagen present in tendon1; tenascin C (Rn01454947_m1), a glycoprotein important during the development and repair of tendons47; scleraxis (Rn01504576_m1), a key transcription factor in tenocyte differentiation48,49; and tenomodulin (Rn00574164_m1), a key glycoprotein in the proliferation and development of tenocytes.50 Chondrocyte and osteoblast genes were also assessed as tenocytes tend to transdifferentiate into such cell types, one of the pathological causes of tendinopathy.51 The assessed genes were collagen type II (Rn01637087_m1), the major structural collagen of cartilage52; aggrecan (Rn00573424_m1), the major glycoprotein in cartilage and a marker of chondrogenesis53; Sp7 (osterix) (Rn02769744_s1), a transcription factor and marker of osteoblast differentiation54; and bone sialoprotein (Rn00561414_m1), a marker of late osteoblast differentiation.55
Monocyte-derived dendritic cell activation assays
PBMCs were purified by gradient density purification from healthy human volunteer blood. Monocytes were isolated from PBMCs by negative selection using the Monocyte Isolation Kit II (Miltenyi Biotec), according to the manufacturer's protocol. Immature monocyte-derived dendritic cells were prepared by culturing the monocytes for 6 days in RPMI medium supplemented with 10% FBS, 100 ng/mL granulocyte-macrophage colony-stimulating factor and 50 ng/mL interleukin (IL)-4 (Peprotech).
To assess the immunogenicity of the biomaterials, immature dendritic cells were harvested on day 6 and seeded in 24-well plates containing RPMI medium with 10% FBS only (untreated control), 5 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich) as a positive control56 or a well containing the biomaterial indicated. The cells were cultured for 40 h and assessed for the expression of cell surface markers associated with dendritic cell maturation. Supernatants were collected for the assessment of cytokine release.
Harvested dendritic cells were incubated on ice for 20 min with mouse anti-human antibodies against PE/Cy7-anti-CD80 (clone 2D10) (Cat. # 305217), PerCP/Cy5.5 anti-CD83 (clone HB15e) (Cat. # 305320) and APC anti-CD86 (clone IT2.2) (Cat. # 305406) (All supplied by Biolegend). Excess antibody was removed by washing with PBS containing 1% FBS. Cell surface markers were analyzed by flow cytometry using the FACS ARIA II and data analysis was performed using FlowJo (version 7.6) (BD Biosciences).
Cytokine concentrations were measured from collected supernatants using multiplexed cytometric bead arrays (BD Biosciences). The cytokines IL-10, IL-12 p70, IL-1β, IL-6, and tumor necrosis factor (TNF)-α were measured simultaneously. The cytometric bead arrays were performed according to the manufacturer's protocol with one modification; 25 μL supernatant was assayed rather than 50 μL. Cytometric bead array samples were analyzed using a FACS ARIA II and the data analyzed using the FCAP Array software (BD Biosciences).
Statistical analysis
Data were analyzed using either one-way analysis of variance (ANOVA) with post hoc Bonferroni's test or two-way ANOVA with post hoc Bonferroni's test using the GraphPad Prism Software (GraphPad Software).
Results
Scaffold topography
The structure of Spidrex and the comparison materials were visualized by SEM (Fig. 1). SEM images of Spidrex showed the expected tightly knitted structure, with single strands of silk, ∼10–20 μm wide, observed in bundles (Fig. 1A, B). The B. mori scaffold showed a similar, but less densely knitted structure. Single strands of B. mori silk were thinner than Spidrex, measuring ∼5–10 μm in width (Fig. 1C, D). SEM images of the tightly woven polyester jacket that surrounds the internal polyethylene core of Fiberwire (Fig. 1E, F) and of the collagen gels which show a fibrillar arrangement at the higher magnification (Fig. 1H) are presented for comparison.
FIG. 1.
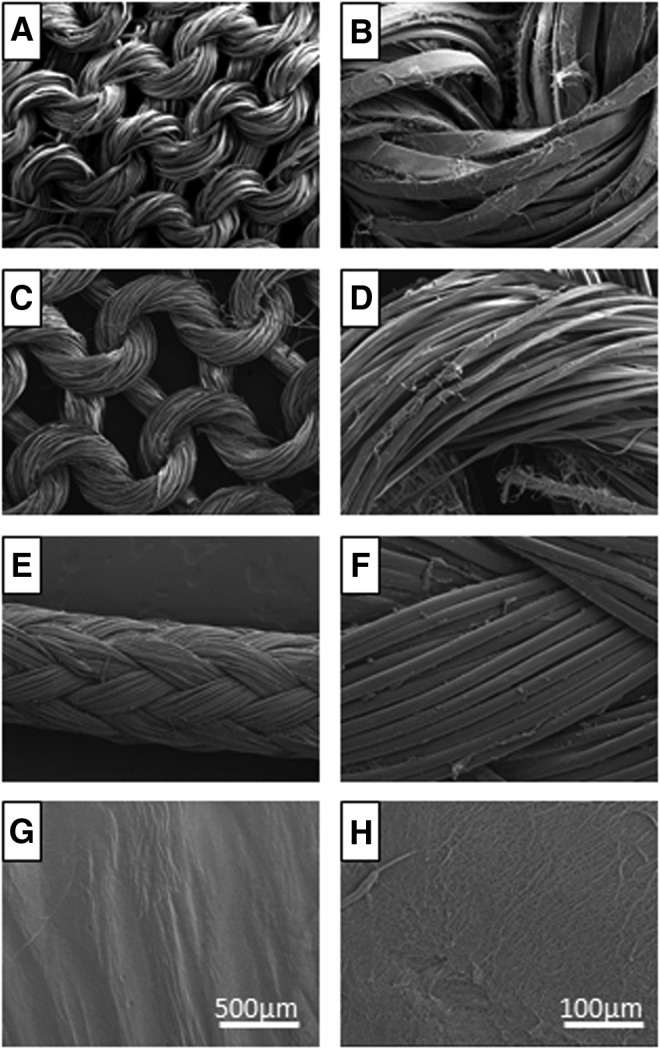
Scaffold topography. Scanning electron microscopy (SEM) images of knitted Spidrex® (A, B), knitted Bombyx mori scaffold (C, D), Fiberwire® suture material (E, F) and 3D collagen gel (G, H).
Analysis of tenocyte cytocompatibility
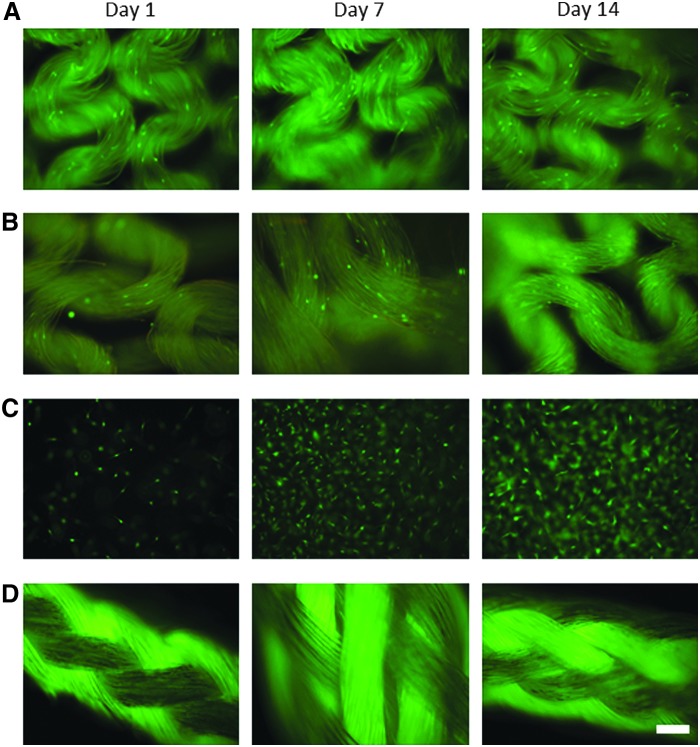
Cytocompatibility was initially assessed qualitatively by calcein-AM fluorescent staining of live primary human tenocytes. Cells were cultured for 1, 7, and 14 days on Spidrex, and compared with cells grown on a knitted B. mori scaffold, in 3D collagen gels and on Fiberwire (Fig. 2). These controls were chosen as B. mori is the most widely characterized silk material, particularly in the tendon field, the collagen gels closely simulate the natural collagenous extracellular environment of tenocytes, and Fiberwire is a suture material often used in human rotator cuff repairs and, therefore, represents the current clinical surgical option.
FIG. 2.
Qualitative analysis of human tenocyte viability on the different materials. Fluorescent images of calcein-AM-stained primary human tenocytes cultured for 1, 7, and 14 days on Spidrex (A), on a knitted B. mori silk scaffold (B), within a 3D collagen gel (C) and on Fiberwire suture material (D). Scale bar=200 μm. Observations were performed three times with four scaffolds assessed per treatment. Data presented are representative of these biological repeats.
After 24 h of culture on Spidrex, human tenocytes adhered to the silk and began to elongate along the individual silk fibers. The number of viable cells appeared to increase over the 14 day period (Fig. 2A). A similar pattern of growth was observed with human tenocytes cultured on the B. mori scaffold (Fig. 2B). Human tenocytes cultured within the collagen gel (Fig. 2C), appeared to rapidly increase in number between day 1 and 7, with a small number of cells visible after 24 h and the gel almost fully confluent after 7 and 14 days of culture. All attempts to culture tenocytes on Fiberwire proved unsuccessful, as cells did not adhere to this polyethylene/polyester suture material (Fig. 2D).
Quantitative assessment of cell viability using the alamarBlue assay demonstrated that human tenocytes cultured on the Spidrex and the B. mori scaffold continued to proliferate over the 14 days of culture (Fig. 3). There were significant increases in cell viability between day 1 and days 7 and 14 for both silk scaffolds (p<0.05 for both), and between day 7 and 14 for human tenocytes cultured on Spidrex (p<0.05). Human tenocytes cultured within 3D collagen gels grew at a greater rate during the 14 day culture period, with statistically significant increases between all time points (p<0.05 for all).
FIG. 3.
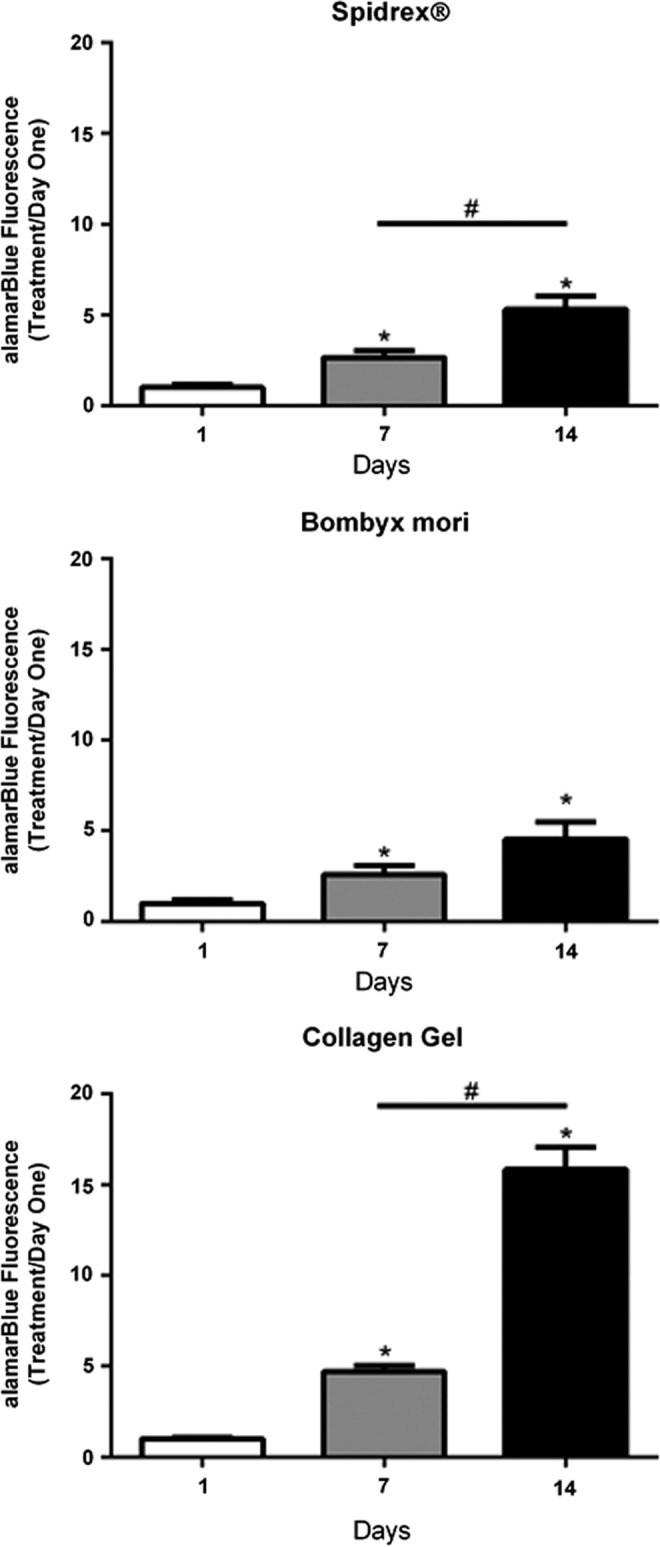
Quantitative assessment of human tenocyte viability on the different materials. alamarBlue® assay demonstrating viability of primary human tenocytes cultured on Spidrex, a knitted B. mori scaffold and within collagen gels over a 14 day period. One-way analysis of variance (ANOVA) (p<0.01) with post hoc Bonferroni's test. *p<0.05 compared with day 1. #p<0.05 between days 7 and 14. Cell viability was expressed as treatment to day 1 ratios. Each experiment was performed three times with four scaffolds assessed per treatment. Data presented are representative of these biological repeats.
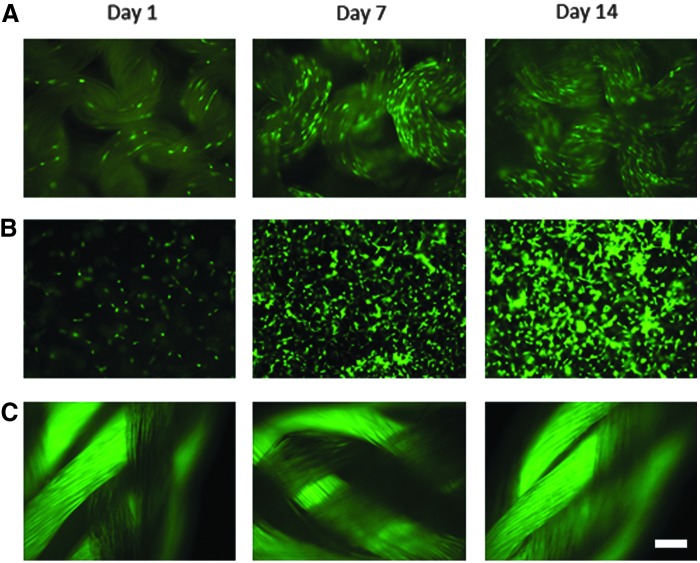
Similar to the human tenocytes, after 24 h of culture on Spidrex, rat tenocytes also successfully adhered to the silk (Fig. 4A). After 7 and 14 days, Spidrex appeared to be completely covered by the rat tenocytes. A similar pattern of growth was seen in rat tenocytes cultured within the collagen gel as with the human tenocytes (Fig. 4B), with a small number of cells visible after 24 h and the gel appearing fully confluent after 7 and 14 days of culture. As with the human tenocytes, all attempts to culture rat tenocytes on Fiberwire proved unsuccessful (Fig. 4C)
FIG. 4.
Qualitative analysis of rat tenocyte viability on the different materials. Fluorescent images of calcein-AM-stained primary rat tenocytes cultured for 1, 7, and 14 days on Spidrex (A), within a 3D collagen gel (B) and on Fiberwire suture material (C). Scale bar=200 μm. Observations were performed three times with four scaffolds assessed per treatment. Data presented are representative of these biological repeats.
Quantitative assessment of primary rat tenocyte viability demonstrated that tenocytes cultured on the Spidrex proliferated rapidly during the first 7 days of culture, after which their growth appeared to plateau (Fig. 5). There were significant increases in the number of viable cells between day 1 and days 7 and 14 (p<0.05 for both). Primary rat tenocytes cultured within 3D collagen gels grew at a steady rate during the 14 day culture period, with statistically significant increases between days 1, 7, and 14 (p<0.05 for all).
FIG. 5.
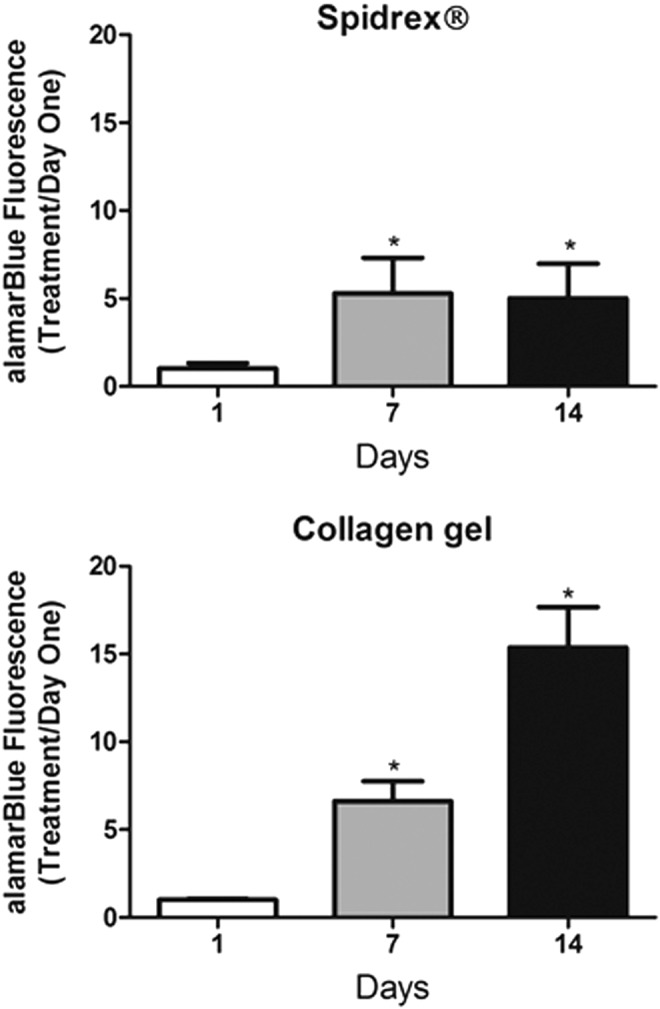
Quantitative assessment of rat tenocyte viability on the different materials. alamarBlue assay demonstrating viability of primary rat tenocytes cultured on Spidrex and within collagen gels over a 14 day period. One-way ANOVA (p<0.01) with post hoc Bonferroni's test. *p<0.05 compared with day 1. Cell viability was expressed as treatment to day 1 ratios. Each experiment was performed three times with four scaffolds assessed per treatment. Data presented are representative of these biological repeats.
Spidrex promotes a tenocytic morphology
Morphological analysis of human tenocyte cell shape when cultured on the different silk scaffolds and within collagen gels was carried out using the ImageJ software. This analysis demonstrated that when cultured on Spidrex, human tenocytes were significantly less circular than those cultured on the B. mori scaffold and those within the collagen gel on days 1, 7, and 14 (p<0.05 for all), as denoted by their cell shape index, whereby 1 equals a pure circle (Fig. 6). Similarly, human tenocytes cultured on Spidrex had a significantly higher aspect ratio (length of longest axis/length of shortest axis) compared with human tenocytes cultured on the B. mori scaffold or within collagen gels on day 7 and 14 of culture (p<0.05 for all) (Fig. 6) suggesting they maintained their tenocytic morphology better than those on the B. mori scaffold or within the collagen gels.
FIG. 6.
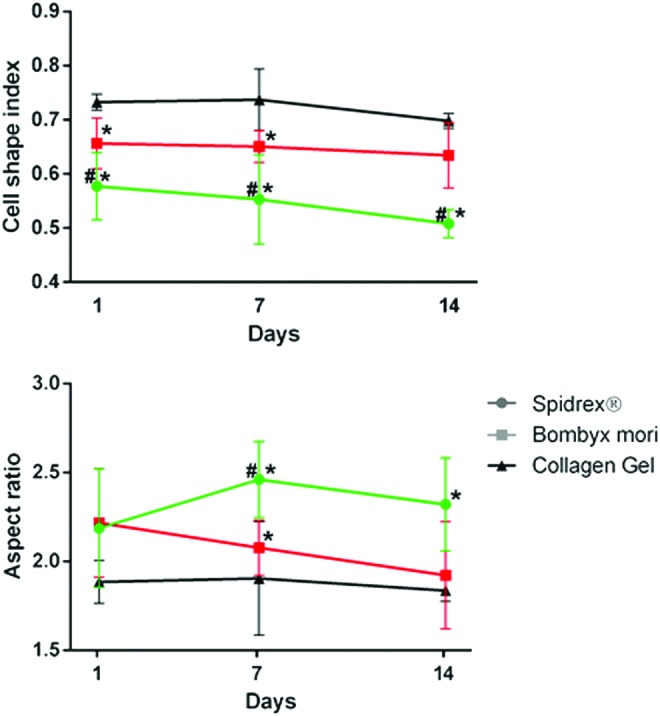
Assessment of human tenocyte cell shape on the different materials. Morphological analysis of primary human tenocytes cultured on Spidrex, a knitted B. mori scaffold and within collagen gels over a 14 day period. Two-way ANOVA (p<0.01) with post hoc Bonferroni's test. *p<0.05 compared with collagen gel. #p<0.05 compared with B. mori. Color images available online at www.liebertpub.com/tea
Human tenocytes cultured on the B. mori scaffold were significantly less circular than human tenocytes cultured within the collagen gels on day 1 and 7 of culture (p<0.05) and had a greater aspect ratio on day 7 of culture (p<0.05).
Spidrex inhibits transdifferentiation of tenocytes
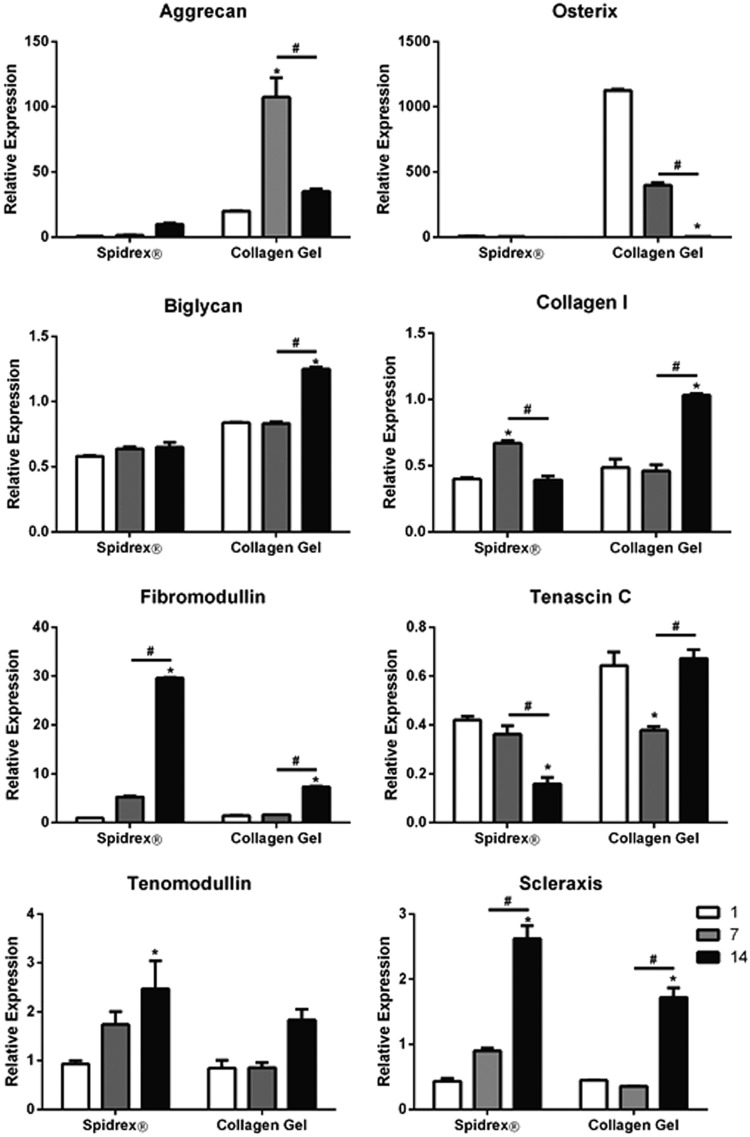
Having determined that Spidrex is highly cytocompatible and allows tenocyte attachment and growth, real-time PCR was used to determine the expression levels of genes important in tenocyte biology.
Aggrecan was expressed at very low levels and osterix was not expressed in rat tenocytes cultured on Spidrex, whereas rat tenocytes cultured within the collagen gels showed high levels of both genes (Fig. 7). All assessed tenocyte genes were expressed by cells in both culture systems. Expression of biglycan and collagen type I were significantly increased in the collagen gel at day 14 (p<0.05), but not in rat tenocytes cultured on Spidrex. Tenascin-C expression was significantly reduced in rat tenocytes cultured on Spidrex (p<0.05), but not in the collagen gels. Fibromodulin, tenomodulin, and scleraxis expression all significantly increased over the 14 days of culture on Spidrex to a higher level than rat tenocytes cultured in the collagen gels (p<0.05 for all). Collagen type II and bone sialoprotein were undetected in all samples.
FIG. 7.
Expression of tenocytic, chondrocytic, and osteoblastic genes in primary rat tenocytes. Cells were cultured on Spidrex or within collagen gels over a 14 day period and gene expression was determined by real-time PCR. Two-way ANOVA with post hoc Bonferroni. *p<0.05 compared with day 1. #p<0.05 between days 7 and 14. Each experiment was performed three times and the real-time PCR was repeated at least twice in triplicate. Data presented are representative of these biological repeats.
Spidrex enhances dendritic cell maturation and cytokine release
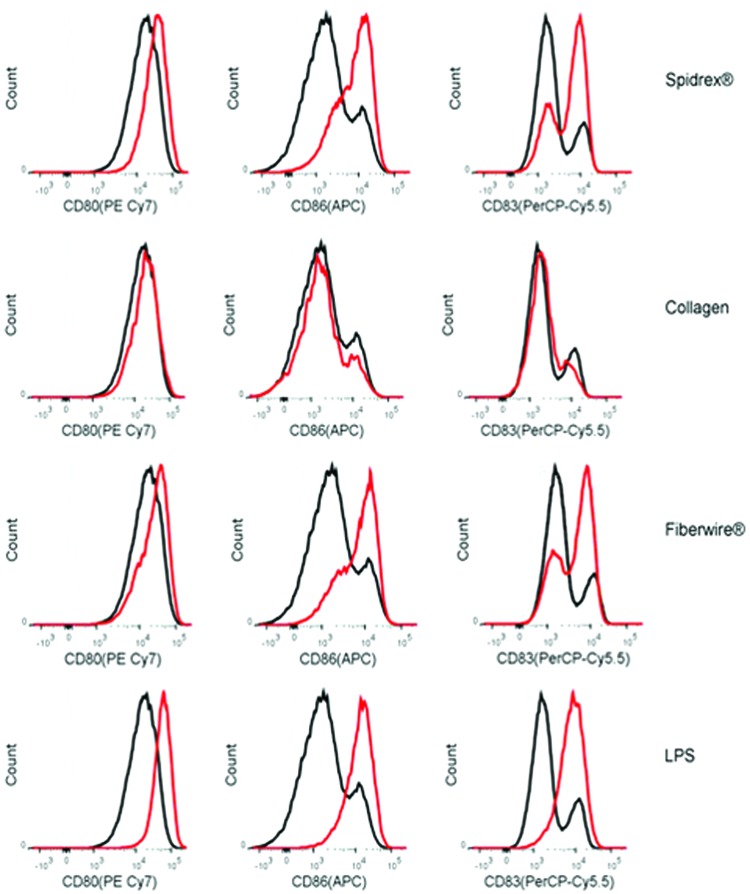
Biomaterial immunogenicity has been strongly correlated to the maturation of antigen-presenting dendritic cells, including the upregulation of dendritic cell surface proteins such as CD80, CD83, and CD86. Therefore, to assess the effect Spidrex has on dendritic cell maturation, the expression of these surface markers was assessed by flow cytometry, with LPS used as a positive control.
Following 40 h of exposure to Spidrex, dendritic cells upregulated all three maturation markers assessed, compared with the low levels of these proteins expressed by untreated dendritic cells (Fig. 8). However, the level of upregulation of surface markers by Spidrex was lower than that observed in cells treated with the positive control LPS. By comparison, dendritic cells incubated in the presence of the collagen gel did not alter maturation marker expression above levels observed with the untreated cells. Cells exposed to Fiberwire appeared to express similar levels of maturation markers as observed with Spidrex.
FIG. 8.
Maturation of dendritic cells exposed to the different materials. Representative FACS plots of CD80, CD86, and CD83 expression, as markers of dendritic cell maturation, following 40 h of culture with Spidrex, collagen gels, Fiberwire and lipopolysaccharide (LPS). The shaded area represents the unstained control, the black line is the untreated control, and the red line represents the treatment group as indicated on the right. Three different patient samples were analyzed, each run in duplicate. Data presented for are from one patient as representative for the three patients. Color images available online at www.liebertpub.com/tea
Dendritic cell inflammatory cytokine release
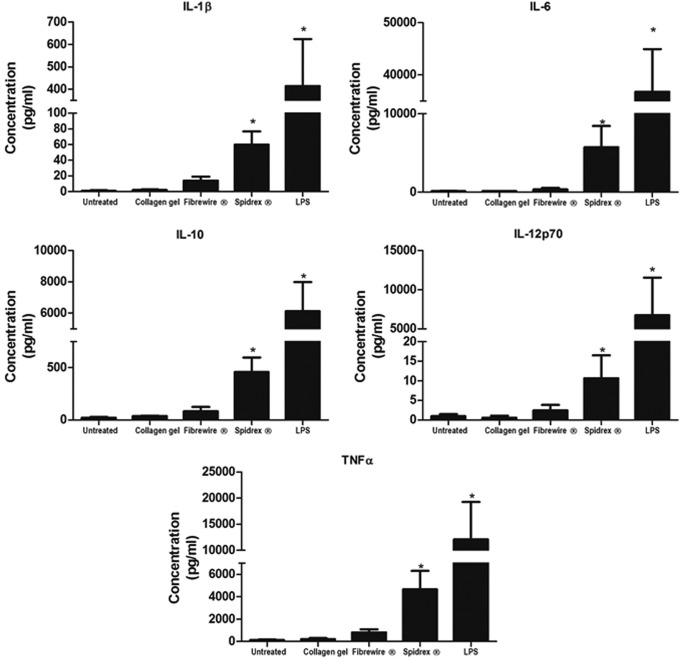
The levels of IL-1β, IL-6, IL-10, IL-12p70, and TNF-α secreted into culture medium from dendritic cells exposed to Spidrex were measured using cytometric bead array analysis and compared with collagen gels, Fiberwire and LPS, to assess the cytokine profile of the dendritic cells following culture for 40 h.
Dendritic cells exposed to Spidrex secreted significantly higher levels of IL-1β, IL-6, IL-10, and TNF-α than the untreated dendritic cells (p<0.05 for all) (Fig. 9). IL-12p70 secretion also appeared to increase following exposure to Spidrex, although this was not statistically significant, likely due to the high variability between individual patients. The levels of cytokines secreted following exposure to Spidrex were lower than the levels of secretion induced by the positive control, LPS. The release of cytokines following dendritic cell exposure to collagen gels was approximately the same as that of untreated dendritic cells, whereas those exposed to Fiberwire did not give significantly higher levels than the untreated dendritic cells.
FIG. 9.
Inflammatory cytokine release from dendritic cells exposed to the different materials. Cytometric Bead Array analysis of inflammatory cytokines released from primary human dendritic cells following 40 h of exposure to Spidrex, collagen gels, Fiberwire and LPS. One-way ANOVA (p<0.05) with post hoc Dunnett's test. *p<0.05 compared with untreated control. Three different patient samples were analyzed, each run in duplicate. Data presented are the three patients' data combined.
Discussion
In order for a biomaterial scaffold to be used clinically it must pass a number of regulatory tests. As such, initial in vitro biomaterial studies tend to focus on assays of cytotoxicity, relating data to regulatory guidelines such as the International Organization of Standardization (ISO) protocols for the Biological Evaluation of Medical Devices 10993.57 These assays give an indication of toxicity to tissues in direct contact with the biomaterial. While this may be sufficient for long-term medical implants, novel biomaterial scaffolds are designed to interact with host tissue to facilitate appropriate cellular responses, such as new tissue formation. Furthermore, one of the main failings of biomaterials results from chronic immune reactions rather than cellular toxicity.58,59 Therefore, a more comprehensive in vitro assessment of the biomaterial would be beneficial.59 In this study, we have utilized in vitro assays of cytocompatibility, tenocyte differentiation, and scaffold immunogenicity to assess a novel knitted, non-mulberry silk fibroin scaffold, to provide a preliminary indication of its potential use in tendon tissue regeneration.
Silk is a readily available, natural polymer that is being investigated for a number of biomedical and nonbiomedical applications.25,26 Recently, the fibroin fibers of the B. mori mulberry silkworm have been assessed for use in tendon/ligament regeneration.28,29,35–38,60,61 These silk fibers have also been used as sutures since the late 19th century62 and when implanted subcutaneously, B. mori silk fibroin elicits very mild immune responses.63,64 Indeed, the majority of silk based tissue engineered constructs utilize the fibroin from B. mori, most likely due to its high availability and good mechanical properties.26–28 Spidrex is a novel material in this field, fabricated from commercially available non-mulberry silk, which undergoes degumming to remove the immunogenic sericin and is knitted into a structure reminiscent of the natural crimp pattern found in tendon fibrils which straighten as force is applied, increasing the tendon's mechanical resistance.1 In this study, we compared Spidrex to a similarly knitted B. mori silk scaffold, a 3D collagen gel and Fiberwire suture material.
In vitro, materials based on B. mori silk have proven to be suitable scaffolds for the growth of corneal fibroblasts, mesenchymal stem cells, human articular chondrocytes and human tenocytes,60,61,65,66 and B. mori silk-PLGA hybrid scaffolds are cytocompatible for bone marrow-derived mesenchymal stem cell growth.35 Similarly, non-mulberry silk fibroin-based materials from the Samia ricini and Antharae pernyi silkworms have supported fibroblast-like, osteoblast-like, and bone marrow-derived mesenchymal stem cell growth in vitro.42,43 In this study, we demonstrated the cytocompatibility of Spidrex, showing that primary human and rat tenocyte viability increased over 14 days of culture on Spidrex. Morphological analysis of calcein-AM-stained human tenocytes cultured on the different silk scaffolds and within collagen gels demonstrated that human tenocytes retained their native elongated morphology when cultured on Spidrex, whereas human tenocytes cultured on the knitted B. mori scaffold and within the collagen gels did not appear as tenocytic. It should be noted that while attempts were made to blind the investigators to the treatment groups in the morphological analysis, the inherent visible differences in scaffold structures meant that blinding was unrealistic. However, investigators carried out the assessments independently and were blinded to each other's scores.
The ability of Spidrex to promote a more tenocytic morphology and support tenocyte cell growth is perhaps due to the fact that non-mulberry silks, such as those in Spidrex, contain the cell binding RGD tripeptide motif (Arg-Gly-Asp)39–41 and are, therefore, more tenocyte compatible, compared with the other materials tested in this study. Both the qualitative and quantitative cytocompatibility results suggest that Spidrex would pass the in vitro cytotoxicity tests required for medical implant regulatory bodies.57
The lack of human or rat tenocyte growth on the Fiberwire suture material is perhaps unsurprising as the polyethylene/polyester suture material is highly hydrophobic and has been selected for its chemical and biological inertness; properties sought in materials intended to remain in the body for a long time, as is the case with most suture materials. This is in direct contrast to a material designed for tissue regeneration, which needs to be biologically active. Silk fibroin from non-mulberry silkworms, particularly that of Antheraea mylitta and A. pernyi, is thought to achieve this by containing the RGD tripeptide motif that promotes cell attachment by binding to cell surface integrins.39–41 This sequence is absent in mulberry silks, such as B. mori.
The ultimate purpose of a biomaterial scaffold is to aid host tissue regeneration. To achieve this, it must facilitate appropriate cellular responses, namely the secretion of new extracellular matrix, dependent on the development and subsequent maintenance of a host cell phenotype.59 Spidrex appears to be successful in this regard, significantly increasing tenocyte differentiation markers over a 14-day period. These included fibromodulin, an extracellular matrix protein important for the tendon stem cell niche,46 scleraxis, a key transcription factor required for tenocyte development,48,49 and tenomodullin, which is necessary for tenocyte proliferation.50 Commitment of progenitors to the tenocyte lineage and subsequent tenocyte proliferation and differentiation are all necessary for the successful healing of tendon tissue. In addition, Spidrex successfully inhibited tenocyte transdifferentiation by not inducing the expression of chondrocytic or osteoblastic genes, as was seen in the collagen gel control cultures. This retention of a tenocytic gene expression profile seen in primary rat tenocytes cultured on Spidrex is particularly important in a regenerative capacity, as these cells are prone to transdifferentiating into nontenocytic lineages. This transdifferentiation would significantly alter the mechanical properties of the tendon, rendering it susceptible to further damage. Indeed, this maintenance of a tenocytic morphology and gene expression profile seen in host cells cultured on Spidrex is likely due to the nanofilamentous construction of the RGD sequence containing non-mulberry silks providing surface cues which promote the native elongated morphology of tenocytes along the silk fibers. This is reminiscent of the natural behavior of tenocytes in vivo, which elongate along the uniaxially oriented, parallel aligned collagen fibers of tendon.1 In contrast, both human and rat tenocytes cultured in the collagen gels did not elongate, instead they spread in all directions within the gels. This may account for the expression of nontenocytic genes in this culture system, as tenocyte phenotype is closely linked to cell morphology.67
Initiation of tendon healing is characterized by an intense inflammatory response, the first of three overlapping cellular phases, the latter two of which are proliferative and remodeling phases. The initial inflammatory phase involves immune cell invasion, cytokine release, and phagocytosis of necrotic tissue. This lays the foundation for host cell proliferation, vascularization, innervation, and importantly, new matrix formation. The time period for this varies, but it is generally understood that the proliferative phase begins 3 days postinjury, with any underlying immune responses resolving within the first 7 days.68
By its very nature a biomaterial is foreign to its host and interacts with antigen-presenting cells such as dendritic cells and macrophages to enhance host immune responses.69 Its biocompatibility is thus judged on its ability to improve healing, interact with host tissue and utilize the advantageous effects of the early inflammatory reaction, without inciting a site-specific, clinically adverse response.59 In this context, our study used human monocyte-derived dendritic cells to give an indication of the type of early response that Spidrex may elicit in vivo, as dendritic cells are the most potent antigen-presenting cells, share a similar lineage to macrophages and their maturation in response to biomaterials is known to be indicative of in vivo responses.70–72 In this study, we demonstrated that Spidrex increased dendritic cell maturation to similar levels as Fiberwire, the suture material currently used in rotator cuff repair. Spidrex also increased subsequent cytokine release from the dendritic cells, indicating that an in vivo immune response is likely postimplantation. The increase in dendritic cell maturation, we observed, is also similar to that of synthetic polymers currently used as medical implants.69,73 Indeed, the majority of materials, both synthetic and natural, are associated with early intense cellular responses that aid the tissue regeneration process.58,73 The success of a biomaterial is, therefore, determined by whether this immune response resolves early in the healing process and aids the following proliferative phase, or whether the immune response persists and becomes a clinically adverse event; something to be determined in an in vivo, clinically relevant environment.59
Conclusion
In vitro evaluation of potential biomaterial scaffolds is an important first step in determining scaffold potential and is essential for the early exclusion of unsuitable materials, such as those that do not support host cell attachment/growth, those that induce host cell transdifferentiation to undesirable lineages or those that induce damaging immune responses. This early exclusion prevents premature unnecessary in vivo studies and will ultimately help reduce experimental animal usage.
Spidrex has performed well in these studies, demonstrating in vitro cytocompatibility comparable to the better characterized B. mori silk, the bioactivity required to enhance host tenocyte differentiation, and immunogenicity similar to that of suture material currently used in surgical repairs of the rotator cuff. These findings suggest Spidrex is a good candidate for improving the clinical outcomes by promoting and enhancing tendon repair.
Acknowledgments
The authors would like to thank Mr. Greg Gamble for his assistance with the statistical analyses. This study was funded by an Auckland Medical Research Foundation Postdoctoral Fellowship for D.S. Musson and The Maurice Wilkins Centre and supported by the European Union Seventh Framework Programe skelGEN (FP7/2007–2013) under grant agreement no. 318553.
Disclosure Statement
Coauthor Stephanie Lesage is an employee of the Oxford Biomaterials, supplier of Spidrex. All other authors have no conflicts of interest to declare.
References
- 1.Benjamin M., and Ralphs J.R.The cell and developmental biology of tendons and ligaments. Int Rev Cytol 196,85, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Liu Y., Ramanath H.S., and Wang D.A.Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol 26,201, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Riley G.Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol 4,82, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Fehringer E.V., Sun J., VanOeveren L.S., Keller B.K., and Matsen F.A., 3rd Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elbow Surg 17,881, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Minagawa H., Yamamoto N., Abe H., Fukuda M., Seki N., Kikuchi K., et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop 10,8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19,116, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Vitale M.A., Vitale M.G., Zivin J.G., Braman J.P., Bigliani L.U., and Flatow E.L.Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg 16,181, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Milgrom C., Schaffler M., Gilbert S., and van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br Vol 77,296, 1995 [PubMed] [Google Scholar]
- 9.Tempelhof S., Rupp S., and Seil R.Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 8,296, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Longo U.G., Lamberti A., Rizzello G., Maffulli N., and Denaro V.Synthetic augmentation in massive rotator cuff tears. Med Sport Sci 57,168, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Galatz L.M., Ball C.M., Teefey S.A., Middleton W.D., and Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am Vol 86-A,219, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Xu J., Wang A., and Zheng M.Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices 6,61, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Morse K., Davis A.D., Afra R., Kaye E.K., Schepsis A., and Voloshin I.Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med 36,1824, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Lu H.H., and Thomopoulos S.Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng 15,201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakimi O., Mouthuy P.A., and Carr A.Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol 94,287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weeks K.D., 3rd, Dines J.S., Rodeo S.A., and Bedi A.The basic science behind biologic augmentation of tendon-bone healing: a scientific review. Instruct Course Lect 63,443, 2014 [PubMed] [Google Scholar]
- 17.Atesok K., Fu F.H., Wolf M.R., Ochi M., Jazrawi L.M., Doral M.N., et al. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am Vol 96, 513, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Bogdanowicz D., Erisken C., Lee N.M., and Lu H.H.Biomimetic scaffold design for functional and integrative tendon repair. J Shoulder Elbow Surg 21,266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung E.V., Silverio L., and Sperling J.W.Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res 468,1476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derwin K.A., Badylak S.F., Steinmann S.P., and Iannotti J.P.Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg 19,467, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ricchetti E.T., Aurora A., Iannotti J.P., and Derwin K.A.Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg 21,251, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Barber F.A., Burns J.P., Deutsch A., Labbe M.R., and Litchfield R.B.A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28,8, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Burkhead W.Z., Schiffern S.C., and Krishnan S.G.Use of graft jacket as an augmentation for massive rotator cuff tears. Semin Arthroplasty 18,11, 2007 [Google Scholar]
- 24.Wong I., Burns J., and Snyder S.Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg 19,104, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Omenetto F.G., and Kaplan D.L.New opportunities for an ancient material. Science 329,528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundu B., Rajkhowa R., Kundu S.C., and Wang X.Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 65,457, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Rajkhowa R., Gil E.S., Kluge J., Numata K., Wang L., Wang X., et al. Reinforcing silk scaffolds with silk particles. Macromol Biosci 10,599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy N., and Yang Y.Morphology and tensile properties of silk fibers produced by uncommon Saturniidae. Int J Biol Macromol 46,419, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Ayoub N.A., Garb J.E., Tinghitella R.M., Collin M.A., and Hayashi C.Y.Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS One 2,e514, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vendrely C., and Scheibel T.Biotechnological production of spider-silk proteins enables new applications. Macromol Biosci 7,401, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Xu M., and Lewis R.V.Structure of a protein superfiber: spider dragline silk. Proc Natl Acad Sci U S A 87,7120, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIntosh A.C., Kearns V.R., Crawford A., and Hatton P.V.Skeletal tissue engineering using silk biomaterials. J Tissue Eng Regen Med 2,71, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Panilaitis B., Altman G.H., Chen J., Jin H.J., Karageorgiou V., and Kaplan D.L.Macrophage responses to silk. Biomaterials 24,3079, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Kasoju N., and Bora U.Silk fibroin in tissue engineering. Adv Healthc Mater 1,393, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Sahoo S., Toh S.L., and Goh J.C.PLGA nanofiber-coated silk microfibrous scaffold for connective tissue engineering. J Biomed Mater Res Part B Appl Biomater 95,19, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Teh T.K., Toh S.L., and Goh J.C.Aligned fibrous scaffolds for enhanced mechanoresponse and tenogenesis of mesenchymal stem cells. Tissue Eng Part A 19,1360, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Chen X., Yin Z., Chen J.L., Liu H.H., Shen W.L., Fang Z., et al. Scleraxis-overexpressed human embryonic stem cell-derived mesenchymal stem cells for tendon tissue engineering with knitted silk-collagen scaffold. Tissue Eng Part A 20,1583, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Kwon S.Y., Chung J.W., Park H.J., Jiang Y.Y., Park J.K., and Seo Y.K.Silk and collagen scaffolds for tendon reconstruction. Proc Inst Mech Eng H 228,388, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Kim H.J., Vunjak-Novakovic G., and Kaplan D.L.Stem cell-based tissue engineering with silk biomaterials. Biomaterials 27,6064, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Minoura N., Aiba S., Higuchi M., Gotoh Y., Tsukada M., and Imai Y.Attachment and growth of fibroblast cells on silk fibroin. Biochem Biophys Res Commun 208,511, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Datta A., Ghosh A.K., and Kundu S.C.Purification and characterization of fibroin from the tropical Saturniid silkworm, Antheraea mylitta. Insect Biochem Mol Biol 31,1013, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Luan X.Y., Wang Y., Duan X., Duan Q.Y., Li M.Z., Lu S.Z., et al. Attachment and growth of human bone marrow derived mesenchymal stem cells on regenerated Antheraea pernyi silk fibroin films. Biomed Mater 1,181, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Pal S., Kundu J., Talukdar S., Thomas T., and Kundu S.C.An emerging functional natural silk biomaterial from the only domesticated non-mulberry silkworm Samia ricini. Macromol Biosci 13,1020, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Kundu S.C., Kundu B., Talukdar S., Bano S., Nayak S., Kundu J., et al. Invited review nonmulberry silk biopolymers. Biopolymers 97,455, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W., et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13,1219, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Riley G.P., Harrall R.L., Cawston T.E., Hazleman B.L., and Mackie E.J.Tenascin-C and human tendon degeneration. Am J Pathol 149,933, 1996 [PMC free article] [PubMed] [Google Scholar]
- 48.Cserjesi P., Brown D., Ligon K.L., Lyons G.E., Copeland N.G., Gilbert D.J., et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121,1099, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Schweitzer R., Chyung J.H., Murtaugh L.C., Brent A.E., Rosen V., Olson E.N., et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128,3855, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Docheva D., Hunziker E.B., Fassler R., and Brandau O.Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 25,699, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lui P.P., Fu S.C., Chan L.S., Hung L.K., and Chan K.M.Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J Histochem Cytochem 57,91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller E.J., and Gay S.The collagens: an overview and update. Methods Enzymol 144,3, 1987 [DOI] [PubMed] [Google Scholar]
- 53.Kiani C., Chen L., Wu Y.J., Yee A.J., and Yang B.B.Structure and function of aggrecan. Cell Res 12,19, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108,17, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ganss B., Kim R.H., and Sodek J.Bone sialoprotein. Crit Rev Oral Biol Med 10,79, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Raetz C.R., and Whitfield C.Lipopolysaccharide endotoxins. Annu Rev Biochem 71,635, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ISO (ed.) Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process. ISO 10993-1:20092009
- 58.Anderson J.M., Rodriguez A., and Chang D.T.Foreign body reaction to biomaterials. Semin Immunol 20,86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams D.F.On the mechanisms of biocompatibility. Biomaterials 29,2941, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Altman G.H., Horan R.L., Lu H.H., Moreau J., Martin I., Richmond J.C., et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 23,4131, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Qiu Y., Wang X., Zhang Y., Carr A.J., Zhu L., Xia Z., et al. In vitro two-dimensional and three-dimensional tenocyte culture for tendon tissue engineering. J Tissue Eng Regen Med 2013. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 62.Kearns V., MacIntosh A., Crawford A., and Hatton P.Silk-based biomaterials for tissue engineering. Top Tissue Eng 4,5, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Rudym D.D., Walsh A., Abrahamsen L., Kim H.J., Kim H.S., et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 29,3415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meinel L., and Kaplan D.L.Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev 64,1111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil E.S., Park S.H., Marchant J., Omenetto F., and Kaplan D.L.Response of human corneal fibroblasts on silk film surface patterns. Macromol Biosci 10,664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Blasioli D.J., Kim H.J., Kim H.S., and Kaplan D.L.Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials 27,4434, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Zhu J., Li J., Wang B., Zhang W.J., Zhou G., Cao Y., et al. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials 31,6952, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Hope M., and Saxby T.S.Tendon healing. Foot Ankle Clin 12,553 v, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Park J., and Babensee J.E.Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater 8,3606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foti M., Granucci F., Pelizzola M., Beretta O., and Ricciardi-Castagnoli P.Dendritic cells in pathogen recognition and induction of immune responses: a functional genomics approach. J Leukoc Biol 79,913, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Norton L.W., Park J., and Babensee J.E.Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Controlled Release 146,341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kou P.M., Pallassana N., Bowden R., Cunningham B., Joy A., Kohn J., et al. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 33,1699, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikos A.G., McIntire L.V., Anderson J.M., and Babensee J.E.Host response to tissue engineered devices. Adv Drug Deliv Rev 33,111, 1998 [DOI] [PubMed] [Google Scholar]