Abstract
Background: Iodinated contrast media (ICM) is a source of excess iodine that may induce thyroid dysfunction. A prospective cohort study was conducted to assess the effects of ICM on urinary iodine clearance and serum thyroid function tests (TFTs) in adults.
Methods: In this prospective cohort study of 54 adults undergoing elective computed tomography (CT) scans at an academic medical center, serial urinary iodine concentrations (UIC) and serum TFTs were obtained until UIC normalized following ICM administration. Thyroid volume/nodularity were assessed by ultrasound. Associations between covariates and peak UIC, duration for UIC to peak and normalize, and thyroid dysfunction risk were assessed.
Results: The mean±standard deviation (SD) iodine administered was 34.6±6.0 g. Baseline median (range) UIC was 105.1 (17.0–866.1) μg/L, and serum thyrotropin (TSH) concentration was 1.26 (0.5–11.2) mIU/L. The mean±SD times to achieve peak UIC (median [range]: 3519 [233–157,500] μg/L] and normalized UIC were 1.1±0.5 and 5.2±4.0 weeks, respectively. Four subjects had elevated baseline TSH, and one had missing baseline TSH values. Of the remaining 49 subjects, 11 (22%) developed an abnormal TSH within one to four weeks (six elevated and five decreased). Administered iodine amount correlated with peak UIC following ICM administration (p<0.001). Increasing age and administered iodine amount predicted peak UIC (p=0.024 and p<0.001, respectively). Age, sex, race/ethnicity, smoking status, family history of thyroid disease, personal or family history of thyroid autoimmunity, thyroid volume, presence of thyroid nodules ≥1 cm, iodine dose, baseline UIC, and baseline TFTs were not predictive of durations to achieve peak or normalized UIC.
Conclusion: Peak UIC occurred at 1.1 weeks and normalized by 5.2 weeks following ICM administration for outpatient CT scans. Because thyroid dysfunction developed in 22% of individuals following a single ICM dose, monitoring of thyroid function should be considered in at-risk patients.
Introduction
Iodine is an essential micronutrient for the thyroid gland to synthesize thyroid hormones. A normal adult utilizes about 80 μg/day of iodine to maintain adequate thyroid hormone production, which represents an intake of approximately 150 μg/day of iodine after accounting for fecal and urinary losses (1). The recommended daily allowance (RDA) of iodine by the U.S. Institute of Medicine and World Health Organization (WHO) is 150 μg/day for non-pregnant adults and 200–290 μg/day for pregnant or lactating women (2,3). With the increased use of computed tomography (CT) scans using iodinated contrast media (ICM) in recent decades, excess iodine exposure has increased. The use of CT scans increased by 19% annually in the United States between 1991 and 2002 (4), with approximately 70 million CT scans being performed annually by 2007 (5). Worldwide, it is estimated that 80 million doses of ICM are administered annually (6). ICM used for CT scans contain between 320 and 370 mg/mL of iodine. A typical dose of ICM contains approximately 15–37 g of total iodine, corresponding to several hundred thousand times the RDA. Most of the iodine in the commonly used water-soluble radiographic contrast media is in the form of organically bound iodine, and only a fraction of the iodine enters the body as a free iodide to be taken up by the thyroid gland. The upper limit of free iodide in water-soluble radiographic contrast media is generally <50 μg/mL, per production regulations. Still, the total amount of iodide in a typical dose of radiographic ICM for routine CT scans is much greater than the RDA, as many patients receive more than 50 mL of intravenous contrast media. In addition, 0.01–0.15% of the bound iodine in ICM can be further deiodinated in the body to produce additional free iodide after administration (7). The tolerable upper limit recommended by the U.S. Institute of Medicine for iodine is 1100 μg/day in adults (2).
Because 90% of dietary iodine is excreted in the urine, urinary iodine concentration (UIC) serves as a biomarker for recent iodine intake. Median population UIC can be assessed using spot urine samples and is recommended by the WHO for assessing iodine status in populations (3). Adequate iodine intake correlates with a median population UIC of 100–199 μg/L (8).
Iodine-induced thyroid dysfunction due to iodinated contrast agents has been described over the past several decades (9–15). Determining the effects of ICM on urinary iodine clearance and serum thyroid function remains important, as these have implications for planning thyroid radioiodine uptakes, scans, and therapy, as well as the need to monitor patients at risk for developing hyperthyroidism or hypothyroidism following exposure to ICM.
In 1948, Wolff and Chaikoff first described a transient inhibition of thyroid hormone synthesis in rats exposed to a large amount of intraperitoneal iodine, known as the acute Wolff–Chaikoff effect (16). However, normal thyroid hormone synthesis resumed in approximately 24–48 h, even with continued excess iodide exposure, a phenomenon described as the escape from the acute Wolff–Chaikoff effect (17). This escape phenomenon is thought to occur as a result of a decrease in expression of the sodium/iodine symporter (NIS), which is responsible for the active transport of iodine into thyroid follicular cells (18). Patients with underlying compromised thyroid function, such as those with a history of partial thyroidectomy, treated Graves' disease, postpartum or subacute thyroiditis, or Hashimoto's thyroiditis, may be susceptible to iodine-induced hypothyroidism resulting from the failure to escape from the acute Wolff–Chaikoff effect (13,14). Those with increased risk of thyroid autonomy, such as a history of nontoxic nodular goiter, mild Graves' disease, or residence in an area of endemic iodine deficiency, are at increased risk of developing iodine-induced hyperthyroidism upon exposure to excess iodine (14).
Recent observational studies have investigated the association between ICM exposure and the development of thyroid dysfunction. Two nested case-control studies by Rhee et al. conducted in Boston, Massachusetts, showed significant associations between ICM exposure and the development of overt and subclinical hyperthyroidism and overt hypothyroidism (15,19). These studies were limited by their retrospective designs, variability in intervals between serum thyroid function testing, and limited availability of peripheral serum thyroid hormone measurements.
There have been other limited studies of urinary iodine clearance following ICM exposure from iodinated CT scans. A prospective study by Padovani et al. in Brazil assessed urinary iodine clearance in 25 athyreotic patients at baseline, one week, and then monthly up to two months after ICM administration, and reported that UIC returned to baseline levels one month after exposure to iodinated contrast agents (20). Another prospective study by Nimmons et al. in the United States showed that the median duration for UIC to return to baseline among 21 patients without known thyroid dysfunction was approximately six weeks (21). A retrospective study by Sohn et al. in South Korea reported that there were no significant differences in UIC at one month compared to six months after ICM exposure in 1032 athyreotic patients who had CT scans with ICM prior to total thyroidectomy, suggesting that UIC returned to baseline by one month (22). However, the time period between ICM administration and total thyroidectomy is unclear in this study. Most recently, Ho et al. compared urinary iodine clearance between six athyreoitic patients and seven euthyroid controls in Canada, measuring urinary iodine clearance every two weeks for the first eight weeks, then monthly up to six months after CT scan with iodinated contrast. They found that urinary iodine clearance returned to baseline by four weeks, without any significance differences between the two groups (23). Iodine nutrition varies by region of the world, and iodine deficiency confers an additional risk for the development of thyroid dysfunction following iodine excess. There have been no prospective studies assessing the effects of ICM on both UIC and serum thyroid function in the United States, which is considered generally iodine sufficient (24).
In the present study, the aim was to conduct the largest prospective study on the effects of ICM on urinary iodine clearance and serum thyroid function in clinically euthyroid adults with intact thyroid glands, with assessment of other potential predictors such as thyroid volume, thyroid nodularity, and the presence of serum thyroid peroxidase (TPO) antibodies.
Materials and Methods
A prospective cohort study was conducted to determine the time required for UIC to peak and normalize and the risk of thyroid dysfunction following ICM exposure in 54 clinically euthyroid adults. Institutional Review Board approval for the study was obtained from Boston University Medical Campus and Boston Medical Center, where the study was conducted.
Study subjects
Adults at least 18 years of age scheduled for elective outpatient CT scans with intravenous contrast at the Boston Medical Center from March 2013 to February 2014 were included. Exclusion criteria were: (a) ICM administration within the past six months; (b) known baseline thyroid dysfunction; (c) current use of thyroid hormone or antithyroidal drug therapy; (d) use of amiodarone or other iodine-containing medications within the past two years; (e) lithium use within the past six months, since lithium can cause thyroid dysfunction; (f) current pregnancy or lactation; and (g) inability to provide informed consent because of language barrier or cognitive impairment. A total of 56 subjects were initially recruited, and two withdrew after obtaining consent. Two subjects did not complete the full four-week follow-up, but their data as available were included in the analyses. One subject was not included in the analyses involving thyroid function tests, as the baseline serum thyroid function tests were unavailable.
Study measures
Serum and spot urine samples were obtained from the subjects on the day of the CT scan prior to the administration of ICM. During this baseline visit, a questionnaire was administered to obtain the following data: age, sex, race/ethnicity, place of birth, family history of thyroid disease (thyroid dysfunction and/or thyroid nodules), personal and family history of autoimmune disease, smoking history, and medication list. Of the 54 subjects, 51 received Isovue 370 (iodine concentration: 370 mg iodine/mL) and three received Optiray 350 (iodine concentration: 350 mg iodine/mL). The amount of contrast received ranged from 70 to 100 mL per person.
Subjects returned for follow-up weekly for four weeks and provided spot urine samples and sera at each visit. The normalization of UIC was defined as a return to within 1.5 times the baseline levels or <164 μg/L, which was the 2004–2008 National Health and Nutrition Examination Survey (NHANES) median population UIC (24). From subjects in whom UIC did not normalize within the initial four weeks, urine and sera were obtained biweekly up to 10 weeks, and then every four weeks up to 24 weeks until UIC normalized. A brief questionnaire was administered at each follow-up visit to obtain information regarding any interim excess iodine exposure, any new medications started, or new medical diagnoses made since the last visit. Thyroid ultrasound was performed during the initial four weeks of the study to assess thyroid volume (as calculated by the sum of 0.479×length in cm×width in cm×thickness in cm of each lobe as presented by Brunn et al. (25,26)) and the presence of thyroid nodules.
Laboratory methods
Laboratory measurements of UIC and serum thyroid function were performed at the Boston University Iodine, Perchlorate, and Thyroid Function Test Research Laboratory. Sera were stored at −80°C until measurement. Serum thyrotropin (TSH), free thyroxine (fT4), total triiodothyronine (TT3), and TPO antibody levels were measured after completion of subject recruitment by enzyme-linked immunosorbent assay (ELISA; Immuno-Biological Laboratories, Inc., Minneapolis, MN and Calbiotech, Inc., Spring Valley, CA). The interassay CV for these methods is 4.2–7.3%. The reference ranges for the assays are: TSH 0.4–4.2 mIU/L, fT4 0.8–2.0 ng/dL, and TT3 0.52–1.85 ng/mL. The TPO antibody limit of detection was 5 IU/mL, and levels >75 IU/mL were considered positive. Thyroid function tests from all serum samples from each subject were measured in duplicate in the same assay. All thyroid function measurements were initially done using ELISA from Immuno-Biological Laboratories, Inc. There were three subjects who had elevated TSH and elevated TT3 values, suspected to be from the presence of heterophilic antibodies. TSH and TT3 of these subjects as well as TSH of 10 subjects with elevated baseline TSH values were re-measured using ELISA from Calbiotech, Inc. One of these three subjects did not have adequate baseline serum samples available for repeat measurements, and was subsequently excluded from final analyses involving thyroid function tests. Five randomly selected baseline TSH values of the subjects whose baseline TSH values were within the reference range using the first assay were also re-measured using the second assay to ensure correlation of values between the two assays. Spot UIC was measured in duplicate spectrophotometrically using a Technicon Autoanalyzer (Technicon Instrument, Inc., Tarrytown, NY) by a modification of the method by Benotti et al. (27).
Statistical analyses
Descriptive characteristics are reported as mean±standard deviation (SD) or median (range). Pearson's rank correlation and binomial analyses were used to determine univariate associations between baseline subject characteristics, amount of iodine received, thyroid volume, thyroid nodules ≥1 cm, baseline UIC, peak UIC, duration to reach peak UIC, duration to normalization of UIC, baseline TSH concentration, and development of incident thyroid dysfunction. Mixed effects models were used to assess potential changes in TSH, fT4, and TT3 during the study period for each subject. Subjects with abnormal serum thyroid function tests at baseline were excluded from the analyses assessing development of incident thyroid dysfunction and change in thyroid function over time.
Multivariate linear regression models were used to assess predictors (age, amount of iodine administered, thyroid volume, presence of thyroid nodules ≥1 cm, sex, race/ethnicity, smoking status, place of birth, personal or family history of autoimmune disease, family history of thyroid disease, baseline UIC, baseline TSH concentration, baseline fT4 concentration, and baseline TT3 concentration) of peak UIC, duration to achieve peak UIC, and duration to normalization of UIC. Multivariate logistic regression models were used to assess predictors (age, amount of iodine administered, thyroid volume, presence of thyroid nodules ≥1 cm, sex, baseline TSH concentration, baseline fT4 concentration, and baseline TT3 concentration) for the development of incident thyroid dysfunction.
All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC), and results were considered statistically significant if the two-tailed p-value was <0.05.
Results
Fifty-four subjects (Table 1) were included in the analyses, of whom 57% were men, 48% white, 37% black, and 11% Hispanic. On ultrasound, 15% of the subjects had a thyroid nodule ≥1 cm in the largest dimension. Only one subject had a positive TPO antibody titer.
Table 1.
Baseline Characteristics of Subjects
| Mean (SD) or median (range) | |
|---|---|
| Age (years) | 50.3 (12.6) |
| Amount of iodine administered (g) | 34.6 (6.0) |
| UIC at baseline (μg/L) | 105.1 (17.0–866.1) |
| TSH at baseline (mIU/L)* | 1.26 (0.5–11.2) |
| fT4 at baseline (ng/dL)* | 0.83 (0.47–1.34) |
| TT3 at baseline (ng/mL)* | 1.40 (0.71–2.34) |
| # (%) | |
| Sex | |
| Male | 31 (58) |
| Female | 22 (42) |
| Race/ethnicity | |
| White | 25 (47) |
| Black | 20 (38) |
| Hispanic | 6 (11) |
| Other | 2 (4) |
| Place of birth | |
| United States | 41 (77) |
| Caribbean Islands | 7 (13) |
| Other | 5 (10) |
| Current cigarette smoking status | |
| Non-smoker | 29 (55) |
| Smoker | 24 (45) |
| History of autoimmune disease | |
| No | 50 (94) |
| Yes | 3 (6) |
| Family history of thyroid disease | |
| No | 44 (83) |
| Yes | 9 (17) |
| Family history of autoimmune disease | |
| No | 48 (91) |
| Yes | 5 (9) |
| Number of thyroid nodules >1 cm | |
| 0 | 45 (85) |
| ≥1 | 8 (15) |
n=54 except *n=51, excluding those with likely thyroid function test interferences.
UIC, urinary iodine concentration; TSH, thyrotropin; fT4, free thyroxine; TT3, total triiodothyronine.
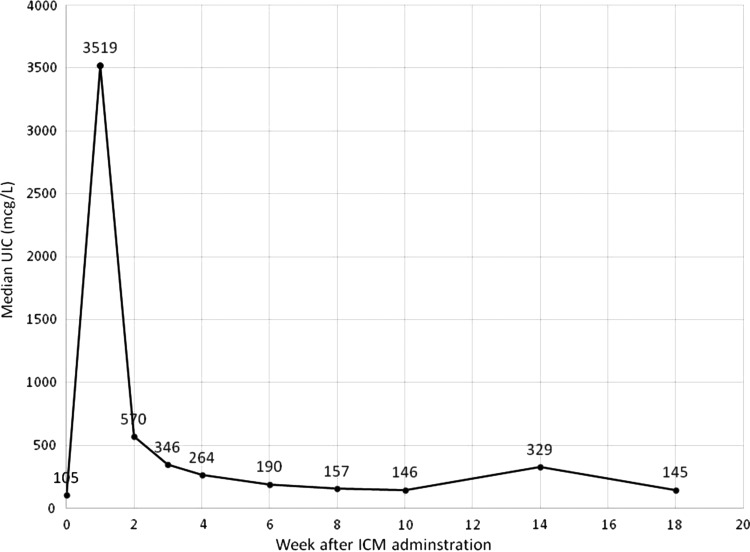
The mean±SD age of the subjects was 50.3±12.6 years. The mean±SD amount of iodine administered for the CT scans was 34.6±6.0 g. The baseline median (range) UIC was 105 (17–866) μg/L, which peaked at a median of 3519 (233–157,500) μg/L. Median UIC by time (weeks) following ICM administration is shown in Figure 1. The mean±SD duration to achieve peak UIC was 1.1±0.5 weeks. Mean±SD duration for UIC to normalize was 5.2±4.0 weeks. Baseline median (range) serum TSH concentration was 1.26 (0.5–11.2) mIU/L, fT4 0.83 (0.47–1.34) ng/dL, and TT3 1.40 (0.71–2.34) ng/mL. Serum TSH, fT4, and TT3 levels did not change significantly over the study period (p=0.96, 0.82, and 1.00, respectively).
FIG. 1.
Median urinary iodine concentrations following iodinated contrast media administration. UIC, urinary iodine concentrations; ICM, iodinated contrast media. Week 0 represents baseline UIC before administration of ICM.
Of the 54 subjects, two were not included in the assessment of baseline thyroid function due to likely interference in the TSH and TT3 assays, presumably from heterophilic antibodies (measured TSH concentrations: 8.3 and 5.6 mIU/L; measured TT3 concentrations: 4.8 and 2.5 ng/mL, respectively). One subject was not included in analyses involving thyroid function tests because of lack of baseline thyroid function values on repeat measurements using the second assay. Two other subjects had elevated baseline TSH values (11.2 and 8.7 mIU/L) but normal fT4 and TT3 concentrations.
Of the remaining 49 subjects, 11 (22%) developed an abnormal TSH concentration within one to four weeks after ICM administration: five developed suppressed TSH concentrations (range 0.05–0.31 mIU/L), and six developed slightly elevated serum TSH concentrations (range 4.50–5.60 mIU/L). Of the five subjects with suppressed serum TSH concentrations, one developed normal fT4 but elevated TT3 levels, two developed slightly low fT4 and normal TT3 levels, and two had normal fT4 and TT3 levels. Of the six subjects who developed elevated serum TSH concentrations, four had low serum fT4 values, and two continued to have normal serum fT4 concentrations throughout the study period. Serum TT3 concentrations remained normal in all six subjects. Two of the subjects who developed suppressed TSH concentrations (one with an elevated TT3 level and one with normal fT4 and TT3 levels) had persistently suppressed TSH concentrations at the end of follow-up at four weeks after ICM administration. The serum thyroid function tests normalized in all of the remaining nine patients who developed abnormal TSH concentrations after ICM administration by the end of their follow-up. None of the subjects was treated with thyroid hormone replacement or antithyroid medications during the study period.
There were no significant correlations between age, amount of iodine received, thyroid volume, baseline UIC, peak UIC, duration to achieve peak UIC, baseline TSH, and the duration to normalization of UIC (Table 2). There was a moderate positive correlation between amount of iodine administered and peak UIC (r=0.649; p<0.001). There was a moderate negative correlation between baseline TSH concentrations and time to achieve peak UIC (r=−0.283; p=0.049), but no significant correlations were found between age, amount of iodine received, thyroid volume, baseline UIC, peak UIC, duration to achieve peak UIC, duration to normalization of UIC, and development of thyroid dysfunction (Table 3).
Table 2.
Univariate Correlations Between Covariates and Duration to Normalization of UIC After ICM Administration
| Covariates | Correlation coefficient | p |
|---|---|---|
| Age (years) | 0.024 | 0.87 |
| Amount of iodine received (g) | −0.207 | 0.137 |
| Thyroid volume (cm3) | 0.017 | 0.90 |
| Baseline UIC (μg/L) | −0.219 | 0.116 |
| Peak UIC (μg/L) | −0.047 | 0.74 |
| Duration to peak UIC (weeks) | 0.026 | 0.86 |
| Baseline TSH (μIU/L)* | 0.075 | 0.61 |
n=53 except *n=49.
ICM, iodinated contrast media.
Table 3.
Univariate Correlations Between Covariates and Development of Incident Thyroid Dysfunction After ICM Administration
| Covariates | Correlation coefficient | p |
|---|---|---|
| Age (years) | 0.047 | 0.75 |
| Amount of iodine received (g) | 0.096 | 0.51 |
| Thyroid volume (cm3) | −0.091 | 0.53 |
| Baseline UIC (μg/L) | −0.143 | 0.33 |
| Peak UIC (μg/L) | −0.056 | 0.70 |
| Duration to peak UIC (weeks) | −0.056 | 0.70 |
| Duration to normalization of UIC (weeks) | 0.076 | 0.60 |
| Baseline TSH (μIU/L) | 0.194 | 0.18 |
n=49.
Age and amount of administered iodine were significant predictors of peak UIC (p=0.024 and <0.001, respectively; Table 4). There were no significant predictors of the duration to achieve peak UIC (p=0.55), duration to normalization of UIC (p=0.44), and incident thyroid dysfunction (p=0.29).
Table 4.
Multivariate Linear Regression Model Predicting Peak UIC After ICM Administration (Overall Model p=0.035; R2=0.67)
| Predictors | F-value | p-Value |
|---|---|---|
| Age (years) | 5.76 | 0.024* |
| Amount of iodine received (g) | 23.26 | <0.001* |
| Thyroid volume (cm3) | 0.09 | 0.77 |
| Presence of nodules ≥1 cm | 0.19 | 0.67 |
| Sex | 0.94 | 0.34 |
| Race/ethnicity | 0.37 | 0.77 |
| Smoking status | 0.00 | 0.98 |
| Place of birth | 0.65 | 0.76 |
| History of autoimmune disease | 0.72 | 0.40 |
| Family history of thyroid disease | 0.53 | 0.47 |
| Family history of autoimmune disease | 4.06 | 0.05 |
| Baseline UIC (μg/L) | 0.05 | 0.83 |
| Baseline TSH (μIU/L) | 0.10 | 0.76 |
| Baseline fT4 (ng/dL) | 0.02 | 0.90 |
| Baseline TT3 (ng/mL) | 0.33 | 0.57 |
n=53.
Statistically significant at two-tailed p-value <0.05.
Discussion
There have been only a few studies assessing the rate of urinary iodine clearance after radiographic iodinated contrast administration (20–23). The interest in the rate of urinary iodine clearance after ICM administration has been primarily for the timing of radioactive iodine ablation after thyroidectomy for thyroid cancer and for treating patients with hyperthyroidism with radioactive iodine. Two of the three studies assessed urinary iodine clearance only in athyreotic patients after total thyroidectomies (20,22). Although Ho et al. found no significant difference in urinary iodine clearance after ICM administration between athyreotic patients and euthyroid patients with intact thyroid glands, the overall sample size was small with a total of 13 patients (23). The recent study by Nimmons et al. is the largest data set available among individuals with intact thyroid glands (21). Nimmons et al. conducted a prospective study on 21 clinically euthyroid subjects residing in the United States with UIC measurement every two weeks after ICM administration up to 12 weeks. They reported the median time for normalization of UIC was 43 days, with 90% of subjects returning to baseline by 75 days (21). The time to normalize UIC was longer in the study by Nimmons et al. compared to the other two studies, likely because the subjects had intact thyroid glands, which concentrate iodine and thus retain total body iodine for a longer period of time.
One limitation of the present study is the lack of data on the subjects' thyroid radioactive iodine uptake, which would have added information on the iodine saturation status in the thyroid gland to correlate with the measured UIC. However, UIC is a good measure of recent iodine exposure, and with close follow-up of UIC in the study subjects, the data provide a good representation of the thyroidal iodine status after ICM administration.
The present study represents the largest number of individuals studied prospectively to assess urinary iodine clearance after ICM administration. The mean time to normalization of UIC in this study was 5.2 weeks, similar to the 43 days reported by Nimmons et al. (21). Subjects were followed until UICs were normalized, which required up to 18 weeks in some individuals. However, there were no significant associations between covariates and the duration to normalization of UIC. The baseline median UIC in the sample was 105.6 μg/L, lower than the most recent NHANES U.S. population median UIC of 164 μg/L (24), but still representing overall iodine sufficiency of the study population. The majority of subjects achieved peak UIC one week after ICM administration, with the median peak UIC at 3518.9 μg/L, but the peak UIC in some subjects were as high as 157,500 μg/L after administration of ICM for a routine CT scan.
Although the study was not powered to assess the development of incident thyroid dysfunction, 22% (11) of the subjects who did not have abnormal serum thyroid function at baseline developed an abnormal TSH over the course of the study. Among those who developed incident thyroid dysfunction, four of six subjects who developed an elevated serum TSH also had a low serum fT4 concentration, consistent with overt hypothyroidism. The remaining two subjects had subclinical hypothyroidism with isolated TSH elevations and no decrease in fT4. One subject developed T3 thyrotoxicosis with suppressed serum TSH, elevated TT3, and normal fT4 levels. Four subjects developed subclinical hyperthyroidism with suppressed serum TSH only. One subject who developed T3 thyrotoxicosis and one of four subjects who developed subclinical hyperthyroidism had a suppressed serum TSH at the end of the study follow-up (week 4 for both subjects); the serum thyroid function tests of the remaining nine subjects who developed incident thyroid dysfunction returned to normal by the end of the study follow-up period.
In summary, peak UIC occurred at a mean of 1.1 weeks and normalized by a mean of 5.2 weeks after administration of ICM for routine CT scans in clinically euthyroid patients with intact thyroid glands. Although previous studies of athyreotic patients showed that the UIC returned to baseline by one month after ICM administration, it would be prudent to wait at least two months before radioactive iodine is given for thyroid scans or therapy. As thyroid dysfunction developed in 22% of subjects following a single ICM dose, monitoring of thyroid function should be considered in at-risk patients.
Acknowledgments
This research was supported by the Thyroid Research Fund at BMC, BU-CTSI Grant NIH U54TR001012, NIH T32DK007201-37 (S.Y.L.), and NIH K23HD068552 (A.M.L.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would like to thank the radiology department at Boston University Medical Center and the staff at General Clinical Research Unit at Boston University Medical Center for their help in conducting this study. The abstract and the results of this study were presented as a poster at the 84th Annual Meeting of American Thyroid Association, October 29–November 2, 2014, Coronado, CA, and won the 2014 Trainee Poster Contest in Clinical Research Award.
Author Disclosure Statement
The authors disclose no conflict of interest.
References
- 1.Glinoer D.2004The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab 18:133–152 [DOI] [PubMed] [Google Scholar]
- 2.Otten JJ, Hellwig JP, Meyers LD.2006DRI, Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academies Press, Washington, DC [Google Scholar]
- 3.WHO, UNICEF, ICCIDD 2007 Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. Third edition. Available at: http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf (accessed September19, 2014)
- 4.Kalra MK, Maher MM, D'Souza R, Saini S.2004Multidetector computed tomography technology: current status and emerging developments. J Comput Assist Tomogr 28:S2–6 [DOI] [PubMed] [Google Scholar]
- 5.Smith-Bindman R, Lipson J, Marcus R, Kim K-P, Mahesh M, Gould R, Berrington de González A, Miglioretti DL.2009Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169:2078–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson PB.2005Editorial: contrast medium-induced nephropathy. Nephrol Dial Transplant 20:i1. [DOI] [PubMed] [Google Scholar]
- 7.Molen AJ, van der Thomsen HS, Morcos SK.2004Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 14:902–907 [DOI] [PubMed] [Google Scholar]
- 8.Andersson M, Karumbunathan V, Zimmermann MB.2012Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–750 [DOI] [PubMed] [Google Scholar]
- 9.Conn JJ, Sebastian MJ, Deam D, Tam M, Martin FIR 1996 A prospective study of the effect of nonionic contrast media on thyroid function. Thyroid 6:107–110 [DOI] [PubMed] [Google Scholar]
- 10.Fassbender WJ, Vogel C, Doppl W, Stracke H, Bretzel RG, Klör HU.2001Thyroid function, thyroid immunoglobulin status, and urinary iodine excretion after enteral contrast-agent administration by endoscopic retrograde cholangiopancreatography. Endoscopy 33:245–252 [DOI] [PubMed] [Google Scholar]
- 11.Gartner W, Weissel M.2004Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week? Thyroid 14:521–524 [DOI] [PubMed] [Google Scholar]
- 12.Martin FIR, Tress BW, Colman PG, Deam DR.1993Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med 95:78–82 [DOI] [PubMed] [Google Scholar]
- 13.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG.2001Iodine-induced hypothyroidism. Thyroid 11:501–510 [DOI] [PubMed] [Google Scholar]
- 14.Braverman LE.1994Iodine and the thyroid: 33 years of study. Thyroid 4:351–356 [DOI] [PubMed] [Google Scholar]
- 15.Rhee CM, Bhan I, Alexander EK, Brunelli SM.2012Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 172:153–159 [DOI] [PubMed] [Google Scholar]
- 16.Wolff J, Chaikoff IL.1948The inhibitory action of iodide upon organic binding of iodine by the normal thyroid gland. J Biol Chem 172:855. [PubMed] [Google Scholar]
- 17.Wolff J, Chaikoff IL.1949The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology 45:504–513, illust [DOI] [PubMed] [Google Scholar]
- 18.Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, Chin WW, Braverman LE.Escape from the acute Wolff–Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 140:3404–3410 [DOI] [PubMed] [Google Scholar]
- 19.Rhee CM, Lynch KE, Zandi-Nejad K, Pearce EN, Alexander EK, Brunelli SM. 2013Iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism in a community-based cohort. Endocrinol Stud 3:e8:27–30 [Google Scholar]
- 20.Padovani RP, Kasamatsu TS, Nakabashi CCD, Camacho CP, Andreoni DM, Malouf EZ, Marone MMS, Maciel RMB, Biscolla RPM.2012One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid 22:926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimmons GL, Funk GF, Graham MM, Pagedar NA.2013Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg 139:479–482 [DOI] [PubMed] [Google Scholar]
- 22.Sohn SY, Choi JH, Kim NK, Joung JY, Cho YY, Park SM, Kim TH, Jin SM, Bae JC, Lee SY, Chung JH, Kim SW.2014The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid 24:872–877 [DOI] [PubMed] [Google Scholar]
- 23.Ho JD, Tsang JF, Scoggan KA, Leslie WD.2014Urinary iodine clearance following iodinated contrast administration: a comparison of euthyroid and postthyroidectomy subjects. J Thyroid Res 2014:580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY.2011Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21:419–427 [DOI] [PubMed] [Google Scholar]
- 25.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC.1981[Volumetric analysis of thyroid lobes by real-time ultrasound (author's transl)]. Dtsch Med Wochenschr 106:1338–1340 [DOI] [PubMed] [Google Scholar]
- 26.Ying M, Yung DMC, Ho KKL.2008Two-dimensional ultrasound measurement of thyroid gland volume: a new equation with higher correlation with 3-D ultrasound measurement. Ultrasound Med Biol 34:56–63 [DOI] [PubMed] [Google Scholar]
- 27.Benotti J, Benotti N, Pino S, Gardyna H.1965Determination of total iodine in urine, stool, diets, and tissue. Clin Chem 11:932–936 [PubMed] [Google Scholar]