Abstract
MicroRNA-15b (miR-15b) has been demonstrated to suppress proliferation by arresting cell cycle progression and inducing apoptosis in glioma cells. However, the prognostic value of miR-15b expression in human gliomas remains unclear. In the present study, the authors examined the expression profile in glioma specimens and the prognostic value of miR-15b in patients with gliomas. Real-time polymerase chain reaction assay was employed to detect the expression levels of miR-15b in 92 glioma tissues categorized by World Health Organization (WHO) histopathological grades. However, the prognostic value of miR-15b in human glioma has not been evaluated yet. MiR-15b expression in human glioma tissues was distinctly lower than in normal brain tissues. Furthermore, the expression of miR-15b notably decreased with the ascending histopathological grade of gliomas. Additionally, Kaplan–Meier survival analysis showed that low miR-15b expression was associated with poor overall survival in patients with gliomas. Similarly, miR-15b reduction occurred with increasing frequency in glioma patients with lower Karnofsky performance scale (KPS) scores than in those with higher KPS scores. No significant difference was observed between miR-15b expression and gender, age, and tumor location. These findings revealed that a lower expression level of miR-15b was closely related to a shorter overall survival, suggesting that miR-15b could be an intrinsic factor that plays an important role in the malignant progression of gliomas.
Key words: : glioma, microRNA, miR-15b, overall survival
Introduction
Gliomas are the most common malignant primary brain tumors in humans, accounting for 1.9% of all new cancer cases and 2.3% of cancer deaths worldwide.1 In the past few decades, despite great advances in clinical treatments, including surgery, operative navigation system, chemotherapy, and radiotherapy, the unfavorable prognosis of patients with gliomas has not improved significantly. For example, glioblastoma is the most malignant glioma with a median survival of 14.6 months and a 9.8% 5-year survival.2 Thus, it is highly necessary to explore novel biomarkers for the early identification of a more effective, clinical therapeutic strategy against malignant gliomas.
MiRNAs are small, highly conserved, noncoding RNA molecules of ∼18–24 nucleotides in length that negatively regulate gene expression by binding to the 3′untranslated region of targeted messenger RNA (mRNA).3 Recently, emerging studies have indicated that miRNAs play a key role in multiple cellular biological processes, including cell differentiation, proliferation, apoptosis, metabolism, and angiogenesis. Furthermore, the functions of miRNAs involved in the initiation and progression of human cancers have been extensively verified. There is no doubt that miRNAs could function as oncogenic miRNAs or tumor suppressor miRNAs through pairing with the corresponding mRNAs.4 As an oncogenic miRNA, the effect of miR-21 involved in diverse cancers has been well established.5–7 Recently, Wu et al. analyzed 152 pairs of human gliomas and non-neoplastic brain tissues to evaluate the miR-21 expression level using real-time polymerase chain reaction (PCR) and found that overexpression of miR-21 in gliomas might be correlated with poor outcome.8 The results suggested that miR-21 was more highly expressed in glioma tissues compared to the corresponding non-neoplastic brain tissues. MiR-21 expression was significantly associated with high pathological grades and lower Karnofsky performance scores of glioma patients. Many miRNAs have been reported to be oncogenic miRNAs or tumor suppressor miRNAs in gliomas, including miR-10b, miR-137, miR-145, miR-125b, and others.9–13 Given that miRNAs are highly conserved, they could be used as signatures in cancer diagnosis. In fact, MiR-34a, miR-203, miR-372, and miR-17 were identified as independent diagnostic, and prognostic biomarkers in patients with gliomas, in particular, those with high pathological grades.14–17
Previously, the authors showed that overexpression of microRNA-15b (miR-15b) inhibited proliferation by arresting cell cycle progression and inducing apoptosis by directly targeting cyclin D1 in gliomas.18 Those results correspond to the findings of other studies. Xia et al. reported that overexpression of miR-15b resulted in cell cycle arrest at the G0/G1 phase, whereas suppression of the miR-15b expression resulted in a simultaneous decrease in cell populations in G0/G1 and increase in cell populations in the S phase in gliomas.19 Zheng et al. reported that miR-15b reduced glioma cell invasion and angiogenesis through NRP and MMP-3. These results show that there is a close relationship between miR-15b and gliomagenesis.20 However, the clinical significance of miR-15b in human gliomas has not been elucidated. Therefore, in the present study, they explored the diagnostic and prognostic value of miR-15b in glioma tissues of different World Health Organization (WHO) grades.
Materials and Methods
Tumor specimens
The study was approved by the Ethics Committee of Nantong University. Informed consent was acquired from patients in advance. All specimens were handled and made anonymous according to ethical and legal standards.
A total of 92 glioma specimens were obtained at the Fifth Affiliated Hospital of Nantong University from May 2006 to May 2011. All human glioma tissues were collected at the time of surgery and were categorized according to the WHO classification. The tissue specimens included 29 grade I–II tumors, 39 grade III tumors, and 24 grade IV tumors (glioblastomas). None of the patients received chemotherapy and radiotherapy before initial surgical treatment. Twelve normal brain tissues were obtained from patients who underwent internal decompression for traumatic brain injury. All tissues were snap-frozen in liquid nitrogen and stored at −80°C for the real-time quantitative RT-PCR assay. The clinicopathological features, including the Karnofsky performance scale (KPS) score, of all patients are listed in Table 1. Males and females were enrolled in the present study, ranging from 7 to 74 years of age with a mean age of 45 years.
Table 1.
Relationship Between miR-15b Expression Level in Glioma Specimens and Gender, Age, Tumor Location, KPS Score and WHO Grade
| miR-15b expression | ||||
|---|---|---|---|---|
| Clinical features | No. of case | High (n, %) | Low (n, %) | p-Value |
| Gender | 0.821 | |||
| Male | 58 | 27 | 31 | |
| Female | 34 | 15 | 19 | |
| Age | 0.763 | |||
| <55 | 41 | 18 | 23 | |
| ≥55 | 51 | 24 | 27 | |
| Tumor location | 0.641 | |||
| Frontal | 35 | 16 | 19 | |
| Temporal | 29 | 15 | 14 | |
| Other | 28 | 11 | 17 | |
| KPS | 0.001 | |||
| <80 | 52 | 16 | 36 | |
| ≥80 | 40 | 26 | 14 | |
| WHO grade | <0.001 | |||
| WHO I–II | 29 | 25 | 4 | |
| WHO III | 39 | 17 | 22 | |
| WHO IV | 24 | 0 | 24 | |
KPS, Karnofsky performance scale; miR-15b, microRNA-15b; WHO, World Health Organization.
For the analysis of survival and follow-up, the date of surgery was used to represent the beginning of the follow-up. Follow-up information for all patients was obtained every 3 months by telephone until August 2013. All patients who died from other diseases or from unexpected events were excluded from the data collection.
Quantitative real-time PCR analysis of miR-15b
Total RNA was extracted from clinical tissues and transfected cells using the Trizol Reagent (Invitrogen). The ABI 7300 HT Sequence Detection system (Applied Biosystems) was used for TaqMan-based real-time reverse transcription–polymerase chain reaction (RT-PCR) assays to detect the relative levels of miR-15b in glioma samples. MiR-15b primers and probes for TaqMan miRNA assays were purchased from Applied Biosystems. The quantitative miR-15b expression data were calculated using a 2−ΔΔCt method.
Statistics analysis
Data were analyzed using SPSS 13.0 statistical software. Data are expressed as the mean±standard deviation. The t-test and chi-square tests were used to evaluate the significant differences among the groups with different clinicopathological data. Kaplan–Meier analysis was employed to assess survival according to the miR-15b expression. Differences with p<0.05 were considered to be statistically significant.
Results
Downregulation of miR-15b in human glioma tissues
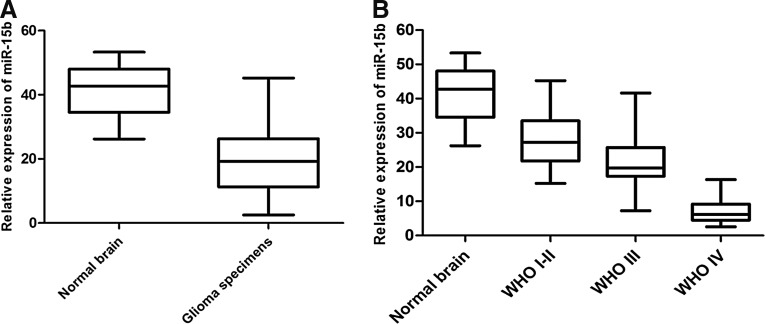
The real-time PCR assay was employed to detect the expression level of miR-15b in 92 human glioma specimens and 12 normal brain tissues. As shown in Figure 1A, miR-15b expression in human glioma tissues was distinctly lower than in normal brain tissues (p<0.01). Moreover, the expression of miR-15b in both grade III and IV gliomas tissues was significantly lower than in grade I–II gliomas (p<0.01), suggesting that there was a significant negative correlation between the miR-15b expression level and malignant magnitude of gliomas (Fig. 1B).
FIG. 1.
MicroRNA-15b (miR-15b) expression in glioma tissues and normal brain tissues was measured by real-time polymerase chain reaction assay. (A) Expression levels of miR-15b in glioma specimens and normal brain tissues. (B) Expression levels of miR-15b in 92 glioma specimens with different histopathological grades and normal brain tissues.
Association of miR-15b expression with the clinicopathological characteristics of human gliomas
To study the relationship of miR-15b expression and clinicopathological characteristics of human gliomas, clinical follow-up was performed for all patients (Table 1). According to the relative expression of miR-15b, 92 glioma specimens were divided 2 groups: glioma tissues with miR-15b expression less than the median expression level (19.56) were assigned to the low expression group (mean expression value 11.62, n=48); glioma tissues with miR-15b expression higher than the median expression level were assigned to the high expression group (mean expression value 28.22, n=44). As shown in Table 1, there was a significant association between miR-15b and the histopathological grade of glioma (p<0.001). A lower expression of miR-15b was seen more frequently in grade III–IV gliomas, whereas a higher expression of miR-15b was seen more commonly in grade I–II gliomas. Similarly, miR-15b reduction occurred with increasing frequency in glioma patients with lower KPS scores than in those with higher KPS scores (p=0.001). In addition, no significant differences were observed between miR-15b expression and gender, age, or tumor location.
Significant prognostic value of miR-15b expression in glioma patients
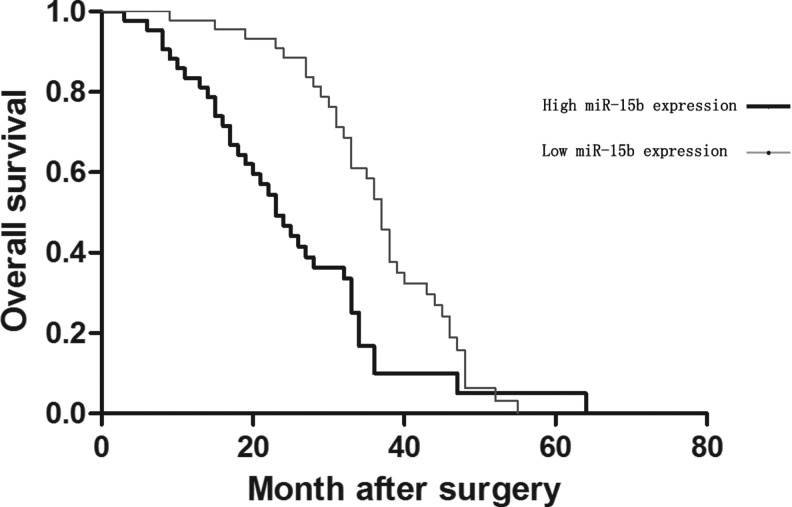
To determine the correlation between miR-15b and prognosis of patients with gliomas, the authors reviewed the clinical follow-up data for all 92 glioma patients. The univariate analysis strongly suggested a significant difference between the miR-15b high expression group and the low expression group (p=0.001; Table 2). Patients with high miR-15b expression had a clearly longer survival time than those with low miR-15b expression (Fig. 2). In addition, analysis using the Cox proportional hazards model revealed that miR-15b, WHO grade, and KPS score were closely correlated with overall survival and these were independent prognostic factors for patients with gliomas.
Table 2.
Univariate and Cox Multivariable Analysis of Prognostic Parameters for Patients with Gliomas
| Variables | Univariate log-rank test (p) | Cox multivariable analysis (p) | Relative risk (RR) |
|---|---|---|---|
| Gender | |||
| Male vs. female | 0.41 | — | — |
| Age | |||
| <55 vs. ≥55 | 0.54 | — | — |
| KPS | |||
| <80 vs. ≥80 | 0.04 | — | — |
| WHO grade | |||
| I–II vs. III–IV | 0.001 | — | — |
| MiR-15b expression | |||
| High vs. low | 0.001 | 0.003 | 16.76 |
FIG. 2.
Kaplan–Meier survival curves for miR-15b expression. Patients with low expression of miR-15b had a significantly worse outcome than the patients with high expression of miR-15b.
Discussion
Despite the standard treatment of gliomas, which consists of maximal safe surgical resection in combination with radiotherapy and chemotherapy, the clinical outcome of patients with gliomas remains poor. Thus, there is an urgent need to explore novel diagnostic, prognostic, and therapeutic modalities arising from the molecular and genetic level for this lethal disease. To date, the diagnosis and treatment of glioma have been based on histopathology, with grading according to the WHO classification system.21,22 With the rapid development of sequencing technologies and large-scale gene expression profiling, identification of novel prognostic biomarkers and therapeutic targets has better elucidated disease mechanisms resulting in glioma. Several genes are well known to be closely correlated to the treatment success and prognosis in patients with gliomas, such as O6-methylguanine DNA-methyltransferase (MGMT) promoter methylation and loss of chromosomes 1p and 19q,8 and mutations in the isocitrate dehydrogenase 1 (IDH1) and IDH2 genes.23–26 With the extensive understanding of miRNAs in gliomagenesis, an increasing number of miRNAs as potential prognostic biomarkers and therapeutic targets have been identified.
MiR-15b is one of the comprehensively studied miRNAs in diverse human tumors. The authors have elucidated that miR-15b can inhibit the proliferation of and promote apoptosis in glioma cells in vitro.18 In the present study, they investigated miR-15b expression in 92 cases of human gliomas and also investigated the relationship between miR-15b expression and clinicopathologic characteristics, such as gender, age, tumor location, KPS score, and WHO grade. The results showed that the expression of miR-15b was significantly decreased in glioma tissues compared to normal brain tissues, which is consistent with previous reports. Furthermore, they found an elevated expression level of miR-15b with decreasing grade of gliomas, suggesting that miR-15b might function as an antioncogene during the progression of human glioma tumorigenesis. However, the decreased expression of miR-15b was strongly related to the KPS score and WHO grade. There were no significant differences between the miR-15b expression level and other clinical features such as gender or age.
With respect to the clinical outcome of patients with gliomas, it is currently recognized that the WHO grade is closely related to prognosis. However, the survival time varies greatly even within the same pathological stage. Therefore, identification of novel prognostic biomarkers and therapeutic targets specific for gliomas might provide a potential breakthrough in improving prognosis and survival. In this finding, the lower expression level of miR-15b was closely related to a shorter overall survival, suggesting that miR-15b could be an intrinsic factor that plays an important role in the malignant progression of gliomas.
In combination with the results of previous studies, these data further elucidate the clinical significance of miR-15b in gliomas and show that miR-15b might be a potential independent prognostic factor for this lethal disease. However, the mechanism involved in tumorigenesis and the biological functions of miR-15b merit further evaluation.
Acknowledgments
All authors have declared all sources of funding for the research reported in this article and have no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest. This work was supported by the China Natural Science Foundation (81201976, 81000963, 81370062), the Jiangsu Province's Natural Science Foundation (BK2012670, BK20141256), the Jiangsu Province's Health Department (z201318), the Yancheng Medical Science Development Foundation (YK2013003, YK2013019), the Jiangsu Province's 333 Talent Program (BRA2011046), and the Kunshan Social Development Foundation (grant number: KS1006, KS1009).
Disclosure Statement
No competing financial interests exist.
References
- 1.Li M, Li J, Ding X, et al. . MicroRNA and cancer. AAPS J 2010;12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459. [DOI] [PubMed] [Google Scholar]
- 3.Gargalionis AN, Basdra EK. Insights in microRNAs biology. Curr Top Med Chem 2013;13:1493. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857. [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Choudhury SN, Hua X, et al. . Interaction of the oncogenic miR-21 microRNA and the p53 tumor suppressor pathway. Carcinogenesis 2013;34:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang BG, Li JF, Yu BQ, et al. . microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep 2012;27:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nouraee N, Van Roosbroeck K, Vasei M, et al. . Expression, tissue distribution and function of miR-21 in esophageal squamous cell carcinoma. PLoS One 2013;8:e73009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, Li G, Feng D, et al. . MicroRNA-21 expression is associated with overall survival in patients with glioma. Diagn Pathol 2013;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Yan W, Wang Y, et al. . MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res 2011;1389:9. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Cao Y, Shi L, et al. . Overexpressed miRNA-137 inhibits human glioma cells growth by targeting Rac1. Cancer Biother Radiopharm 2013;28:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan Y, Sun G, Zhang S, et al. . MicroRNA-125b inhibitor sensitizes human primary glioblastoma cells to chemotherapeutic drug temozolomide on invasion. In Vitro Cell Dev Biol Anim 2013;49:599. [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Wan Y, Sun G, et al. . Functional differences of miR-125b on the invasion of primary glioblastoma CD133-negative cells and CD133-positive cells. Neuromolecular Med 2012;14:303. [DOI] [PubMed] [Google Scholar]
- 13.Shi L, Wang Z, Sun G, et al. . miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med 2014;16:517. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Zhang Z, Tu Y, et al. . Correlation of microRNA-372 upregulation with poor prognosis in human glioma. Diagn Pathol 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S, Wang S, Geng S, et al. . Increased expression of microRNA-17 predicts poor prognosis in human glioma. J Biomed Biotechnol 2012;2012:970761 DOI: 10.1155/2012/970761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Zhao H, Xiang W. Expression level of human miR-34a correlates with glioma grade and prognosis. J Neurooncol 2013;113:221. [DOI] [PubMed] [Google Scholar]
- 17.He J, Deng Y, Yang G, et al. . MicroRNA-203 down-regulation is associated with unfavorable prognosis in human glioma. J Surg Oncol 2013;108:121. [DOI] [PubMed] [Google Scholar]
- 18.Sun G, Shi L, Yan S, et al. . MiR-15b targets Cyclin D1 to regulate proliferation and apoptosis in glioma cells. Biomed Res Int 2014;2014:687826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H, Qi Y, Ng SS, et al. . MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun 2009;380:205. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Chopp M, Lu Y, et al. . MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett 2013;329:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) classification of tumours of the central nervous system: Newly codified entities. Brain Pathol 2007;17:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurnher MM. 2007 World Health Organization classification of tumours of the central nervous system. Cancer Imaging 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Bent MJ, Gravendeel LA, Gorlia T, et al. . A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: A report from EORTC study 26951. Clin Cancer Res 2011;17:7148. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep 2013;13:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wick W, Weller M, van den Bent M, et al. . MGMT testing-the challenges for biomarker-based glioma treatment. Nat Rev Neurol 2014;10:372. [DOI] [PubMed] [Google Scholar]