Abstract
68Ga-NODAGA-THERANOST™ is an αvβ3 integrin antagonist and the first radiolabeled peptidomimetic to reach clinical development for targeting integrin receptors. In this first-in-human study, the feasibility of integrin receptor peptidomimetic positron emission tomography/computed tomography (PET/CT) imaging was confirmed in patients with non-small-cell lung cancer and breast cancer.
Methods: Patients underwent PET/CT imaging with 68Ga NODAGA-THERANOST. PET images were analyzed qualitatively and quantitatively and compared to 2-deoxy-2-(18F) fluoro-d-glucose (18F-FDG) findings. Images were obtained 60 minutes postinjection of 300–500 MBq of 68Ga-NODAGA-THERANOST.
Results: 68Ga-NODAGA-THERANOST revealed high tumor-to-background ratios (SUVmax=4.8) and uptake at neoangiogenesis sites. Reconstructed fused images distinguished cancers with high malignancy potential and enabled enhanced bone metastasis detection. 18F-FDG-positive lung and lymph node metastases did not show uptake, indicating the absence of neovascularization.
Conclusions: 68Ga-NODAGA-THERANOST was found to be safe and effective, exhibiting in this study rapid blood clearance, stability, rapid renal excretion, favorable biodistribution and PK/PD, low irradiation burden (μSv/MBq/μg), and convenient radiolabeling. This radioligand might enable theranostics, that is, a combination of diagnostics followed by the appropriate therapeutics, namely antiangiogenic therapy, image-guided presurgical assessment, treatment response evaluation, prediction of pathologic response, neoadjuvant-peptidomimetic-radiochemotherapy, and personalized medicine strategies. Further clinical trials evaluating 68Ga-NODAGA-THERANOST are warranted.
Key words: : αvβ3 receptor targeting, 68Ga-NODAGA-THERANOST™, positron emission tomography
Introduction
According to the World Health Organization, ∼24.6 million people are living with cancer worldwide, and the American Cancer Society has estimated approximately 1.6 million new cases in the United States in 2015.1 Medical imaging plays a key role in cancer diagnosis and clinical management of patients by highlighting noninvasively cancer presence, spread and metabolism, enabling staging, restaging and therapy monitoring, and contrasting differences between normal and malignant tissues. Positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), and fusion imaging (PET/CT or PET/MRI) represent important evolutions in technology. Clinicians are seeing dramatic improvements in cancer treatment moving toward individually tailored programs of personalized therapy.2 However, despite significant preclinical scientific progress, very few cancer imaging agents are currently available in the clinic.
The most frequently used PET tracer in this setting to date is 2-deoxy-2-(18F) fluoro-d-glucose (18F-FDG). However, several studies have demonstrated its limitations in distinguishing some malignant lesions.3 A theranostic approach could allow targeted tumor detection, while potentially reducing the harmful collateral effects of chemotherapy or radiochemotherapy.
The cell adhesion motif αvβ3 arginine–glycine–aspartate (RGD) integrin receptor was discovered in fibronectin by Pytela, Pierschbacher, and Ruoslahti more than 20 years ago.4 Shortly thereafter, Smith and Cheresh identified the vitronectin receptor as one of the adhesion molecules recognizing this sequence.5 The αvβ3 integrin receptor is overexpressed on endothelial cells and on some tumor cells.
Integrins are amino acid-containing derivatives that are key regulators of tumor angiogenesis and metastasis and are heterodimeric transmembrane proteins involved in cell adhesion and cell signaling, and their expression is upregulated in cancers and inflammatory diseases.6 Integrins play a vital role in angiogenesis, leukocyte function, and tumor growth and are potential targets for therapeutic agents, demonstrating an antiproliferative effect on tumor growth and the selective inhibition of endothelial αvβ3 integrin; also, they have been shown to increase the effectiveness of combination regimens such as chemotherapy and radiochemotherapy.
The αvβ3 integrin is significantly overexpressed in certain types of tumor cells and in almost all tumor vasculature. It is expressed on the luminal surface of neovasculature (but is not found on the endothelial surface of mature capillaries), has been shown to be upregulated in tumor blood vessels that undergo angiogenesis, and has been implicated in metastasis.7 Increased levels of integrin αvβ3 are closely associated with increased cell invasion and metastases. Integrins are upregulated in many malignancies, mediate a variety of tumor integrin-targeting therapeutics, and have activity against many cancer subtypes.8–10
Because αvβ3 is involved in neoangiogenesis in solid tumors and is also directly expressed in cancer cells (e.g., glioblastomas, melanomas, myelomas, ovarian, breast, and prostate cancers), these radiolabeled peptide ligands for αvβ3 targeting might become powerful new tools for molecular imaging and targeted radiotherapy of tumors undergoing angiogenesis.7,10–16
Noninvasive imaging of αvβ3 expression could be of vital importance for patient selection and monitoring of anti-integrin treatment efficacy. The ability to image angiogenesis could potentially be used to identify patients who may benefit from antiangiogenic treatments, prognostication/risk stratification.16 PET imaging with 18F-RGD peptides has been shown to visualize and quantify αvβ3 integrin expression in patients, but radiolabeling is complex and image contrast is limited in some tumor types.17 Low tumor uptake, failure to differentiate inflammation, and high cost and difficulties in routine radiosynthesis have limited some clinical application of RGD agents.18 Compared to the commercially available cyclic peptide RGDfv, this probe has at least 20 times better binding affinity for the αvβ3 receptor.19
The PET tracer 18F-Galacto-RGD has been developed and while this agent shows favorable pharmacokinetics, tracer synthesis is labor intensive (duration 200 minutes), involving steps that cannot be automated. A promising alternative tracer that can be synthesized is 68Ga-NODAGA-RGD, which has been used in animal studies and has the advantage of the availability of 68Ga, convenient radiolabeling, high stability, and good imaging properties.11–13,18,20 Superior preclinical results were obtained in comparison with 18F-Galacto-RGD. Therefore, 68Ga-NODAGA-cyclo-RGDyK may be a suitable replacement for 18F-Galacto-cyclo-RGDfK.12,13,18,21
Clinical data have been reported for the glycosylated peptide, cRGDfK, labeled with 2-18F-fluoropropionate to yield 18F-galacto-RGD (Fluciclatide) in patients with malignant melanoma.16 According to clinicaltrials.gov,22 the 18F-FPPRGD2 (2-fluoropropionyl)-labeled PEGylated dimeric RGD peptide [PEG3{c(RGDyk)}2]), based on the dimeric RGD peptide sequence targeting αvβ3 is in early-stage clinical trials. Lung cancer and cardiovascular imaging trials are underway using 68Ga-BNOTA-PRGD2. Trials are also underway using 68Ga-BNOTA-PRGD2 at the Peking Union Medical College Hospital 6).
While 18F- and 64Cu-labeled RGD peptides have been used for quantification of αvβ3 expression by PET imaging, these are associated with problems, including limited image contrast and complex radiolabeling.12,13,23 Regardless, radiolabeled peptides have been the dominant molecular imaging strategy for imaging integrin expression that has been translated into the clinic.21
A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide or by designing similar systems that mimic peptides, such as peptoids and β-peptides. Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties, such as stability or biological activity. These modifications involve changes to the peptide that will not occur naturally such as altered backbones and the incorporation of unnatural amino acids.
Natural amino acids in peptides can be replaced with nonproteinogenic counterparts (proteinogenic means “protein building”). Proteinogenic amino acids can be condensed into a polypeptide (the subunit of a protein) through a process called translation. Proteinogenic amino acids are amino acids that are precursors to proteins, and are incorporated into proteins cotranslationally—that is, during translation to obtain drug-like targeting molecules that stimulate innovation leading to development of approaches that differ from conventional RGDs and can result in enhanced interactions.24
The αvβ3 integrin antagonist (IAC) peptidomimetic, 4-[2-(3,4,5,6-tetrahydropyrimidine-2-ylamino)ethyloxy]benzoyl-2-[N-(3-amino-neopenta-1-carbamyl)]-aminoethylsulfonyl-amino-β-alanine, THERANOST™, was developed to provide an alternate vector that selectively targets integrin αvβ3 receptor and clears rapidly from the whole body. Based on extensive lead optimization studies, this IAC demonstrated superior binding affinity to cells that express αvβ3. The compound accumulated in angiogenic blood vessels after systemic administration in the murine squamous cell carcinoma model.8,9,25 Thus, this novel integrin-targeting platform was expected to have potential applications for targeted delivery of drugs, and more directly, highly suitable for molecular imaging and possibly therapy applications.26
IAC has been labeled with 99mTc and 111In,9,25 conjugated with a dye, FITC, and evaluated as an optical imaging agent.19,26 Thus, in vivo studies were performed aimed at rapid targeting of integrin receptors to produce a high tumor-to-background ratio and as well in the cardiovascular setting as an optical imaging agent.19
The 111In-labeled compound was evaluated for in vitro receptor binding and carried forward through biodistribution and imaging studies in M21 human melanoma xenograft models demonstrating specific binding that could be blocked by an excess of unlabeled agent indicating receptor-mediated uptake. The radiolabeled agent was excreted primarily through the renal system resulting in high tumor-to-background ratios at 2 hours in imaging studies enabling visualization of receptor-positive tumors at 4 hours. Similarly, 99mTc-labeled IAC resulted in equally positive results demonstrating good tumor-targeting properties with a rapid uptake, prolonged tumor retention, and fast whole-body clearance kinetics.
Overall, the tumor-targeting kinetics combined with the rapid accumulation and prolonged retention justified further studies of molecular imaging of tumor-induced angiogenic vessels and various malignant tumors expressing the receptor in humans.
During the course of the development of a positron-labeled peptidomimetic IAC targeting αvβ3, incorporation of 68Ga as the radionuclide of choice was self-evident. Generator-derived radionuclides for PET/CT imaging are promising for optimizing targeted radiotherapy by an individual patient-based approach, applying pretherapeutic evaluation as well as dosimetric calculations and for measuring treatment response after radionuclide therapy.27
68Ga is available from an in-house generator enabling 68Ga radiopharmacy independent of an on-site cyclotron. 68Ga has an ideal half-life (t1/2=68 minutes) and decays by 89% through positron emission (β+ Emax=1.899 MeV) that provides PET images with high-quality resolution. The parent, 68Ge, is accelerator produced and decays with a half-life of 270.8 days by electron capture. There are at least three commercial and several in-house generators available, positioning this radionuclide for further widespread clinical development. As such, 68Ga was selected by this team as the optimal radionuclide for radiolabeling and clinical translation as a diagnostic agent for molecular imaging.
Appropriate chelation technology that complements the chemistry of Ga(III) and also meets the requirements for in vivo stability for retention of the 68Ga prompted selection of a bifunctional NOTA derivative. Synthesis was performed in a straightforward aseptic procedure. All routine quality control requirements were met.
The stability of the Ga(III)-NOTA complex is well recognized.28 The NOTA derivative 1-(1-carboxy-3-carbo-tert-butoxypropyl)-4,7-(carbo-tert-butoxymethyl)-1,4,7-triazacyclononane [NODAGA(tBu)3] is a bifunctional chelator, which conveniently lends itself to efficient active ester conjugation to the primary amine of IAC. Subsequent acidic deprotection provides an IAC-NODAGA (Fig. 1) suitable for labeling with 68Ga.29 Having established both the conjugation chemistry for appending NODAGA to this novel peptidomimetic as well as radiolabeling with 111In and 67Ga, its efficient translation into the clinic became a logical forward step to follow in its development. Significant potential utility for noninvasive evaluation of angiogenic and metastatic activity in cancer as well as for monitoring tumor therapy using αvβ3 or other angiogenesis inhibitors appears possible, and as such, patient imaging studies were initiated.
FIG. 1.
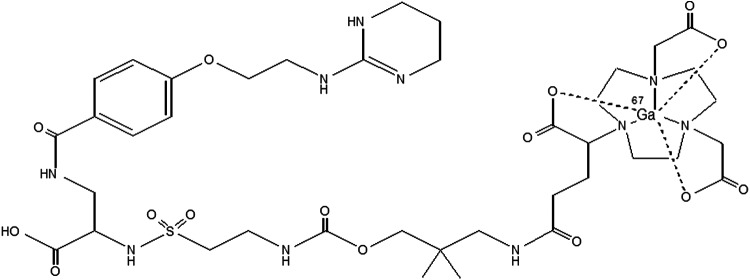
Structure of 68Ga-NODAGA-THERANOST.
68Ga-IAC-NODAGA, which was shown to exhibit high affinity for αvβ3 and high selectivity versus other integrin receptors, demonstrated high tumor uptake and produced excellent PET/CT images. Based on these promising results, investigations moved forward to evaluate NODAGA-IAC and DOTAGA-IAC conjugates for targeting tumor-associated angiogenesis and αvβ3 integrin-positive tumors so as to define their PET and SPECT imaging qualities as well as the potential for delivery of therapeutic radionuclides. Herein, the authors present the result of the first-in-human clinical results of PET/CT imaging using 68Ga-NOTAGA-IAC, 68Ga-NODAGA-THERANOST.
Materials and Methods
Patients
Approval for the use of 68Ga-NODAGA-THERANOST was granted by an in-hospital investigative review board, and written informed consent of patients was obtained in accordance with the Declaration of Helsinki. Patients >18 years with full contractual capability were recruited. Briefly, inclusion criteria were patients with small-cell lung carcinoma or inflammatory breast cancer histologically proven and measurable tumor according to RECIST evaluation criteria.
Standard vital signs and blood sample values were evaluated as was tracer biodistribution, dosimetric properties, time–activity curves, and the stability of laboratory values.
Chemistry and 68Ga radiolabeling
The NODAGA-THERANOST conjugate provided by Advanced Imaging Projects, LLC., was radiolabeled efficiently with 68Ga by the Radiopharmacy Department at the Theranostics Center of the Zentralklinik Bad Berka at 90°C in 10 minutes using 68Ga obtained from an Eckert and Ziegler 68Ge/68Ga generator using a NaCl-based labeling procedure (Mueller's Method30,31). The 68Ga-labeling yield and radiochemical purity were 96% or greater as determined by radio-high-performance liquid chromatography using an H2O-CH3CN gradient and an RP-18 column. The final product solution of 68Ga-NODAGA-THERANOST was sterile filtered and used directly for patient administration.
Patient imaging
68Ga-NODAGA-THERANOST was administered by intravenous injection, and PET/CT scans were obtained at 60 minutes using a Siemens Medical Solutions Biograph duo PET/CT Scanner, which consists of a PET system with a full-ring lutetium oxyorthosilicate and a SOMATOM CT Emotion Duo (Siemens Medical Solutions), a two-row spiral CT system with a maximum continuous scan time of 100 seconds and a maximum rotation speed of 75 rpm. Two patients were initially imaged.
Patient 1 was a 28-year-old female patient who presented with extensive metastatic breast cancer (G3), initial stage pT2m pN3a cM0 L1, initial receptor status ER 80% positive, PR 40% positive, and HER2-Score 3+. This patient had liver metastases 1 year after the initial diagnosis. Patient 2 was a 61-year-old male patient with a neuroendocrine neoplasm of the right lower lobe of the lung, stage T2 N0 M0, G1 (<2 mitoses/10 HPF), s.p. right lower lobectomy and bronchoscopic ablation of metastases, who had received two cycles of peptide receptor radionuclide therapy (PRRT, total administered radioactivity 13.8 GBq of 177Lu-DOTATATE).
Results and Discussion
This is the first report on the ability of 68Ga-NODAGA-THERANOST to detect solid tumors in humans, establish the absence of neovascularization, and predict the antitumor effect. The acronym ‘THERANOSTIC’ epitomizes the inseparability of diagnosis and therapy and takes into account personalized management of disease for a specific patient. Thus, what is hypothesized is that molecular imaging can be effectively followed by personalized treatment utilizing the same molecular vector.
Receptor PET/CT using 68Ga enables imaging of metastases with high tumor-to-background ratio, diagnostic sensitivity, specificity and uptake, and desirable circulation half-life. This provides quantitative and reproducible data that can be used for selecting patients for therapy and, thereafter, evaluation of therapy response with probably low radiation burden (10–12 mSv) to the patient.32
Although assessment of tissue metabolism using 18F-FDG provides useful tumor imaging, it is not tumor specific and usually less useful for imaging tumors that have low growth rates as it actually is a metabolic marker or a guide for delivery of specific molecular therapy. The development of new tracers such as THERANOST that target specific molecular abnormalities is therefore essential for the development and utility of clinically relevant PET, which can be used to assess the efficacy of therapeutic drugs. The goals are to allow early detection and characterization of the disorder, to provide more timely and direct assessment of treatment, and to obtain a more fundamental understanding of the disease process.33
As opposed to benign tumors, malignant ones usually overexpress integrins on cancerous tumors. The purpose of this study was to assess the safety, biodistribution, and dosimetric properties and to establish ligand targeting of αvβ3 integrin receptors with 68Ga-NODAGA-THERANOST, a potent αvβ3 peptidomimetic displaying a strong binding affinity for extracellular surface matrix proteins and epitopes, including αvβ3 and other integrins, fibronectin, vitronectin, fibrinogen, tenascin-C, osteopontin, gastrin-releasing peptide, and neuropilin-1.7,11,20,34
PET studies were evaluated and reported by 2 experienced nuclear physicians. Any disagreements were resolved by reaching a consensus.
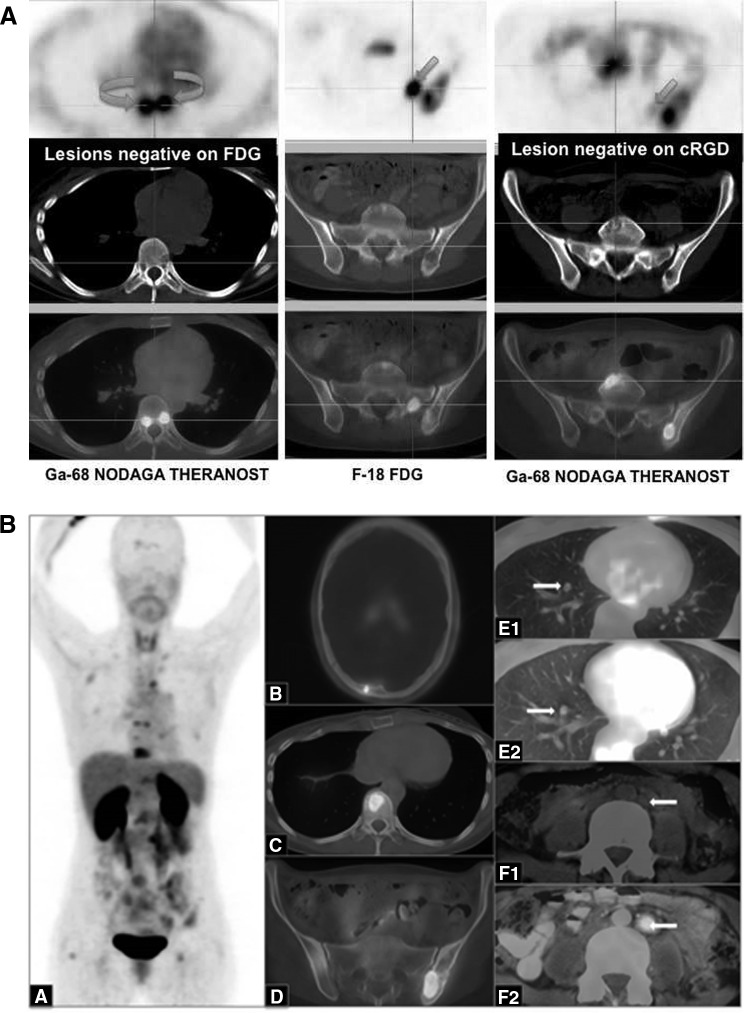
Two patients were imaged using 68Ga-NODAGA-THERANOST. The first patient was a 28-year-old female patient with extensive metastatic breast cancer (G3, HER2 3+) who presented with liver metastases 1 year after the initial diagnosis, indicating cancer with high malignancy potential. Reconstructed fused images of this patient demonstrated enhanced tumor-to-background ratios for 68Ga-NODAGA-THERANOST and PET/CT images obtained 60 minutes postinjection of 472 MBq of 68Ga-NODAGA-THERANOST revealed high uptake, sensitivity, and specificity in tumors at possible neoangiogenesis sites and enhanced detection of multiple skeletal metastases (Fig. 2).
FIG. 2.
(A) PET/CT images using 68Ga-NODAGA-THERANOST (left and right panels) as well as 18F-FDG (middle panel) of patient #1. Overall, 25 lesions were cRGD positive compared to 12 lesions detected by FDG (shown here are skeletal metastases on transverse slices in upper row: PET, middle row: CT, and lower row: fused PET/CT). Curved arrows (left panel) demonstrate cRGD positive vertebral pedicle lesions, which were negative on FDG. Straight arrows indicate FDG-avid lesion in left sacral ala (middle panel), which was cRGD negative (right panel). (B) The maximum intensity projection (A) and reconstructed fused images using 68Ga-NODAGA-THERANOST revealed intense uptake (SUVmax=4.8) at neoangiogenesis sites in multiple skeletal metastases, for example, skull (B), vertebrae (C), and pelvis (D). The 18F-FDG-positive lung (E2) and lymph node (F2) metastases did not show any uptake of 68Ga-NODAGA-THERANOST (E1 and F1, respectively), indicating the absence of neovascularization. CT, computed tomography; PET, positron emission tomography; 18F-FDG, 2-deoxy-2-(18F) fluoro-d-glucose; SUV, standardized uptake value; arrows pointing to the right indicate lung metastasis and to the left indicate lymph node metastasis.
The maximum intensity projection (Fig. 2A) and reconstructed fused images revealed intense uptake (SUVmax 4.8) at the sites of neoangiogenesis in multiple skeletal metastases, for example, the skull (Fig. 2B), vertebrae (Fig. 2C), and pelvis (Fig. 2D). Overall, 25 integrin ανβ3-positive lesions were detected by 68Ga-NODAGA-THERANOST versus 12 by 18F-FDG PET/CT. The 18F-FDG-positive lung (Fig. 2E2) and lymph node (Fig. 2F2) metastases did not show any uptake of 68Ga-NODAGA-THERANOST (Fig. 2E1, F1, respectively), indicating the absence of neovascularization. 68Ga-THERANOST accumulated more specifically than 18F-FDG in primary and metastatic tumors. 18F-FDG-positive lung and lymph node metastases did not show uptake, indicating the absence of neovascularization.
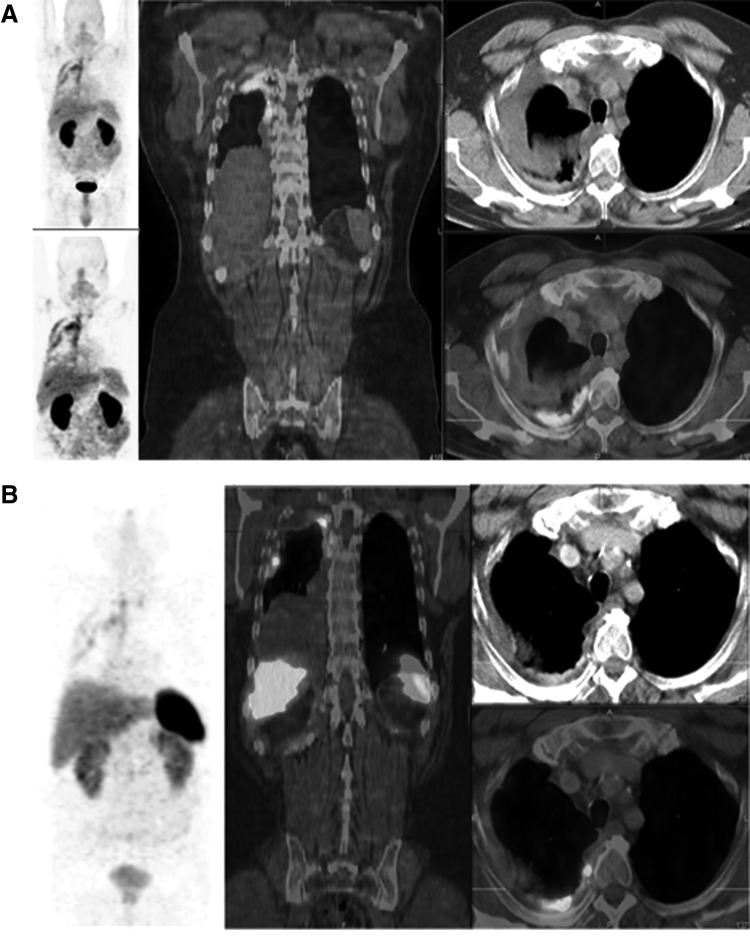
The second patient imaged using 68Ga-NODAGA-THERANOST was a 61-year-old male with a neuroendocrine neoplasm of the right lower lobe of the lung (typical carcinoid), who had received two cycles of PRRT. PET/CT after injection of 496 MBq of 68Ga-NODAGA-THERANOST demonstrated αvβ3 receptor expression and angiogenesis in the right pleural carcinomatosis (Fig. 3A). The SUVmax of the target lesion in the right dorsoapical pleura was 4.0 (tumor-to-liver ratio of SUVmax T/L was 1.43 and tumor-to-spleen ratio of SUVmax [T/S] was 1.48).
FIG. 3.
PET/CT images using 68Ga-NODAGA-THERANOST of patient #2. (A) Demonstrates integrin receptor expression and angiogenesis in the extensive right pleural carcinomatosis. SUVmax of target lesion in the right dorsoapical pleura was 4.0 (tumor-to-liver ratio of SUVmax, T/L was 1.43 and tumor-to-spleen ratio of SUVmax, T/S was 1.48). Pleural uptake was also noted on somatostatin receptor PET/CT with 68Ga DOTATOC (B). SUVmax of the same lesion being 6.9, but with a lower target lesion-to-background ratio (T/L 0.78, T/S 0.17). MIP image on the left, coronal fused PET/CT in the middle, transverse CT on the right above, and transverse fused PT/CT on the right below. MIP images of 68Ga-NODAGA-THERANOST PET/CT are shown in two different color scales.
Pleural uptake was also observed on somatostatin receptor PET/CT with 68Ga-DOTATOC (Fig. 3B), SUVmax of the same lesion being 6.9, but with a lower target lesion-to-background ratio (T/L 0.78, T/S 0.17). This radiotracer was associated with rapid blood clearance and renal clearance. The minimum nonspecific activity accumulation in normal lung tissue and heart rendered high-quality tumor images, enabling clear demarcation of both the primary tumor at the upper lobe of the left lung as well as metastases in the mediastinum, contralateral lung, and diaphragm. Biodistribution, quantitative tissue uptake, and time–activity in tumor and normal organs appear acceptable. As a comparison, 18F-FDG scans were only able to identify the primary tumor, with the metastatic lesions masked by intense cardiac uptake.
The mean and standard deviation of the administered mass of 68Ga-NODAGA-THERANOST was 37.5±12.5 μg (range, 25–50 μg). The mean administered activity was 333.5±162.5 MBq (range, 171–496 MBq). Mean standardized uptake values (SUVs) were calculated from each region of interest using the formula SUV=measured activity concentration (Bq/g)×body weight (g)/injected activity (Bq).
There were no adverse or clinically detectable pharmacological effects in any of the subjects. No significant changes in the vital signs or the results of laboratory studies or electrocardiograms were observed. With an injected dose of 370 MBq and a 1 hour voiding interval, a patient would be exposed to an effective radiation dose of 1.5 rem (15 mSv). Time–activity curves showed rapid clearance from the vasculature with most of the activity occurring in the plasma relative to cells.
Uptake was highest in the tumor, kidney, and urinary bladder. The compound appears safe and well tolerated in patients, exhibits rapid blood clearance, stability, good clearance kinetics and renal excretion, favorable biodistribution, pharmacokinetics, and pharmacodynamics to provide high-contrast PET images of integrin-expressing tumors, recorded 1 hour after injection.
Quantitatively, there were relevant differences in the biodistribution between 68Ga-NODAGA-THERANOST and 18F-FDG resulting in a higher tumor lesion detection rate than 18F-FDG. Remarkably, this radioligand accumulated more specifically than 18F-FDG in both primary and metastatic tumor lesions enabling detection of more than twice the number of possible bone metastases in a breast cancer patient, accumulating more specifically in angiogenic tumors than 18F-FDG and accumulating in malignant tumors more specifically than 18F-FDG or 68Ga-DOTATOC. The high tumor-to-lung contrast probably results from a lack of integrin expression in normal tissues and organs and minimal nonspecific cardiac accumulation. This makes 68Ga-NODAGA-THERANOST an attractive alternative to more costly 18F-labeled compounds.
68Ga-NODAGA-THERANOST, a nonpeptide αvβ3 targeting vector, demonstrated high diagnostic sensitivity, specificity, and binding to integrin-positive tumors. Imaging with 68Ga-NODAGA-THERANOST dramatically improved image contrast, because this compound is only slowly cleared from αvβ3 integrin-expressing tumors, whereas clearance from normal tissues appears to be much faster.
Only 2 patients were included in this pilot study because the aim was to obtain clinical data on the potential usefulness of imaging integrin-positive tumors and differentiating angiogenic tumors and not to determine the sensitivity and specificity in a specific patient population. Regardless, even in this limited patient number study, a strong positive correlation of uptake and proliferation was detected as assessed by Ki-67 indexing.
Ki-67 is a nuclear protein that is associated with and may be necessary for cellular proliferation. It is an excellent marker to determine the growth fraction of a given cell population. The fraction of Ki-67-positive tumor cells (the Ki-67-labeling index) is often correlated with the clinical course of cancer. The best-studied examples in this context are carcinomas of the prostate, brain, lung, and breast. For these types of tumors, the prognostic value for survival and tumor recurrence has repeatedly been proven in uni- and multivariate analyses.
This study provides the first clinical evidence that 68Ga-NODAGA-THERANOST is a useful positron-emitting radionuclide tracer for visualization of tumors and metastases demonstrating that PET imaging using 68Ga-NODAGA-THERANOST is sensitive enough to detect αvβ3 integrin expression and may be a superior approach for imaging and therapy, as well as for selection of patients who could potentially benefit from therapy. Data, so far, justify further evaluation in larger cohorts, especially with regard to integration of 68Ga-NODAGA-THERANOST for early response imaging and as a predictive or interim value tool in the clinical management of cancer patients.
Future aims would include studies to monitor, noninvasively, changes in vascularity after antiangiogenic therapies, and potentially to determine the clinical and pathological efficacy of neoadjuvant chemo-/immunotherapy or molecular targeted therapy with small molecules, for example, applying therapeutics that specifically inhibit angiogenesis in tumors.
THERANOST, carrying therapeutic cargoes, might also have potent activity with drugs of distinct chemotype, potentially including bevacizumab, sorafenib, paclitaxel, docetaxel, bortezomib, sunitinib, bosutinib, or dasatinib. Thus, coadministration of THERANOST in a drug delivery mode could potentially enhance the efficacy of anticancer drugs. Molecular targeted radionuclide therapy (TRNT) could have important applications for treating disseminated diseases with the cytotoxic radionuclide being specifically directed to the malignancy, sparing normal tissues. Radiolabeled with particle emitters such as 177Lu, 90Y, 213Bi, or 212Pb, the DOTAGA conjugate of THERANOST might be employed for TRNT or neoadjuvant peptidomimetic radiotherapy.29
On this basis, THERANOST might be a suitable drug for efficacious diagnosis, and further clinical trials and patient management evaluation before and after therapy may be warranted. In this groundbreaking investigation, the potential utility of this high-affinity tumor-penetrating ligand is suggested. In summary, PET/CT imaging provides clinicians with a noninvasive tool for assessment and response evaluation in the oncological setting. 68Ga is an alternative generator-produced PET tracer, which has the potential to be more cost effective with fewer limitations compared to 18F-FDG.
Conclusions
Synthesis and clinical evaluation of 68Ga-NODAGA-THERANOST for imaging of integrin receptors signal the potential clinical usefulness of this peptidomimetic to identify responders and nonresponders who may benefit at an early stage, an important factor in determining the most appropriate treatment for cancer patients. The 68Ga-NODAGA-THERANOST probe was evaluated clinically and demonstrated selectively binding with high affinity and rapid accumulation in diseased tissue and tumor neovasculature, reporting biochemical and morphological characteristics. Initial evidence suggests that this radioligand has considerable potential for early tumor detection and noninvasive monitoring of tumor metastasis in a range of diseases.
Specific indications may include tumor staging, identifying patients who would benefit from antiangiogenesis therapy early and treatment regimen modification, and as a molecular marker of chemosensitivity. Noninvasive reproducible quantitative assessment (SUVmax) provides important information for planning and monitoring of antiangiogenic therapies, revealing involvement in tumor processes.
Molecular imaging using 68Ga-NODAGA-THERANOST is a promising diagnostic tool for patient management, has the potential to localize metastatic lesions in vivo, adds qualitative information not available using conventional imaging techniques, and may allow the status to be determined for metastases not amenable to biopsy. These observations are clinically relevant, showing potential for determination of therapeutic response earlier than current methods that rely on changes in tumor size or less sensitive receptor agents. 68Ga-NODAGA-THERANOST could potentially become a suitable substitute for 18F or 99mTc molecular imaging agents and a preferred alternative to RGDfv, RGDyK, and other RGDs due to greater specific accumulation in integrin-expressing tumors. Larger trials with 68Ga-NODAGA-THERANOST are needed to evaluate and support the value of this novel radioligand.
Acknowledgments
The authors thank Rosanne Satz for her logistical efforts and editing. This research was supported, in part, by the Intramural Research Program of the NIH, the National Cancer Institute, and the Center for Cancer Research and Advanced Imaging Projects, LLC.
Disclosure Statement
The costs of publication of this article may be defrayed, in part, by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC Section 1734. No potential conflicts of interest relevant to this article were reported.
References
- 1.American Cancer Society. Cancer Facts and Figures 2015. Available at www.cancer.org/research/cancerfactsfigures/index Accessed April16, 2015
- 2.Margolis DJ, Hoffman JM, Herfkens RJ, et al. Molecular imaging techniques in body imaging. Radiology 2007;245:333. [DOI] [PubMed] [Google Scholar]
- 3.U.S. National Institutes of Health, www.sbir:cancer.gov/funding/contracts/fy2013_12.asp Accessed December30, 2014
- 4.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kD cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 1985;40:191. [DOI] [PubMed] [Google Scholar]
- 5.Smith JW, Cheresh DA. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity crosslinking implicates amino acid residues 61–203 of the beta subunit. J Biol Chem 1988;263:18726. [PubMed] [Google Scholar]
- 6.McHugh BJ, Murdoch A, Haslett C, et al. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One 2012;7:e40346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhaofei L, Fan W, Xiaoyuan C. Integrin targeted delivery of radiotherapeutics. Theranostics 2011;1:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang BS, Lim E, Hee Park S, et al. Radiolabeled high affinity peptidomimetic antagonist selectively targets αvβ3 receptor-positive tumor in mice. Nucl Med Biol 2007;34:363. [DOI] [PubMed] [Google Scholar]
- 9.Shin IS, Maeng JS, Jang BS, et al. Tc-labeling of peptidomimetic antagonist to selectively target αvβ3 receptor-positive tumor: Comparison of PDA and EDDA as co-ligands. Curr Radiopharm 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reardon DA, Neyns B, Weller M, et al. Cilengitide: An RGD pentapeptide ανβ3 and ανβ5 integrin inhibitor in development for glioblastoma and other malignancies. Future Oncol 2011;7:339. [DOI] [PubMed] [Google Scholar]
- 11.Jeong JM, Hong MK, Chang YS, et al. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J Nucl Med 2008;49:830. [DOI] [PubMed] [Google Scholar]
- 12.Haubner R, Wester HJ, Reuning U, et al. Radiolabeled αvβ3 integrin antagonists: A new class of tracers for tumor targeting. J Nucl Med 1999;40:1061. [PubMed] [Google Scholar]
- 13.Haubner R, Beer AJ, Wang H, et al. Positron emission tomography tracers for imaging angiogenesis. Eur J Nucl Med Mol Imaging 2010;37(Suppl 1):S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittra ES, Goris ML, Iagaru AH, et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: A PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology 2011;260:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer AJ, Haubner R, Sarbia M, et al. Positron emission tomography using [18F]galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin Cancer Res 2006;12:3942. [DOI] [PubMed] [Google Scholar]
- 16.Kurdziel KA, Lindenberg L, Choyke PL. Oncologic angiogenesis imaging in the clinic—how and why. Imaging Med 2011;3:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumont RA, Deininger F, Haubner R, et al. Novel 64Cu- and 68Ga-labeled RGD conjugates show improved PET imaging of αvβ3 integrin expression and facile radiosynthesis. J Nucl Med 2011;52:1276. [DOI] [PubMed] [Google Scholar]
- 18.Pohle K, Notni J, Bussemer J, et al. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol 2012;39:777. [DOI] [PubMed] [Google Scholar]
- 19.Heroux J, Gharib AM, Danthi N, et al. High-affinity αvβ3 integrin targeted optical probe as a new imaging biomarker for early atherosclerosis: Initial studies in Watanabe rabbits. Mol Imaging Biol 2010;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knetsch PA, Petrik M, Griessinger CM, et al. 68Ga-NODAGA-RGD for imaging αvβ3 integrin expression. Eur J Nucl Med Mol Imaging 2011;38:1303. [DOI] [PubMed] [Google Scholar]
- 21.Gaertner FC, Kessler H, Wester HJ, et al. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging 2012;39(Suppl 1):S126. [DOI] [PubMed] [Google Scholar]
- 22.www.clinicaltrials.gov/ Accessed December30, 2014
- 23.Cho HJ, Lee YD, Park JY, et al. First in human evaluation of a newly developed PET tracer, 18F-RGD-K5 in patients with breast cancer: Comparison with 18F-FDG uptake pattern and microvessel density. J Nucl Med 2009;50(Suppl 2):1910 [Google Scholar]
- 24.Hanessian S, McNaughton-Smith G. Synthesis of a versatile peptidomimetic scaffold. Methods Mol Med 1999;23:161. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, Shen Z, Li KC, et al. Tumor angiogenic endothelial cell targeting by a novel integrin-targeted nanoparticle. Int J Nanomed 2007;2:479. [PMC free article] [PubMed] [Google Scholar]
- 26.Burnett CA, Xie J, Quijano J, et al. Synthesis, in vitro, and in vivo characterization of an integrin αvβ3-targeted molecular probe for optical imaging of tumor. Bioorg Med Chem 2005;13:3763. [DOI] [PubMed] [Google Scholar]
- 27.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumors: On the way to Theranostics. Dalton Trans 2011;40:6104. [DOI] [PubMed] [Google Scholar]
- 28.Parker D. Tumor targeting with radiolabeled macrocycle-antibody conjugates. Chem Soc Rev 1990;19:271 [Google Scholar]
- 29.Kim YS, New K, Milenic DE, et al. Synthesis and characterization of αvβ3-targeting peptidomimetic chelate conjugates for PET and SPECT imaging. Bioorg Med Chem Lett 2012;22:5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller D, Klette I, Baum RP, et al. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem 2012;23:1712. [DOI] [PubMed] [Google Scholar]
- 31.Schultz M, Mueller D, Baum RP, et al. A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl Radiat Isot 2013;76:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baum RP, Kulkarni HR. Theranostics: From molecular imaging using 68Ga labeled tracers and PET/CT to personalized radionuclide therapy—the Bad Berka experience. Theranostics 2012;2:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirsch M, Schackert G, Black PM. Metastasis and angiogenesis. Cancer Treat Res 2004;117:285. [DOI] [PubMed] [Google Scholar]
- 34.Lewis MR. Radiolabeled RGD peptides move beyond cancer: PET imaging of delayed-type hypersensitivity reaction. J Nucl Med 2005;46:2. [PubMed] [Google Scholar]