Abstract
Fracture nonunions represent one of many large bone defects where current treatment strategies fall short in restoring both form and function of the injured tissue. In this case, the use of a tissue-engineered scaffold for promoting bone healing offers an accessible and easy-to-manipulate environment for studying bone formation processes in vitro. We have previously shown that mechanical prestimulation using confined compression of differentiating osteoblasts results in an increase in mineralization formed in a 3D collagen-I scaffold. This study builds on this knowledge by evaluating the short and long-term effects of blocking gap junction-mediated intercellular communication among osteogenic cells on their effectiveness to mineralize collagen-I scaffolds in vitro, and in the presence and absence of mechanical stimulation. In this study, confined compression was applied in conjunction with octanol (a general communication blocker) or 18-α-glycerrhetinic acid (AGA, a specific gap junction blocker) using a modified FlexCell plate to collagen-I scaffolds seeded with murine embryonic stem cells stimulated toward osteoblast differentiation using beta-glycerol phosphate. The activity, presence, and expression of osteoblast cadherin, connexin-43, as well as various pluripotent and osteogenic markers were examined at 5–30 days of differentiation. Fluorescence recovery after photobleaching, immunofluorescence, viability, histology assessments, and reverse-transcriptase polymerase chain reaction assessments revealed that inhibiting communication in this scaffold altered the lineage and function of differentiating osteoblasts. In particular, treatment with communication inhibitors caused reduced mineralization in the matrix, and dissociation between connexin-43 and integrin α5β1. This dissociation was not restored even after long-term recovery. Thus, in order for this scaffold to be considered as an alternative strategy for the repair of large bone defects, cell–cell contacts and cell–matrix interactions must remain intact for osteoblast differentiation and function to be preserved. This study shows that within this 3D scaffold, gap junctions are essential in osteoblast response to mechanical loading, and are essential structures in producing a significant amount and organization of mineralization in the matrix.
Introduction
Current bone tissue engineering strategies focus primarily on promoting the healing of large defects,1,2 particularly fracture nonunions, which fail to heal naturally after 6–8 months3 causing pain and poor quality of life for patients.1,4,5 Fracture nonunions fail to form a functional soft callus in which osteogenic precursor cells respond to the mechanical, humoral, and cellular signals that ordinarily initiate and propagate mineralization of the matrix.3,6 Whereas bone grafting is currently the treatment standard for large bone defects,1 donor site morbidity and allogeneic reactions remain problematic. An alternative strategy for bridging large bone defects is the use of tissue engineering scaffolds, that is, natural or synthetic scaffolds that can be seeded with osteogenic cells. For example, hard scaffold materials such as polycaprolactone or hydroxyapatite can contribute to measurable bone repair,7,8 as can soft scaffolds composed of natural or synthetic products.7,8 Among the many choices of soft natural biomaterials for bone tissue engineering, type I collagen is popular as it is abundant and is the primary organic component of bone matrix.9 Whether inorganic or organic, natural or synthetic, scaffolds must support living cells to contribute to the long-term repair of fracture nonunions. In clinical practice, small acellular scaffolds can support ingrowth of endogenous cells, particularly in young, healthy patients. In contrast, large acellular scaffolds, including cortical bone grafts, often fail particularly in elderly and medically complicated patients.10–13 In the latter group of patients, adding exogenous cells to supplement or stimulate endogenous cells repopulating a hard scaffold can improve bone healing. Similarly, seeding a soft scaffold with osteogenic cells is another approach for augmenting bone formation. Hence, in developing new strategies for tissue engineering, using stem cells seeded in a scaffold provides an accessible and easy-to-manipulate construct to investigate questions of lineage determination, and assess cell response to external stimuli at different stages of differentiation.14
Previously, gels of type I collagen with beta-glycerol phosphate have been shown to drive the differentiation of murine embryonic stem cells (mESCs) into mineral-producing osteoblasts that have reduced teratoma formation when implanted in vivo.15 When mechanical prestimulation in the form of confined compression is applied to these cells at a Day 5 differentiated state, we have found that this increases the amount and organization of mineralization present in the matrix, and significantly increases connexin-43 (Cx-43) expression.16
It is well established that Cx-43 gap junctions are essential for osteoblast differentiation and function.17,18 Gap junctions are membrane channels that are formed by the docking of two hemichannels on adjacent cells. The gap junctional hemichannel, also called a connexon, is formed by a hexameric arrangement of proteins termed connexins.17,18 Gap junctions allow the diffusion of ions, metabolites, and signaling molecules between adjacent cells.18 The most abundant connexin in bone is Cx-43.17,18
Other studies performed on stem cells of an osteoblast lineage showed that treatment with gap junction inhibitors resulted in decreased expression of osteoblast differentiation markers in vitro, such as alkaline phosphatase, osteocalcin, and type I collagen.18,19 In vivo, connexin 43 mutations in mice have presented phenotypes similar to those presented in humans with oculodentodigital dysplasia.18–20 Characteristics of this mutation include delayed ossification, craniofacial abnormalities, and misshapen ribs, due to nonfunctional gap junctions.18–20 These studies collectively suggest that cell-to-cell communication through functional gap junctions is essential for normal bone matrix formation and mineralization.
Long before it was recognized that bone cells communicate directly by gap junctions, it was clear that bone cells are exquisitely sensitive to specific humoral signals, which is the basis of many metabolic bone diseases. More recently, cell-to-cell communication through paracrine signaling among bone cells has also been recognized.21,22 In conjunction, it has also been demonstrated that mechanically induced fluid flow through osteocytic canaliculi is central to mechanotransduction in bone.23,24 In culture, osteocytes have been shown to respond to fluid flow through the rapid production of secondary messengers such as nitric oxide and prostaglandin E2.23 Less clear is how osteoblasts present on the surface of bone respond to mechanical loading.23 It has been previously shown that both fluid flow and cyclic strain produce an immediate bone cell response to loading beginning with osteoblast recruitment.23,24 Cyclic strain in particular was shown to significantly increase nitric oxide production.23 Given the high sensitivity of osteoblasts to mechanical loading, studies of the relationship between mechanical stimulation and osteoblast communication are needed to understand how these two factors are coupled in mineral formation.
In vivo, osteoblasts form gap junctions with osteocytes, which perceive mechanical signals from bone deformation, and transduce these signals to a biological response to initiate mineralization or resorption of bone.25 In vitro, the initial differentiation of osteogenic cells in 3D collagen-I scaffolds depends on the humoral milieu, yet few studies have characterized the mechanical signals that promote matrix mineralization, which would be important for defining the conditions wherein cell constructs could be implanted to effectively promote repair in fracture nonunions. In this study, confined compression was applied in conjunction with octanol (a general communication blocker) or 18-α-glycerrhetinic acid (AGA, a specific gap junction blocker) using a modified FlexCell plate to collagen-I scaffolds seeded with mESCs stimulated toward osteoblast differentiation using beta-glycerol phosphate. The current in vitro study thus aimed to characterize two factors known to be influential in vivo: mechanical load and cell-to-cell communication.
Materials and Methods
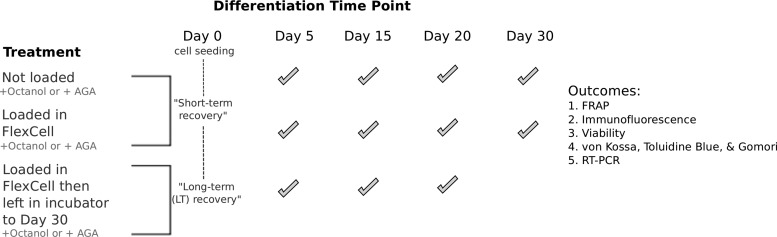
The treatment groups in this study were defined as follows and will be used to discuss results in this article (Fig. 1). Gels that were not subjected to any mechanical load at different time points of differentiation were named Day 5, 15, 20, and 30. For gels that were loaded then immediately stained or analyzed, gels were named Day 5 loaded, Day 15 loaded, Day 20 loaded, and Day 30 loaded. For gels that were loaded in the presence of either octanol (+OCT) or AGA (+AGA) then left for long-term recovery for 30 days before staining or analysis, LT was appended to the name of the treatment group.
FIG. 1.
Treatments (left) of cells at a Day 5–30 state of differentiation with and without loading, and communication inhibitors. Check marks show the time points when treatments were applied, and when the five outcomes (right) were assessed.
Cell preparation
The mESCs (ES-D3 cell line) were maintained in their pluripotent state in media containing high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% fetal bovine serum, 1% nonessential amino acids, 50 U/mL penicillin, 50 mg/mL streptomycin, 0.1 mM β-mercaptoethanol (all supplied from Invitrogen, Life Technologies), and 1000 U/mL leukemia inhibitory factor (LIF; Millipore). The mESCs were passaged in T-75 flasks prepared with 0.1% gelatin, and after three to four passages, cells were counted using a hemocytometer, spun down, and resuspended in 5× DMEM media containing 10 mM pro-osteoblastic beta-glycerol phosphate (BGP; Sigma). No ascorbic acid, 1,25-OH2 VD3, or dexamethasone was added to the media as per the pro-osteoblastic differentiation protocol determined by our group.15 The cells in BGP media were then combined with purified bovine collagen-I (Advanced Biomatrix) with a final volume composed of 80% collagen-I, and 20% cells+BGP. The final cell density in each gel consisting of cells+BGP+collagen-I was 1 million cells/mL. The mixture was pipetted in 1 mL volumes into a 24-well plate and left in a 37°C incubator to gel. Four differentiation time points were investigated in this study: day 5, 15, 20, and 30.
Mechanical loading regime
Confined compression of the gels was performed at these time points using a custom modified BioPress™ FlexCell FX-4000™ (FlexCell International Corp) six-well loading plate with a loading regime of two cycles of 4 h of loading followed by 16 h in a resting state, for a total of 40 h.26 The loading plate has been previously characterized by our group and calibrated to subject the gels to a pressure of 5 kPa with an overall compressive strain of 5% at a load frequency of 1 Hz, and a strain rate of 1%/s. Similar cyclic loading regimes have been widely used to investigate mechanically sensitive cell responses, and has been shown to be representative of physiological mechanical rate and frequency in bone during locomotion.26–29 The rest states in this regime allow the media to be drawn back into the collagen matrix and thus enables recovery following loading.26,30–32 Further, it has been shown that these loading parameters are sufficient to produce measurable differences in osteoblast response when cells are loaded in the FlexCell system.28,29 The FlexCell system has also been widely used to study the effect of mechanical loading on osteoblast differentiation in both 2D and 3D scaffolds.33–35
Communication inhibition
At each time point of differentiation, gels were treated with either 1 mM 1-octanol (Sigma) or 100 μM 18-α-glycerrhetinic acid (AGA; Sigma). Both octanol and AGA have been shown previously to be reversible communication inhibitors.36–39 Further, AGA has been shown to specifically inhibit gap junction channels and disassembles connexons.36,37 Octanol and AGA inhibition of cells was performed to assess differences resulting from general communication blocking and specific gap junction blocking, respectively.
Fluorescence recovery after photobleaching
Fluorescence recovery after photobleaching (FRAP) was performed on gels to assess the overall gap junction activity. At day 5, 15, 20, and 30 of differentiation, gels were loaded in the presence of octanol (n=7 for each time point) or AGA (n=7 for each time point). Gels were also left nonloaded and incubated in octanol (n=7 for each time point) or AGA (n=7 for each time point) for 40 h. All gels were incubated at 37°C with 500 μM calcein-AM for 2 h before imaging. Gels in the FlexCell plates were incubated in calcein-AM while loading was taking place. A Zeiss LSM510 microscope was used for imaging (63×1.2 NA water immersion objective, 488 nm argon laser, 1.3 μm optical slice).40,41 An initial image was recorded (tini) at 20% laser intensity. An oval region of interest (ROI) was then fit around the cell body and any visible cell processes. This ROI was then photobleached at 100% laser intensity for 15 s. At least three regions within each gel were imaged. Images were obtained immediately after photobleaching (t0), after 5 min (t5), 10 min (t10), 20 min (t20), and 30 min (t30) of recovery. The mean pixel intensity within cells was determined using the NIH ImageJ 1.43 software and normalized to an uninvolved cell in the image field.40,41 Percent recovery for each image obtained after photobleaching gave an indication of gap junction functionality and was calculated using the following equation40,41:
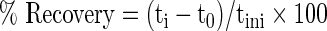 |
To determine the reversibility of gap junction inhibitors within the gels, fluorescence recovery was measured in gels at each time point. After this measurement, gels were incubated in either octanol (n=3 for each time point) or AGA (n=3 for each time point) for at least 2 h before performing FRAP again. After fluorescence recovery was measured following inhibition, the communication inhibitor was washed out of the gels three times in 1×PBS, gels were reimaged, and fluorescence recovery was measured again.
Immunofluorescence staining
At day 5, 15, 20, and 30 of differentiation, unloaded and loaded gels were prepared for immunofluorescence staining. Gels treated with octanol or AGA were stained 2 days following communication inhibition (short-term recovery, n=8 at each time point for each blocker) or at a day 30 state following communication inhibition (long-term recovery, n=8 at each time point for each blocker). Gels were stained for integrin α5β1, a structure that is closely associated with connexin-43.42 Each gel was fixed in filtered 4% paraformaldehyde/PBS overnight at 4°C. The gels were then washed three times in 1×PBS and incubated overnight at 4°C in 0.5% saponin/PBS. The gels were washed again three times in 1×PBS and blocked overnight at 4°C with 3% bovine serum albumin (BSA)/phosphate-buffered saline (PBS). After the blocking step, gels were incubated with mouse connexin-43 primary antibody at a dilution of 1:50 in 3% BSA/PBS. The gels were then washed three times in 1×PBS and blocked again overnight at 4°C in 3% BSA/PBS. Gels were then incubated with rat integrin α5β1 primary antibody with a fluorescein (FITC) conjugate and anti-mouse Alexa568 secondary antibody at a dilution of 1:50 in 3% BSA/PBS (all antibodies supplied from Life Technologies). Finally, gels were washed three times in 1×PBS and visualized using a Zeiss LSM710 microscope. Percentage of coexpression for connexin-43 and integrin α5β1 was calculated using colocalization analysis within the Zen software platform on the Zeiss LSM710 microscope.
Viability study
At day 5, 15, 20, and 30 of differentiation, viability of cells treated with either octanol or AGA within unloaded (total n=24 for each blocker) and loaded (total n=24 for each blocker) collagen gels was determined. To determine viability, gels were incubated in 4 μM calcein–ethidium homodimer (Molecular Probes LIVE–DEAD Kit; Life Technologies) for 45 min before visualization on a Zeiss LSM510 microscope. Manual thresholding was performed to calculate cell counts using the NIH ImageJ 1.43 software. Automatic thresholding was also performed to compare back to manual counts and reduce user bias.
TUNEL assay
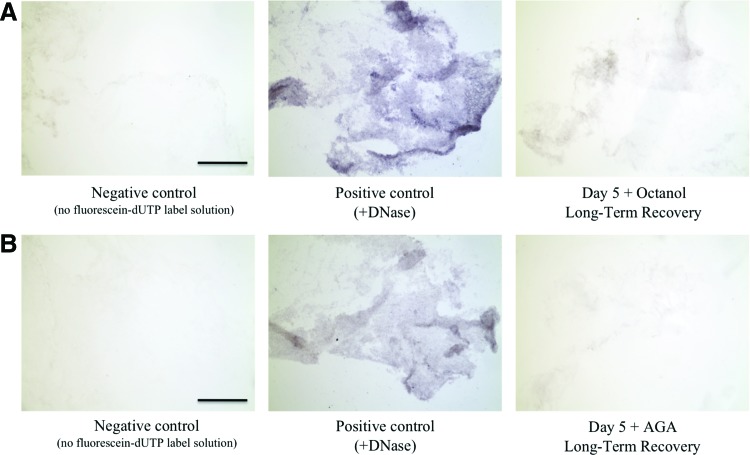
For long-term recovered gels following communication inhibition, the possibility of cell apoptosis through DNA fragmentation was investigated using an in situ AP (ApopTag® Peroxidase) cell death detection kit (Roche Applied Science). Day 5 gels treated with either octanol (n=3) or AGA (n=3) and left to recover to a day 30 state were embedded, frozen, and cut using a cryostat in 10 μm sections. Negative controls for TUNEL staining were prepared by incubating sections in only the label solution instead of the TUNEL reaction mix. Positive controls for TUNEL staining were prepared by incubating sections first in DNase for 15 min at 37°C before labeling.
von Kossa staining
At day 5, 15, 20, and 30 of differentiation, unloaded and loaded gels that were treated with octanol or AGA and left for short-term (n=5 for each time point and blocker) or long-term recovery (n=5 for each time point and blocker) were embedded, frozen, and cut using a cryostat in 10 μm sections. von Kossa staining of gels was performed to assess mineral formation (appears brown–black with this stain) within the gels43 by incubating sections for 1 h under UV light in 1% silver nitrate, neutralizing the reaction with 5% sodium thiosulfate, and counterstaining with nuclear fast red. Images were taken with a Zeiss Axioplan 2 microscope. Negative controls for von Kossa staining were prepared by treating gels with formic acid before incubation with silver nitrate. Sections of 16–18 day old mouse embryos were prepared as positive controls.44 To determine the effect of the collagen matrix on osteoblast activity following communication inhibition, mESCs were cultured in T-75 flasks, stimulated toward osteoblast differentiation using BGP, and treated with either octanol or AGA for 40 h. von Kossa staining was then performed on the flasks following long-term recovery.
Toluidine blue staining
Toluidine Blue staining has been shown to be an effective stain for distinguishing osteoblasts (nuclei stain blue/black) and osteoid (light blue) in a specimen, and for highlighting mineralization fronts (dark purple).45,46 At day 5, 15, 20, and 30 of differentiation, unloaded and loaded gels that were treated with octanol or AGA and left for short (n=5 for each time point and blocker) or long-term recovery (n=5 for each time point and blocker) were embedded, frozen, and cut using a cryostat in 10 μm sections. Toluidine Blue staining was performed by incubating sections in Toluidine Blue working solution for 2–3 min before dehydrating, clearing, and mounting the sections using resinous mounting medium.
Gomori trichrome staining
Gomori trichrome staining has been shown to be an effective stain for distinguishing osteoid (red) and type-I collagen (green) formation both in vivo and in vitro.47,48 At day 5, 15, 20, and 30 of differentiation, unloaded and loaded gels that were treated with octanol or AGA and left for short (n=5 for each time point and blocker) or long-term recovery (n=5 for each time point and blocker) were embedded, frozen, and cut using a cryostat in 10 μm sections. Gomori trichrome staining was performed by incubating sections in Hematoxylin for 5 min, washing the sections with distilled water, then incubating sections for 15 min in Gomori one-step trichrome stain.47,48 Sections were then dehydrated, cleared, and mounted using a resinous mounting medium.
mRNA expression study
At day 5, 15, 20, and 30 of differentiation, quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for connexin-43 (Gja1) and osteoblast cadherin (Cdh11) was performed on unloaded and loaded gels treated with either octanol or AGA and left for short (n=5 for each time point, load condition, and blocker) or long-term recovery (n=5 for each time point, load condition, and blocker) using the iCycler iQ Multicolor Real-Time PCR detection system (Bio Rad Laboratories, Inc.). The following genes were also considered for all groups described above: Oct-4, Sox2, Cathepsin B (Ctsb), Runx2, and osteocalcin (OCN). A protocol previously demonstrated to be sufficient in isolating RNA and generating cDNA for gene expression studies on these gels was used.15 RNA isolation of gels was performed using TRIzol (Invitrogen) with the addition of glycogen for complete dissociation. Thirty microliters of ultrapure water was used to dissolve RNA pellets and RNA was stored at −70°C until complementary DNA (cDNA) extraction was performed. RNA was quantified using a NanoVue spectrometer and bioanalyzer (Bio-Rad). The RNA yield obtained using the NanoVue spectrometer was only used for cDNA analysis if 260/280 ratios were greater than or equal to 1.7 and if 260/230 ratios were greater than or equal to 1.5. cDNA was prepared using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies). RT-PCR was performed using the TaqMan® Universal PCR Master Mix with no AmpErase (Applied Biosystems, Life Technologies) to ensure that PCR products could not be reamplified and, therefore, result in false positive results. All PCR samples were run in triplicate for each gene using a 96-well plate. All gene expression data were analyzed using the comparative CT method, which involves normalization to a reference gene followed by normalization to a cell control.49 In this study, gene expression data were normalized to 18S rRNA, as this has been found to be a stable reference gene for normalizing PCR data for ex vivo studies.50–54 Following normalization, samples were then compared against undifferentiated mESC gene expression data.
Statistical analyses
For the methods described above, the indicated sample sizes denote the number of individual gels that were tested. For FRAP and viability measures, all statistical evaluations at each time point between mean fluorescence recovery values and average viability of unloaded and loaded samples were performed using independent two-sample and paired t-tests, respectively, with a significance level of 0.05. For RT-PCR measures, one-way ANOVA was performed between time points of differentiation for fold induction in unloaded and loaded samples treated with communication inhibitors (GraphPad Prism 6.0) with a significance level of 0.05. If differences within groups were significant, Bonferroni's Multiple Comparison Test was performed.
Results
The different treatment groups and experiments performed on each group are summarized in Figure 1.
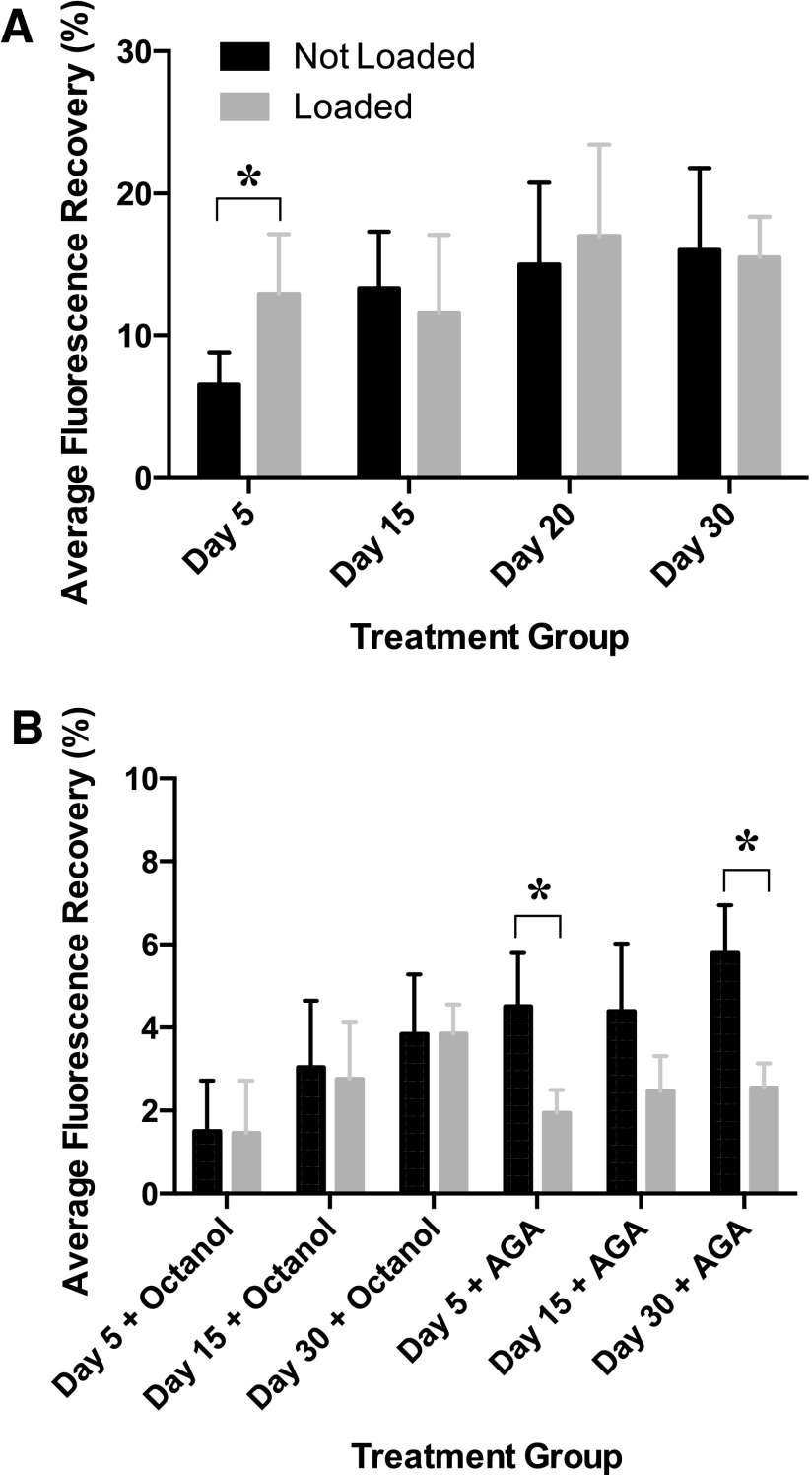
In a previous study performed by our group, FRAP showed that there was a significant difference between average maximum percent recovery in early (Day 5) and late-differentiated (Days 15–30) gels in the presence and absence of loading (Fig. 2A, a previously published result).16 In this study, gels treated with octanol and AGA showed a significant reduction in fluorescence recovery values (Fig. 2B). Differences between early and late differentiated gels that were treated with either octanol or AGA were not statistically significant (Fig. 2B). There was no significant difference in fluorescence recovery between loaded and unloaded samples when treated with octanol (Fig. 2B). Fluorescence recovery showed a significant decrease in day 5 and 30 gels when treated with AGA (Fig. 2B).
FIG. 2.
Average fluorescence recovery in Day 5, 15, 20, and 30 differentiated constructs in (A) the presence and absence of loading, and (B) the presence and absence of communication inhibitors and loading. Asterisks show significant differences at α=0.05.
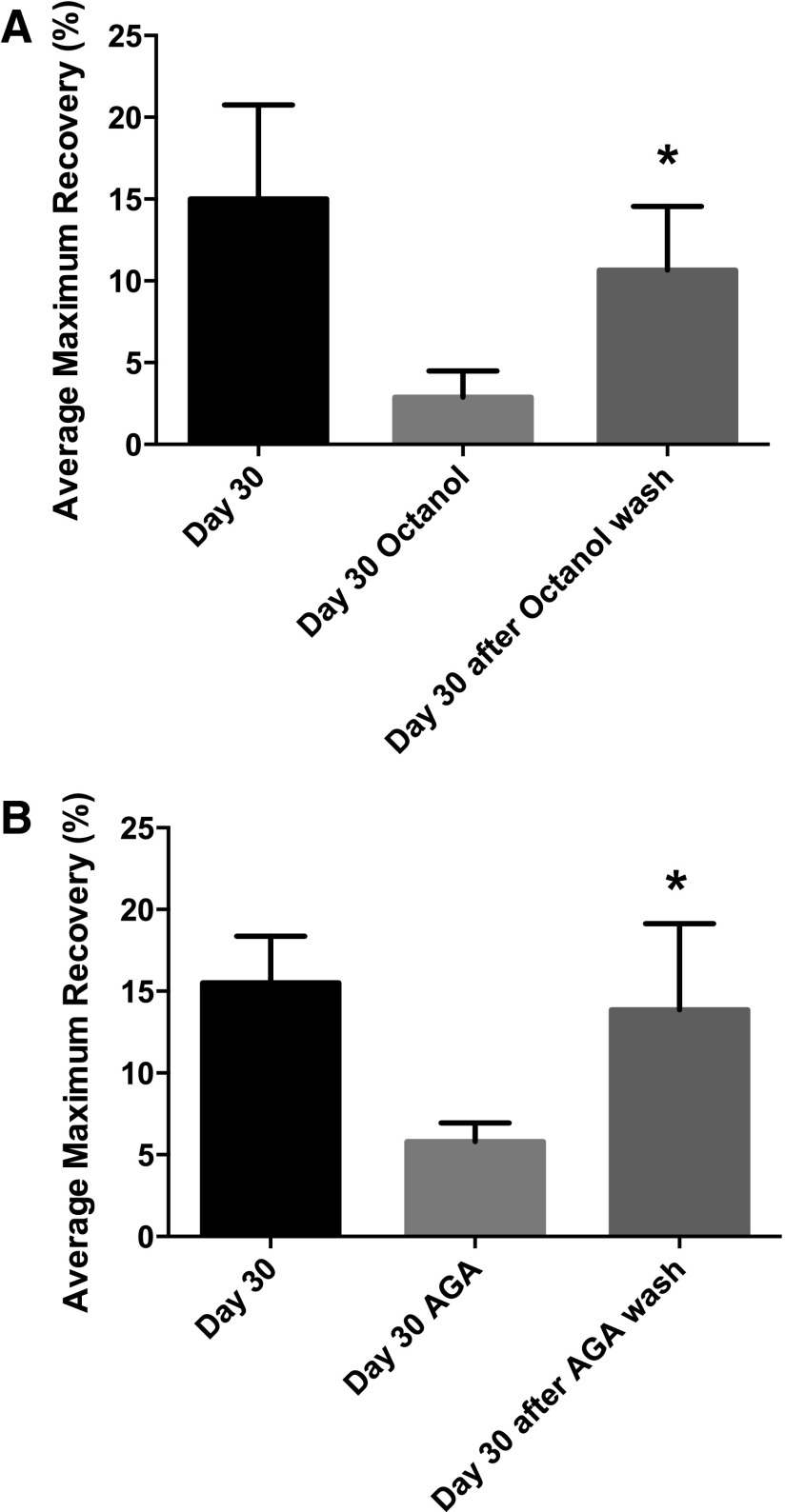
Fluorescence recovery experiments performed to test the permeability and reversibility of octanol and AGA within the gels showed that fluorescence recovery values increased to their original states following communication inhibition (Fig. 3).
FIG. 3.
FRAP to evaluate recovery of fluorescence in Day 30 differentiated constructs when (A) octanol and (B) AGA is washed out of the matrix. Asterisks show significant differences at α=0.05.
Gels treated with octanol and AGA showed a slight decrease in viability compared with untreated constructs. Difference in the range in viability between day 5–30 AGA and day 5–30-loaded AGA was significantly different (Table 1). Specifically, loading in conjunction with AGA decreases the viability of cells by approximately 21% (Table 1).
Table 1.
Viability of Cells With and Without Communication Inhibitors, and in the Presence and Absence of Mechanical Loading
| Differentiation interval | Communication inhibitor applied | Range in viability (%±standard deviation) | Average decrease (%) |
|---|---|---|---|
| Day 5–30 | None | 66.0±5.7→46.9±4.8 | 19.1 |
| Day 5 Loaded–Day 30 Loaded | None | 57.8±7.9→36.0±4.8 | 21.8 |
| Day 5–30 | Octanol | 57.6±4.8→40.7±3.8 | 16.9 |
| Day 5 Loaded–Day 30 Loaded | Octanol | 52.8±4.6→35.7±4.0 | 17.1 |
| Day 5–30 | AGA | 58.3±4.5→41.3±4.7 | 17.0 |
| Day 5 Loaded–Day 30 Loaded | AGA | 58.7±2.8→37.8±2.4 | 20.9a |
Range in viability statistically significant from Day 5–30 AGA range, α=0.05.
TUNEL staining revealed no appreciable positive staining in Day 5 gels treated with either octanol or AGA and left to recover to a Day 30 state (Fig. 4).
FIG. 4.
(Left to right) TUNEL staining of (A) negative control (no fluorescein-dUTP labeling solution), positive control (gel treated with DNase before labeling), and Day 5 gel treated with octanol and left for long-term recovery, and (B) negative control (no fluorescein-dUTP labeling solution), positive control (gel treated with DNase before labeling), and Day 5 gel treated with AGA and left for long-term recovery. Dark blue staining indicates cells undergoing apoptosis, scale bar is 250 μm. Color images available online at www.liebertpub.com/tea
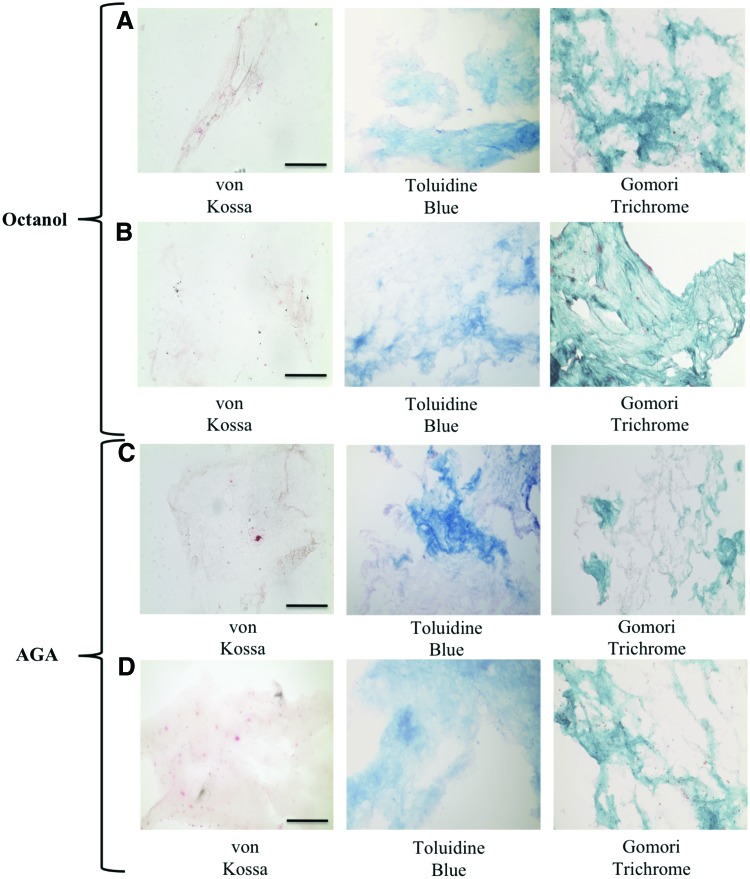
Cells did not initiate concentrated or structured mineralization of the matrix after long-term recovery when treated with octanol in both nonloaded and loaded constructs as observed with von Kossa staining (Fig. 5). Day 5 gels showed staining for osteoid as observed with Toluidine Blue and Gomori Trichrome staining whereas other time points in both unloaded and loaded gels that were treated with octanol and left to recover over a long-term period did not show staining for osteoid (Fig. 5).
FIG. 5.
Histology of Day 5 gels treated with octanol and allowed to recover over a long-term period (A) in the absence of loading, and (B) in the presence of loading. Histology of Day 5 gels treated with AGA and allowed to recover over a long-term period (C) in the absence of loading, and (D) in the presence of loading. Scale bar is 250 μm. Brown/black staining in von Kossa indicates positive staining for mineralization, light blue staining in Toluidine blue indicates osteoid, and green staining in Gomori indicates collagenous matrix. Color images available online at www.liebertpub.com/tea
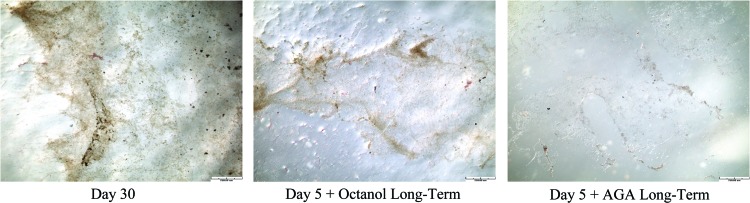
Similarly, von Kossa staining of gels with AGA treatment showed that cells did not initiate concentrated and structured mineralization of the matrix after long-term recovery in the presence and absence of mechanical loading (Fig. 5). Day 5 gels showed staining for osteoid as observed with Toluidine Blue and Gomori Trichrome whereas other time points in both unloaded and loaded gels that were treated with AGA and left to recover over a long-term period did not show staining for osteoid (Fig. 5. At all other time points, no positive von Kossa staining was observed in both short and long-term recovered gels following octanol and AGA treatment in the presence and absence of loading. Finally, von Kossa staining of cultured cells in T-75 flasks following octanol and AGA incubation showed decreased concentration of mineralization compared with a Day 30 differentiated flask following long-term recovery (Fig. 6).
FIG. 6.
von Kossa staining of cells cultured in 2D T-75 flasks at Day 30, Day 5 treated with octanol and left to recover over a long-term period, and Day 5 treated with AGA and left to recover over a long-term period. Brown/black staining indicates positive staining for mineralization, scale bar is 250 μm. Color images available online at www.liebertpub.com/tea
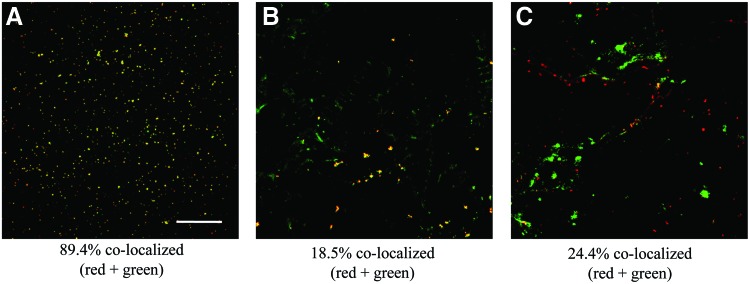
At all time points, immunofluorescence for integrin α5β1 showed that without communication inhibition, integrin α5β1 and connexin-43 were coexpressed (Fig. 7A). Treatment with octanol or AGA appeared to disrupt the coexpression of both integrin α5β1 and connexin-43 even after long-term recovery (Fig. 7B, C). This shift was especially apparent in Day 5 constructs, but was present at all time points under octanol or AGA treatment in the presence or absence of loading.
FIG. 7.
Immunofluorescence staining for integrin α5β1 (green) and connexin-43 (red) in (A) Day 5-loaded gel left to differentiate to Day 30, (B) Day 5-loaded gel treated with octanol left for long-term recovery, and (C) Day 5-loaded gel treated with AGA left for long-term recovery. Scale bar is 200 μm. Color images available online at www.liebertpub.com/tea
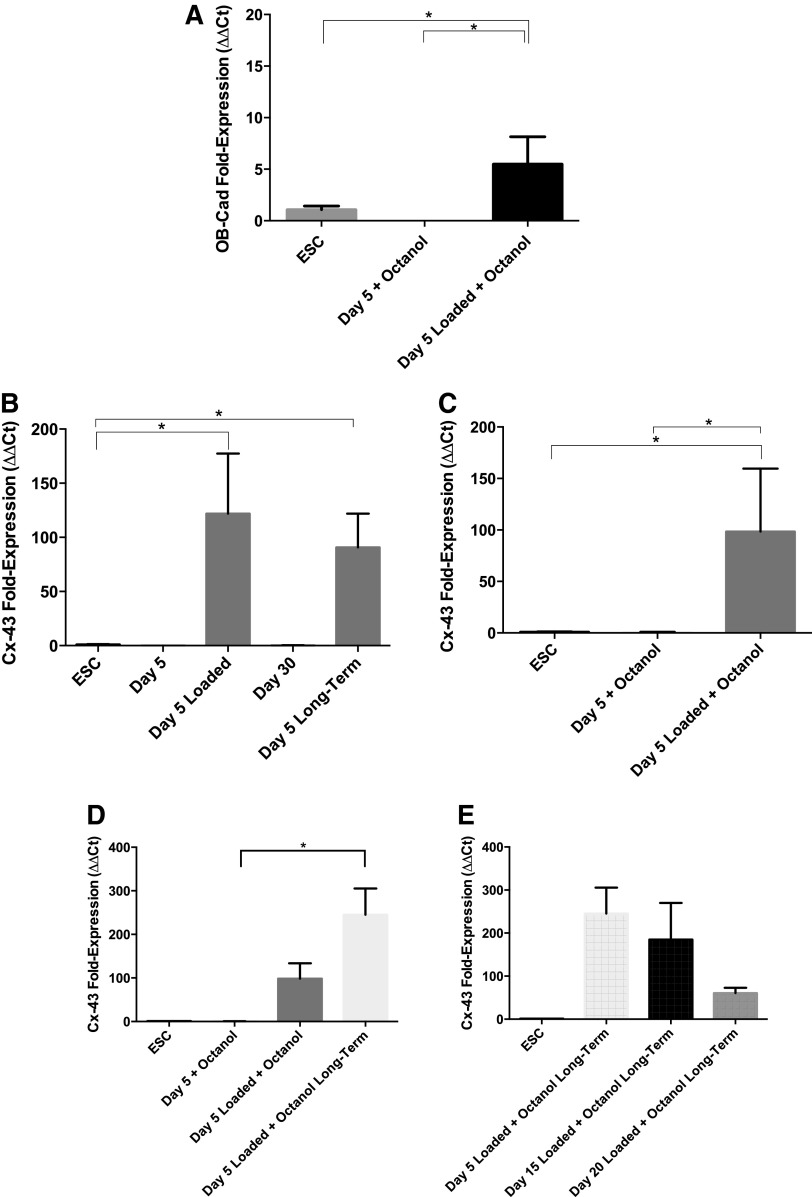
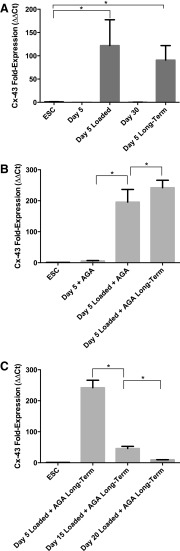
There was no measurable osteoblast cadherin expression in nonloaded gels treated with octanol. No significant upregulation of connexin-43 was observed in nonloaded octanol treated gels days 5–30 comparedwith untreated gels. However, under loading with octanol, osteoblast cadherin was slightly upregulated in Day 5-loaded gels (Fig. 8A) following short-term recovery. Connexin-43 expression increased significantly in octanol-treated Day 5-loaded gels compared with unloaded Day 5 gels (Fig. 8B, C). Further, octanol-treated gels that were loaded and left for long-term recovery maintained upregulation of connexin-43 (Fig. 8D). Differences between short-term and long-term recovery of Day 5-loaded gels under octanol inhibition was not significant (Fig. 8D). At all other time points of differentiation following long-term recovery, connexin-43 expression decreased as cells were loaded and treated in a more committed state (Fig. 8E).
FIG. 8.
RT-PCR results showing (A) osteoblast cadherin expression in Day 5 gels treated with octanol, (B) connexin-43 expression in Day 5, Day 5-loaded, Day 30, and Day 5 long-term gels without communication inhibitors, (C) connexin-43 expression in Day 5 gels treated with octanol, (D) differences in connexin-43 expression between short and long-term recovered Day 5 gels treated with octanol, and (E) connexin-43 expression in Day 5, 15, and 20 long-term recovered gels loaded with octanol. Asterisks show significant differences at α=0.05.
There was no measurable osteoblast cadherin expression in gels treated with AGA. However, nonloaded Day 5 gels treated with AGA showed slight upregulation of connexin-43. Day 5-loaded gels treated with AGA showed the greatest increase in connexin-43 expression (Fig. 9A). This upregulation was maintained in loaded AGA-treated gels left to differentiate to 30 days (Fig. 9B). Connexin-43 expression decreased significantly as cells treated with AGA were loaded and treated in a more committed state (Fig. 9C).
FIG. 9.
RT-PCR results showing (A) connexin-43 expression in Day 5, Day 5-loaded, Day 30, and Day 5 long-term gels without communication inhibitors, (B) connexin-43 expression in Day 5 gels treated with AGA, and (C) connexin-43 expression in Day 5, 15, and 20 long-term recovered gels loaded with AGA. Asterisks show significant differences at α=0.05.
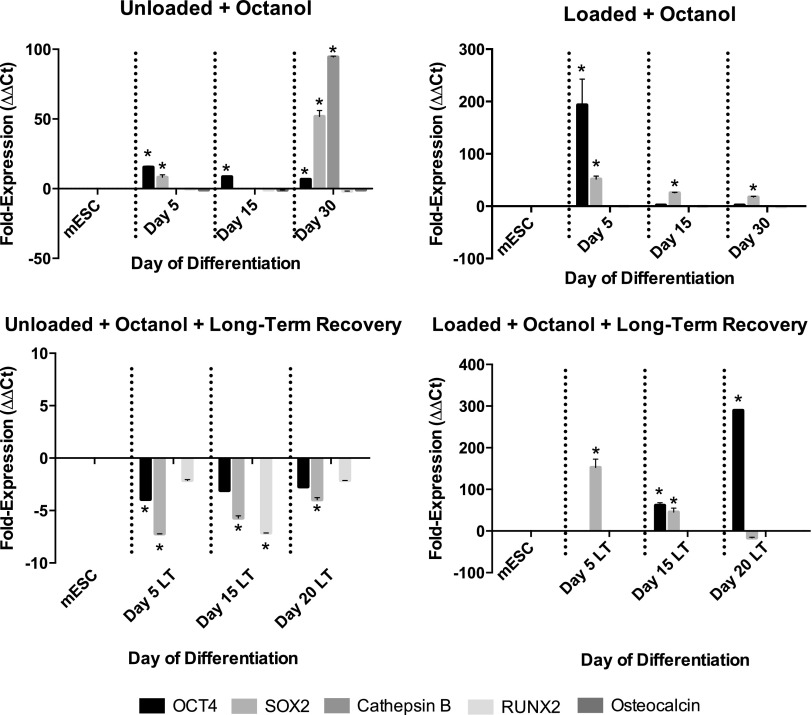
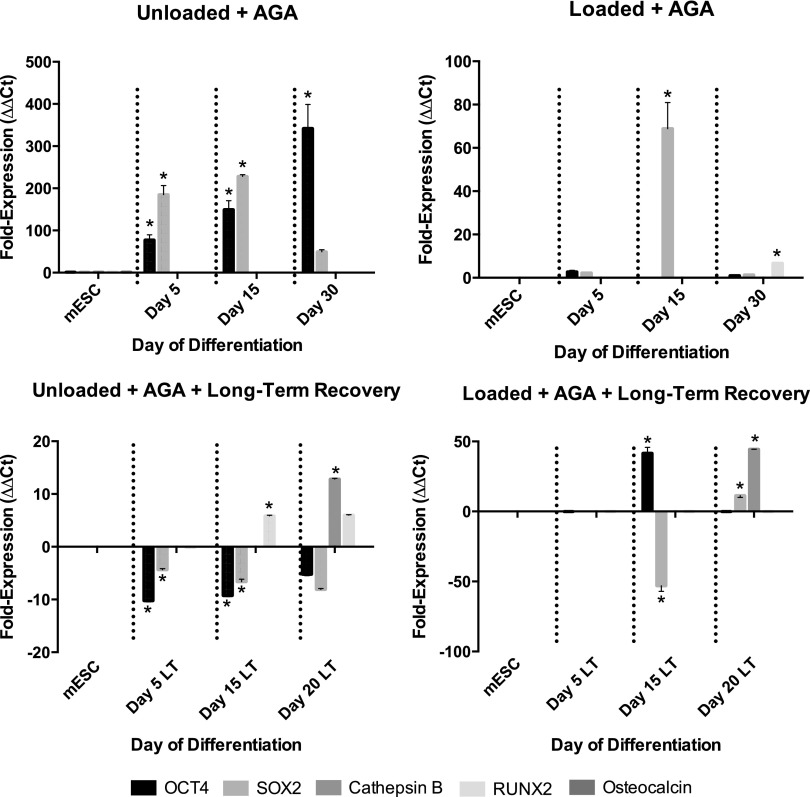
In both nonloaded and loaded conditions, cells treated with octanol coexpressed Oct-4 and Sox2 throughout differentiation. No octanol-treated gels expressed Runx2 or osteocalcin following long-term recovery in the presence or absence of loading (Fig. 10). Day 15 and 20 constructs treated with AGA significantly upregulated Runx2 expression, as did Day 30-loaded constructs treated with AGA (Fig. 11). However, similar to octanol-treated constructs, in both nonloaded and loaded conditions, cells treated with AGA also coexpressed Oct-4 and Sox2 as differentiation progressed (Fig. 11).
FIG. 10.
Expression of pluripotent (Oct-4, Sox2 when coexpressed with Oct-4), early (Cathepsin B, Runx2), and late (osteocalcin) osteoblast markers in gels treated with octanol in the presence and absence of loading, and following short or long-term recovery. Asterisks show significant differences at α=0.05.
FIG. 11.
Expression of pluripotent (Oct-4, Sox2 when coexpressed with Oct-4), early (Cathepsin B, Runx2), and late (osteocalcin) osteoblast markers in gels treated with AGA in the presence and absence of loading, and following short or long-term recovery. Asterisks show significant differences at α=0.05.
Discussion
The goal of this study was to determine the effect of inhibiting of cell communication on osteoblast response to a mechanical stimulus in a 3D type-I collagen scaffold. Our group has previously shown that mechanical prestimulation of day 5 cells within this scaffold produced a drastic increase in the amount and organization of mineralization present.16 Specifically, confined compression of early-differentiated cells produced a honeycomb structure to the mineralization present, a quality associated with late osteoblast/early osteocyte activity.16 Prestimulated cells also upregulated early and late osteoblast markers.16 In this current study, gap junction-mediated cell communication was found to be necessary for directing cells to an osteogenic fate, and for osteoblast ability to perceive a mechanical stimulus and initiate mineralization of the matrix. Combined with the loading regime applied in this study, blocking communication demonstrated the importance of cell–cell communication in osteoblast mechanotransduction. The significant impairment of osteoblast activity within this scaffold, even after communication inhibition was removed, was possibly due to alteration of lineage determination as well as disruption of other cell communication structures such as integrin α5β1. This finding suggests that coexpression of connexin-43 and integrin α5β1 is necessary for cells within this scaffold to differentiate to osteoblasts and maintain their potential to initiate mineralization.
Previous studies in 2D culture investigating the coupling of intercellular communication and mechanical loading on bone cell activity have found similar results. Studies using cultured osteoblasts or osteocytes have confirmed the necessity of gap junction-mediated cell communication, and a specific role of connexin-43 in enabling cells to transduce a mechanical signal to a biological response.55–58 Additionally, overexpression of connexin-43 has been shown to promote proliferation and differentiation of an osteoblast-like cell line, and increase the amount of mineralization present in the extracellular matrix.59 Few studies, however, have translated this to a 3D setting. This study introduces a 3D collagen scaffold that does not induce teratoma formation following implantation,15 where relevant external mechanical load and communication inhibitors can be applied, and where cell–cell contacts still play a major regulatory role in mineral formation.
In this study, blocking communication with octanol resulted in a significant decrease in measured gap junction activity, indicating the high permeability of this inhibitor within this scaffold, and also the effectiveness of octanol to block communication. Blocking of gap junctions with AGA also resulted in a decrease in fluorescence recovery. Viability results indicated that treatment with octanol and AGA did not detrimentally affect cells. The applied loading regime in the presence of AGA slightly decreased the viability of cells within the gels. TUNEL staining, however, revealed that cell apoptosis was not the reason for the decreased mineralization present in the matrix following communication inhibition.
Gels treated with octanol or AGA in the presence or absence of loading did not show concentrated positive staining for mineralization, even after long-term recovery. Similarly, von Kossa staining of cells cultured in T-75 flasks following octanol or AGA treatment showed reduced staining for mineralization. Independent of the matrix used, treatment with octanol and AGA impaired cell ability to initiate mineralization of the matrix even after long-term recovery. Therefore, the collagen matrix used in this study had no effect on producing the reduced mineralization observed following octanol or AGA treatment.
Significant upregulation of osteoblast cadherin and connexin-43 only in Day 5-loaded gels treated with octanol showed that cells in an early state of differentiation may be more likely to modulate the expression of communication structures important for osteoblast differentiation. This was further supported by the downregulation of connexin-43 observed in later-differentiated loaded gels under octanol treatment. Upregulation of connexin-43 in Day 5-loaded gels treated with AGA may also suggest a compensatory mechanism by cells to counteract the disassembly of existing gap junctions by AGA. At later time points, loaded gels treated with AGA showed decreased expression of connexin-43, perhaps indicating that only early-differentiated cells increase connexin-43 expression as a means to compensate for communication inhibition.
Gene expression data for pluripotent and early to late osteoblast markers showed that under octanol and AGA treatment, cells were not consistently expressing osteogenic markers (Runx2, OCN). Instead, heterogeneous populations of cells persisted even after communication inhibitors were removed. Therefore, in the presence and absence of loading, blocking communication significantly impaired osteoblast differentiation within this scaffold. In fact, treatment with communication inhibitors appears to have altered lineage determination of these cells and the ability of cells to initiate mineralization of the matrix even after inhibition was removed. Therefore, long-term culture following the use of communication inhibitors seems to favor an undifferentiated population. Future research, including immunofluorescence for each of the differentiation markers in this study could be performed to better understand the effect of these inhibitors on lineage determination of these cells. The decrease in osteoblast-specific markers following treatment with gap junction inhibitors such as AGA has been previously demonstrated in culture.37,60
Immunofluorescence showed that integrin α5β1 was not coexpressed with connexin-43 in gels not treated with communication inhibitors. Gels treated with octanol and AGA showed a disruption in the coexpression of integrin α5β1 and connexin-43, indicating that the disconnect between these two structures may be a possible mechanism explaining the reduced mineralization observed in this study when communication is inhibited.
In this study, we demonstrated the close coupling between intercellular communication and mechanical stimulation that regulates osteoblast differentiation and ability to initiate mineralization of the matrix within a collagen-I scaffold. Despite cells upregulating connexin-43 expression following octanol or AGA treatment, we hypothesize that the inability of cells to recover was a result of altered lineage determination and disconnect between integrin α5β1 and connexin-43. The crucial role of integrin α5β1 in mechanotransduction pathways has been demonstrated in cultured osteocytes and osteoblasts.36,47 It has been previously shown that mechanical stimulation causes a conformational change in the structure of integrin α5β1, and that this conformational change directly causes the opening of connexin-43 hemichannels.36,47 Given this knowledge, by interfering with connexin-43 gap junctions using either octanol or AGA, we have demonstrated that the close coupling between integrin α5β1 and connexin-43 remains an essential component to the functioning of differentiating cells in an ex vivo collagen-I scaffold.
Overall, this study provides further support for the importance of gap junctions and other cell communication structures in regulating the lineage and function of differentiating cells. It also reveals that in this scaffold preparation, once communication has been inhibited, lineage determination of osteoblasts is impaired and cells are unable to recover their function. Thus, the potential use of this scaffold for the repair of bone defects such as fracture nonunions relies heavily on maintaining cell–cell contacts and cell–matrix interactions throughout the healing process. This study provides insight on the necessity of cell communication in the ability of osteoblasts to perceive a mechanical stimulus in a 3D environment, and enables further exploration of whether this scaffold could be optimized for bone repair using mechanical prestimulation, or secondary messengers such as nitric oxide to modulate cell communication, differentiation, and function.
Acknowledgments
The custom designed loading plate was developed and calibrated by Olesja Hazenbiller (MSc), and histology protocols were provided by Dragana Ponjevic. This research is supported by the Canadian Institutes of Health Research Skeletal Regenerative Medicine Team Grant (Grant No.: RMF-82497), Natural Sciences and Engineering Research Council of Canada (Grant No.: 203436-2010), and Alberta Innovates Technology Futures (Graduate Student Scholarship to Swathi Damaraju).
Disclosure Statement
No competing financial interests exist.
References
- 1.Drosse I., Volkmer E., Capanna R., De Biase P., Mutschler W., and Schieker M.Tissue engineering for bone defect healing: an update on a multi-component approach. Inj Int J Care Inj 39S2,S9, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Schroeder J.E., and Mosheiff R.Tissue engineering approaches for bone repair: concepts and evidence. Injury 42,609, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Catagni M.A., Guerreschi F., Holman J.A., and Cattaneo R.Distraction osteogenesis in the treatment of stiff hypertrophic nonunions using the Ilizarov apparatus. Clin Orthop Relat Res 301,159, 1994 [PubMed] [Google Scholar]
- 4.Cancedda R., Giannoni P., and Mastrogiacomo M.A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28,4240, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Mckibbin B.The Biology of Fracture Healing in Long Bones. J Bone Jt Surg 60-B,150, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Geris L., Sloten J.V., and Van Oosterwyck H.Connecting biology and mechanics in fracture healing: an integrated mathematical modeling framework for the study of nonunions. Biomech Model Mechanobiol 9,713, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Igwe J.C., Mikael P.E., and Nukavarapu S.P.Design, fabrication and in vitro evaluation of a novel polymer-hydrogel hybrid scaffold for bone tissue engineering. J Tissue Eng Regen Med 8,131, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Tan F., Xu X., Deng T., Yin M., Zhang X., and Wang J.Fabrication of positively charged poly(ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold. Biomed Mater 7,055009, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira A.M., Gentile P., Chiono V., and Ciardelli G.Collagen for bone tissue regeneration. Acta Biomater 8,3191, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Dumont C., Burchhardt H., Dresing K., Rudy T., Bohr S., and Stürmer K.M.Free scapular or parascapular flaps for soft tissue damage accompanying talus or calcaneus fractures. Chirurg 78,643, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dumont C., Fuchs M., Burchhardt H., Appelt D., Bohr S., and Stürmer K.M.Clinical results of absorbable plates for displaced metacarpal fractures. J Hand Surg Am 32,491, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fuchs M., Burchhardt H., Dresing K., Radebold T., and Stürmer K.M.Resection of the calcaneus as a treatment option in osteitis following an open calcaneus fracture. Unfallchirurg 103,602, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Balcarek P., Burchhardt H., and Stürmer K.M.Minimally invasive removal of a broken femoral nail. Unfallchirurg 108,419, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Keller G.Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 19,1129, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Krawetz R.J., Taiani J.T., Wu Y.E., Liu S., Meng G., Matyas J.R., et al. Collagen I scaffolds cross-linked with beta-glycerol phosphate induce osteogenic differentiation of embryonic stem cells in vitro and regulate their tumorigenic potential in vivo. Tissue Eng 18,1014, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Damaraju S., Matyas J.R., Rancourt D., and Duncan N.The effect of mechanical stimulation on mineralization in differentiating osteoblasts in collagen-I scaffolds. Tissue Eng Part A 20,3142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batra N., Kar R., and Jiang J.X.Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta 1818,1909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J.X., Siller-Jackson A.J., and Burra S.Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci 12,1450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale B., Gualtieri R., Talevi R., Tosti E., Santella L., and Elder K.Intercellular communication in the early human embryo. Mol Reprod Dev 29,22, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Stains J.P., and Civitelli R.Cell-to-cell interactions in bone. Biochem Biophys Res Commun 328,721, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Sims N.A., and Martin T.J.Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep 3,481, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonna S., and Sims N.A.Talking among ourselves: paracrine control of bone formation within the osteoblast lineage. Calcif Tissue Int 94,35, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Mullender M., El Haj A., Yang Y., van Duin M., Burger E., and Klein-Nulend J.Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput 42,14, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Boppart M.D., Kimmel D.B., Yee J.A., and Cullen D.M.Time course of osteoblast appearance after in vivo mechanical loading. Bone 23,409, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Chen J.-H., Liu C., You L., and Simmons C.A.Boning up on Wolff's Law: Mechanical regulation of the cells that make and maintain bone. J Biomech 43,108, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hazenbiller O.Micro-environmental factors directing differentiation of murine embryonic stem cells down osteogenic and chondrogenic lineages [master's thesis]. University of Calgary, p. 203, 2013. Available at http://theses.ucalgary.ca/handle/11023/554, accessed May, 2014 [Google Scholar]
- 27.Sanchez C., Gabay O., Salvat C., Henrotin Y.E., and Berenbaum F.Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthritis Cartilage 17,473, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez C., Pesesse L., Gabay O., Delcour J.-P., Msika P., Baudouin C., et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum 64,1193, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Gabay O., Hall D., Berenbaum F., Henrotin Y., and Sanchez C.Osteoarthritis and obesity: experimental models. Jt Bone Spine 75,675, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson A.D., Marvel S.W., Bernacki S.H., Banes A.J., van Aalst J., and Loboa E.G.Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng 37,955, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Gross T.S., Poliachik S.L., Ausk B.J., Sanford D.A., Becker B.A., and Srinivasan S.Why rest stimulates bone formation: a hypothesis based on complex adaptive phenomenon. Exerc Sport Sci Rev 32,9, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan S., Ausk B.J., Poliachik S.L., Warner S.E., Richardson T.S., and Gross T.S.Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol 102,1945, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kang M.-N., Yoon H.-H., Seo Y.-K., and Park J.-K.Effect of mechanical stimulation on the differentiation of cord stem cells. Connect Tissue Res 53,149, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Li S., Jia X., Duance V.C., and Blain E.J.The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. Eur Cell Mater 21,508, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Qi J., Chi L., Wang J., Sumanasinghe R., Wall M., Tsuzaki M., et al. Modulation of collagen gel compaction by extracellular ATP is MAPK and NF-kappaB pathways dependent. Exp Cell Res 315,1990, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Davidson J.S., and Baumgarten I.M.Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther 246,1104, 1988 [PubMed] [Google Scholar]
- 37.Schiller P.C., D'Ippolito G., Balkan W., Roos B.A., and Howard G.A.Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone 28,362, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Thorpe S.D., Buckley C.T., Steward A.J., and Kelly D.J.European Society of Biomechanics S.M. Perren Award 2012: the external mechanical environment can override the influence of local substrate in determining stem cell fate. J. Biomech 45,2483, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Proulx A., Merrifield P.A., and Naus C.C.Communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet 20,133, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Hunter C.J., Matyas J.R., and Duncan N.A.The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine (Phila. Pa. 1976) 29,1099, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Desrochers J., and Duncan N.A.Intercellular communication via gap junctions affected by mechanical load in the bovine annulus fibrosus. Comput Methods Biomech Biomed Engin 17,37, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Batra N., Burra S., Siller-Jackson A.J., Gu S., Xia X., and Weber G.F.Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. PNAS 109,3359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song I.-H., Caplan A.I., and Dennis J.E.In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J Orthop Res 27,916, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Sheehan D.C., and Hrapchak B.B.Theory and Practice of Histotechnology. Columbus, OH: Battelle Press, 1987. [Google Scholar]
- 45.Villanueva A.R., Kujawa M., Mathews C.H., and Parfitt A.M.Identification of the mineralization front: comparison of a modified toluidine blue stain with tetracycline fluorescence. Metab Bone Dis Relat Res 5,41, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Möller B., Wiltfang J., Acil Y., Gierloff M., Lippross S., and Terheyden H.Prevention of the surface resorption of bone grafts by topical application of bisphosphonate on different carrier materials. Clin Oral Investig 18,2203, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Halpern J., Lynch C.C., Fleming J., Hamming D., Martin M.D., Schwartz H.S., et al. The application of a murine bone bioreactor as a model of tumor: bone interaction. Clin Exp Metastasis 23,345, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Moursi A.M., Globus R.K., and Damsky C.H.Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci 110 (Pt 1),2187, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Schmittgen T.D., and Livak K.J.Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3,1101, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Kuchipudi S.V., Tellabati M., Nelli R.K., White G.A., Perez B.B., Sebastian S., et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol J 9,230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyström K., Biller M., Grahn A., Lindh M., Larson G., and Olofsson S.Real time PCR for monitoring regulation of host gene expression in herpes simplex virus type 1-infected human diploid cells. J Virol Methods 118,83, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Bas A., Forsberg G., Hammarström S., and Hammarström M.-L.Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol 59,566, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Wredenberg A., Wibom R., Wilhelmsson H., Graff C., Wiener H.H., Burden S.J., et al. Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci USA 99,15066, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker B.A., Rao K.M.K., Mercer R.R., Geronilla K.B., Kashon M.L., Miller G.R., et al. Quantitative histology and MGF gene expression in rats following SSC exercise in vivo. Med Sci Sports Exerc 38,463, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Cheng B., Zhao S., Luo J., Sprague E., Bonewald L.F., and Jiang J.X.Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res 16,249, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Waggett A.D., Benjamin M., and Ralphs J.R.Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol 85,1145, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Genetos D.C., Kephart C.J., Zhang Y., Yellowley C.E., and Donahue H.J.Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212,207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor A.F., Saunders M.M., Shingle D.L., Cimbala J.M., Zhou Z., and Donahue H.J.Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol 292,C545, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Gramsch B., Gabriel M., Wiemann M., Grummer R., Winterhager E., Bingmann D., and Schirrmacher K.Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res 264,397, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Lecanda F., Towler D.A., Ziambaras K., Cheng S.L., Koval M., Steinberg T.H., et al. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell 9,2249, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]