Abstract
The aim of this study was to systematically review clinical studies examining biofluid biomarkers of brain injury for concussion in athletes. Data sources included PubMed®, MEDLINE®, and the Cochrane Database from 1966 to October 2013. Studies were included if they recruited athletes participating in organized sports who experienced concussion or head injury during a sports-related activity and had brain injury biomarkers measured. Acceptable research designs included experimental, observational, and case-control studies. Review articles, opinion papers, and editorials were excluded. After title and abstract screening of potential articles, full texts were independently reviewed to identify articles that met inclusion criteria. A composite evidentiary table was then constructed and documented the study title, design, population, methods, sample size, outcome measures, and results. The search identified 52 publications, of which 13 were selected and critically reviewed. All of the included studies were prospective and were published either in or after the year 2000. Sports included boxing (six studies), soccer (five studies), running/jogging (two studies), hockey (one study), basketball (one study), cycling (one study), and swimming (one study). The majority of studies (92%) had fewer than 100 patients. Three studies (23%) evaluated biomarkers in cerebrospinal fluid (CSF), one in both serum and CSF, and 10 (77%) in serum exclusively. There were 11 different biomarkers assessed, including S100β, glial fibrillary acidic protein, neuron-specific enolase, tau, neurofilament light protein, amyloid beta, brain-derived neurotrophic factor, creatine kinase and heart-type fatty acid binding protein, prolactin, cortisol, and albumin. A handful of biomarkers showed a correlation with number of hits to the head (soccer), acceleration/deceleration forces (jumps, collisions, and falls), postconcussive symptoms, trauma to the body versus the head, and dynamics of different sports. Although there are no validated biomarkers for concussion as yet, there is potential for biomarkers to provide diagnostic, prognostic, and monitoring information postinjury. They could also be combined with neuroimaging to assess injury evolution and recovery.
Key words: : biomarkers, concussion, sports, systematic review, traumatic brain injury
Introduction
Concussion is also known as mild traumatic brain injury (TBI) and is an unfortunately common occurrence in athletes. Diagnosis of concussion acutely depends on a variety of measures, including neurological examination, neuropsychological evaluation, and neuroimaging. Neuroimaging techniques, such as computed tomographic scanning (CT scan) and magnetic resonance imaging (MRI) are used to provide objective information. However, CT scanning has low sensitivity to diffuse brain damage and confers exposure to radiation. MRI can provide information on the extent of diffuse injuries, but its widespread application is restricted by cost, availability, and its yet undefined role in management of mild TBI (mTBI).1,2 Moreover, conventional neuroimaging techniques and neuropsychological tests often fail to adequately detect injury, in particular, the recognition of diffuse axonal injury, also known as traumatic axonal injury.3 There are promising new neuroimaging techniques being examined that include functional MRI, diffusion tensor imaging, magnetic resonance spectroscopy, and positron emission tomography.4–15 However, the role of these techniques in the clinical management of concussion has not yet been established.16
Research in the field of TBI biomarkers has increased exponentially over the last 20 years,17,18 with most of the publications on the topic of TBI biomarkers occurring in the last 10 years.18–20 Accordingly, studies assessing biomarkers in TBI have looked at a number of potential markers that could lend diagnostic, prognostic, as well as monitoring information. Early and tailored management of athletes after a concussion would provide them with the best opportunity to avoid further injury. Early detection of concussion would be invaluable given that individuals with concussion are acutely at risk for bleeding and axonal injury21,22 and long term can suffer impairment of physical, cognitive, and psychosocial functioning.23–27 Repeated episodes of mTBI can lead to chronic traumatic encephalopathy (CTE), a term used to describe clinical changes in cognition, mood, personality, behavior, and/or movement occurring years after concussion.28,29 With the growing incidence of CTE among athletes, strategies that reduce the risk of becoming injured need to be developed and diagnostic tools that could identify injuries earlier need to be explored.
This systematic review will review the current literature on biofluid biomarkers of brain injury in athletes after sports-related concussion and discuss their potential role.
Methods
A literature search of PubMed®, MEDLINE®, and the Cochrane Database from 1966 to October 2013 was conducted using the MESH search terms athletes, concussion, sports, sports-related, traumatic brain injury, head injury, and biomarkers. Other terms also searched included biochemical markers, neuronal/glial/axonal injury, traumatic intracranial lesions, and expansions of these terms to match synonyms, subterms, or derivatives. These terms were searched in all fields of publication (e.g., title, abstract, and keyword). The search was limited to the English-language articles, clinical “human” studies, and studies that included athletes participating in organized sports. Studies were included if they recruited athletes participating in organized sports who experienced concussion or head injury during a sports-related activity and had brain injury biofluid biomarkers measured. Articles that did not include athletes or did not measure brain-related biofluid biomarkers as a primary focus were excluded. In addition, the bibliographies and reference lists of all articles and all review articles were evaluated for other potentially relevant articles. Acceptable study designs included experimental studies, observational studies, and case-control studies. Review articles, opinion papers, and editorials were excluded. The abstracts of the publications were screened for relevance, and in case of uncertainty regarding the inclusion, the entire text of the article was read. Studies were defined as prospective or retrospective according to whether the method of data collection and the endpoints were defined before patient enrollment began. The full texts of the articles were then pooled and reviewed by two different authors to identify articles that met inclusion criteria. Once the relevant articles were selected, they were reviewed using a standard review form. The review forms allowed the reviewers to objectively assess the content of each article in a consistent fashion. A composite evidentiary table was then constructed. The evidentiary table included the internal identification number, design type, study methods, focus of the article, sample size, TBI severity, biofluid source, collection schedule, clinical variables assessed, outcome measures, results, and conclusions.
Results
The search initially identified 52 articles. Nineteen publications were then selected on the basis of the title and abstract screening. Inclusion criteria were applied to the full text of 19 articles. A review of the bibliographies and reference lists identified an additional four potential articles that had a full text review. In total, 23 (19+4) articles underwent a full text review and 13 of these met all selection criteria and were included in the systematic review. Details of the study selection process are outlined in Figure 1. Studies that were screened, but not included in the review, can be found in Appendix 1. Each of the 13 studies was critically reviewed by at least two investigators using a standard review form. There were no randomized, clinical control studies identified. All of the included studies were prospective and were published either in or after the year 2000. Eight (62%) of the studies utilized control populations. The range in sample size was from 16 to 200. Over 92% of the included studies had fewer than 100 patients. There were three studies (23%) that evaluated biomarkers in cerebrospinal fluid (CSF), one that evaluated both serum and CSF, and 10 (77%) that evaluated serum exclusively.
FIG. 1.
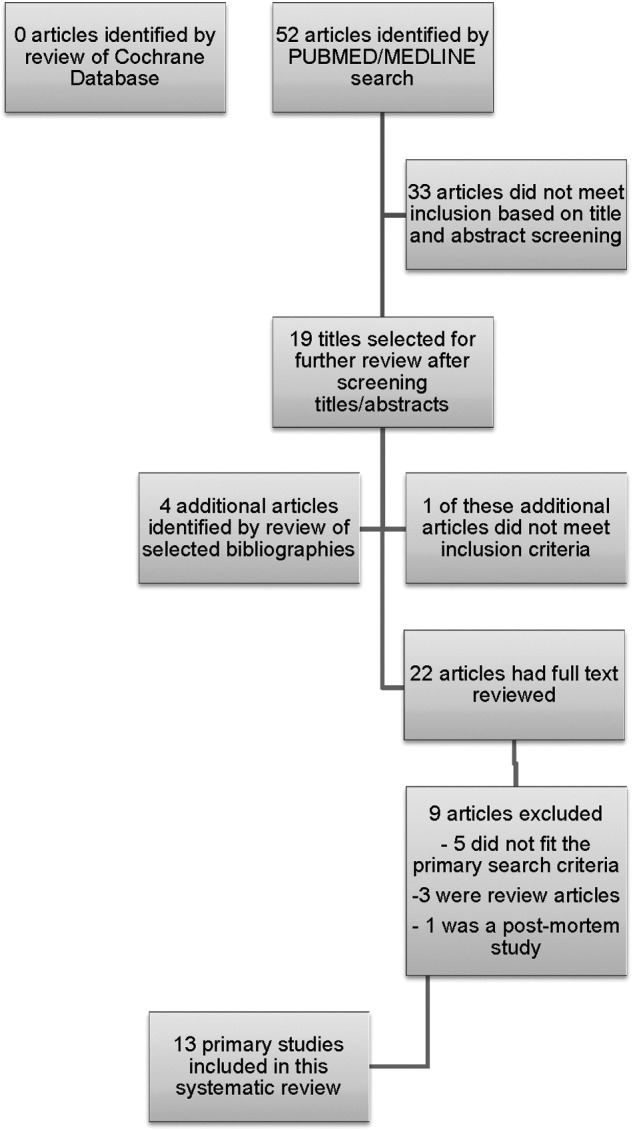
Study selection process. The search initially identified 52 articles. Nineteen publications were then selected on the basis of the title and abstract screening. A review of the bibliographies and reference lists identified an additional four potential articles that had a full text review. In total, 23 (19+4) articles underwent a full text review and 13 of these met all selection criteria and were included in the systematic review.
The age range of subjects in the selected articles was 11–52 years. Seven studies included studies that included both adults and children (defined as younger than 18 years); however, only two studies included subjects younger than 17 years. The performance of the biomarkers in the majority of these studies spanned across ages and did not specifically reflect performance of the biomarker in specific age groups. The evidentiary table (Table 1) summarizes the methods from the included studies and describes the biomarkers that were evaluated in the athletes.30–42 Sports examined in these studies included boxing (six studies), soccer (five studies), running/jogging (two studies), hockey (one study), basketball (one study), cycling (one study), and swimming (one study).
Table 1.
Evidence Table Summarizing Studies Assessing Biomarkers in Athletes
| Studies primarily measuring biomarkers in serum | ||||||
|---|---|---|---|---|---|---|
| Year/ author | Sample size | Sport | Ages (years) | Type of fluid and biomarker | Sample time points and collection times | Outcome measure |
| 2000 Otto | Total, 84 25 amateur boxers 11 runners (25-km race) 12 joggers 12 sprinters 12 cyclists 12 soccer players (headers only) |
Boxing, running, sprinting, cycling, soccer | Boxers 17–40 (median, 20) Runners-25km 20–44 (median, 32) Joggers 23–52 (median, 30) Sprinters 25–52 (median, 30) Cyclists 23–52 (median, 29) Soccer players 20–52 (median, 26) |
Serum S100B |
2 sample time points Pre- and postsport (within 15 min) |
Effect of different sports on levels of S100B Pre- and postsport biomarker levels |
| 2003 Dietrich | Total, 16 swimmers | Swimming | 17–41 (mean, 25) | Serum S100B, prolactin |
2 sample time points Presport (24 h before) and postsport (within 15 min) |
Pre- and postsport biomarker levels |
| 2003 Mussack | Total, 200 61 heading 58 no-heading soccer players 81 trauma patients with TBI |
Soccer | 12–17 (median, 15) TBI/trauma, 27–61 |
Serum S100B |
3 sample time points For soccer: First sample: presoccer training Second sample: postsoccer (60 min) Third sample: postsoccer (360 min) For TBI First sample: 64 and 355 min Second/third samples: 65 and 366 min |
Pre- and postsport biomarker levels Changes in biomarker over time Biomarker levels in sports versus accidental trauma |
| 2003 Stalnacke | Total, 44 26 hockey players 18 basketball players |
Hockey, basketball | Hockey (mean, 28±4) Basketball (mean, 25±4) |
Serum S100B, NSE |
2 sample time points Presport (1–2 h before) and postsport (within 1 h) |
Pre- and postsport biomarker levels Number of acceleration/deceleration events RPSQ |
| 2004 Hasselblatt | Total, 18 runners | Marathon running | 31–47 (mean, 39±8) |
Serum S100B, GFAP, CK |
5 sample time points Pre- and postrace at 0, 1, 3, and 20 h after |
Pre- and postsport biomarker levels Comparison to other markers |
| 2004 Stalnacke | Total, 28 soccer players | Soccer | 21–31 (mean, 26±5) |
Serum S100B, NSE |
2 sample time points Presoccer (1–5 h before) and postsoccer (immediately) |
Pre- and postsport biomarker levels Correlation to headers and other trauma events RPSQ (24–48 h post) |
| 2006 Stalnacke | Total, 44 soccer players | Soccer | Mean, 23±3 | Serum S100B, NSE |
2 sample time points Pre- and postsoccer (immediately) |
Pre- and postsport biomarker levels Effect of other traumatic events (jumps, collisions, falls) |
| 2009 Zetterberg | Total, 67 44 boxers 23 controls |
Boxing | Boxers, 17–28 (median, 19) Controls, 19–50 (median, 28) |
Serum S-100B, BDNF, h-FABP, GFAP, and NSE |
2 sample time points Pre- and postboxing (after 2 months of no boxing activity) |
Athletes vs. controls Chronic biomarker levels |
| 2011 Graham | Total, 16 boxers divided into two groups Punches to head (PTH) Punches to body (PTB) |
Boxing | PTH, 11–29 (mean 18±5) PTB, 16–24 (mean, 19±3) |
Serum S100B, NSE, CK, cortisol |
2 sample time points Preboxing (1 h before) and postboxing (after 5 min) |
Pre- and postsport biomarker levels Effects of PTH versus PTB on biomarkers |
| 2013 Neselius | Total, 55 30 boxers 25 controls |
Boxing | Boxers, 17–34 (mean, 22) Controls, 17–30 (mean, 22) |
Serum S100B, GFAP, BDNF, Aβ1-42, Tau |
2 sample time points Postboxing (after 1–6 days and again after 14 days without any boxing) Controls once |
Athletes vs. controls Subacute biomarker levels |
| Studies primarily measuring biomarkers in CSF | ||||||
|---|---|---|---|---|---|---|
| 2006 Zetterberg | Total, 24 14 boxers 10 controls |
Boxing | Boxers, 22±3.8 Controls, 30±6.3 |
CSF NFLP, T-tau, GFAP, P-tau, β amyloid protein |
2 sample time points Postboxing (after 7–10 days and again after 3 months without any boxing) Controls once |
Athletes vs. controls Chronic biomarker levels |
| 2007 Zetterberg | Total, 33 23 soccer players 10 controls |
Soccer | Soccer players with 10 approved headings, 19–32 (median, 26) Soccer players with 20 approved headings, 20–28 (median, 23) |
Serum S100B, albumin CSF NFLP, T-tau, GFAP, S100B, albumin |
1 sample time point Postsoccer headings (after 7–10 days) |
Athletes vs. controls Correlation to number of headings |
| 2012 Neselius | Total, 5 30 olympic boxers 25 controls |
Boxing | Boxers, 17–34 (mean, 22) Controls, 17–30 (mean, 22) |
CSF NFL, GFAP, T-tau, P-tau181, Aβ1-42, S100B, h-FABP |
2 sample time points Postboxing (after 1–6 days and again after 14 days without any boxing) Controls once |
Athletes vs. controls Subacute biomarker levels |
Aβ1-42, amyloid β1-42; Aβ1-40, amyloid β1-40; BDNF, brain-derived neurotrophic factor; CK, creatine kinase; CSF, cerebrospinal fluid; GFAP, glial fibrillary acidic protein; NFL(P), neurofilament light protein; h-FABP, heart-type fatty acid binding protein; NSE, neuron-specific enolase; P-tau, phosphorylated tau at threonine; RPSQ, Rivermead Post-Concussion Symptom Questionnaire; T-tau, total tau; TBI, traumatic brain injury; TNF, tumor necrosis factor.
There were 11 distinct biomarkers measured in 13 studies, and S100β was the most frequently assessed in 12 studies (92%). Glial fibrillary acidic protein (GFAP) was evaluated in six studies, neuron-specific enolase (NSE) in five studies, tau in four studies, neurofilament light protein (NFL) in three studies, and amyloid beta in three studies. Brain-derived neurotrophic factor (BDNF), creatinine kinase (CK), and heart-type fatty acid binding protein (h-FABP) were each measured in two studies, and prolactin, cortisol, and albumin were each evaluated in one study. Nine studies assessed biomarkers both before and after play or exercise (69%), whereas four studies only evaluated biomarkers afterward (31%). Blood samples were taken at different times postinjury, but all were taken within 3 months postplay or exercise. Time points included baseline levels (11 studies), within 15 min (five studies), 15–60 min (three studies), between 1–24 h (five studies), 24 h to 2 weeks (six studies), and 2–3 months (three studies). Multiple postplay/exercise time points were taken in four different studies. Besides comparing biomarker concentrations pre- and postplay or exercise, other outcome measures included comparison of levels between different sports, comparison of body trauma to head trauma in boxing, results of the Rivermead Post-Concussion Symptom Questionnaire (RPSQ), effect of headers in soccer, comparison to trauma patients, effect of acceleration/deceleration events, and effect of other traumatic events, such as jumps, collisions, and falls. A summary of the results from each of the studies is included in Table 2.
Table 2.
Summary of the Results and Findings from Each of the Included Studies
| Year/author | Results |
|---|---|
| 2000 Otto | - There was no significant difference between the baseline S-100B levels in the groups boxing, jogging, running, or cycling (p=0.12). Baseline levels of S-100B protein in serum ranged between 10 and 169 ng/L (mean, 35 ng/L; median, 22 ng/L). - The increase in S-100B resulting from boxing was significantly higher than that after headers (p<0.0001), cycling (p=0.0002), and sprinting (p<0.02). - There was no significant difference in the rise between the boxing, jogging, and the 25-km race groups (p=0.27). - Competitive boxing resulted in significantly higher levels of S-100B than jogging (p<0.01). - Competitive boxing and 25-km race resulted in S100B elevations that were NOT significantly different (p=0.9). - Sparring boxing was not significantly different from jogging, running, or the 25-km race (p=0.21). - Sprinting caused significantly higher elevations in S-100B than cycling (p=0.021) or headers (p=0.009). There were no difference between cycling and headers (p=0.69). - In boxing, S100B correlated with the number and weight of the punches and boxers fighting without head protectors had higher levels of S-100B than those without protectors. |
| 2003 Dietrich | - S100B was statistically different from baseline 70.7±17.7 pg/mL to after the swimming race 108.13±19.49 pg/mL (p<0.001). Following the race, 4 of 16 (25%) did not have an increase in S100B from their baseline levels. - Prolactin increased from 10.2±0.9 to 16±2.2 ng/dL (p<0.001) pre- and postrace. There was no correlation between S100B and prolactin. |
| 2003 Mussack | - Median S100B serum levels of the heading group increased from 0.15 to 0.18 ng/mL (p<0.05) after training. Levels returned to baseline after 6 h. S100B levels of the no-heading exercise group barely changed from 0.10 to 0.11 ng/mL after training (p>0.05). At 6 h, levels were 0.09 ng/mL. Levels of S100B were significantly lower in the no-heading group, compared to the heading group. - Baseline levels were higher in younger players ages 12–13 (0.20 ng/mL) and 14–15 (0.17 ng/mL), compared to those ages 16–17 (0.06 ng/mL; p=0.006 and p<0.001, respectively). - CT+ levels were higher (0.62 ng/mL) than CT− levels (0.10 ng/mL). Levels in the CT+ group were significantly higher than both the heading and no-heading groups. |
| 2003 Stalnacke | - For ice hockey, S100B levels increased from Pregame=0.22±0.04 μg/L (range, 0.14–0.32) to Postgame=0.30±0.11 μg/L (range, 0.17–0.44; p<0.001). - For basketball, S100B levels increased from Pregame=0.22±0.04 μg/L (range, 0.17–0.28) to Postgame=0.30±0.10 μg/L (range, 0.17–0.51; p=0.001). - For ice hockey, NSE levels increased from Pregame=10.19±3.35 μg/L (range, 7.46–19.75) to Postgame=11.7±3.36 μg/L (range, 7.99–22.39; p=0.13) - For basketball, NSE levels increased from Pregame=9.71±2.93 μg/L (range, 6.4–17.33] to Postgame=10.26±3.06 μg/L (range, 7.22–19.88; p=0.13) |
| 2004 Hasselblatt | - Serum S100B concentrations and serum CK activities increased after the race (p<0.001). After 20 h, serum S100B concentrations decreased and 83% were within the reference levels. - Serum GFAP concentrations remained below detection at all time points. - Three hours after the race, an increase in serum CK activity by 500 U/L was associated with an increase of serum S100B by 0.05 ug/L, compared to baseline. Elevated S100B levels were associated with elevated CK levels immediately postrace, at 1, 3, and 20 h. - Serum S100B and CK levels were not associated with gender, age, or training status. |
| 2004 Stalnacke | - S100B levels increased from Pregame=0.066±0.025 μg/L to Postgame=0.118±0.040 μg/L (p<0.001) - NSE levels increased from Pregame=8.57±2.31 μg/L to Postgame=10.29±2.16 μg/L (p<0.001) - Elevations in S-100B pre- to postgame were significantly correlated with the number of headers (r=0.428; p=0.02) and with the number of other traumatic events (r=0.453; p=0.02). - Changes in NSE were not significantly correlated with headers or other traumatic events. No concussions were observed during the game. - There were no significant correlations between the total RPQ score and changes in S-100B (p=0.130) or changes in NSE (p=0.603). |
| 2006 Stalnacke | - S-100B levels increased from 0.11±0.05 μg/L pregame to 0.18±0.11 postgame (p=0.001). - NSE levels increased from 9.05±1.59 μg/L pregame to 10.14±1.74 μg/L postgame (p=0.001). - The changes in S-100B correlated significantly with the number of headers (without jumps/collisions/falls; r=0.307; p=0.042), number of headers (with jumps/collisions/falls; r=0.474; p=0.001), and with the number of other traumatic events (r=0.517; p=0.001). - The changes in NSE were not significantly correlated with the number of headers or other traumatic events. |
| 2009 Zetterberg | - For S100B, the median level for boxers was 70 ng/L (range, 32–240), compared to controls (65 ng/L; range, 19–13; p>0.05). - For BDNF, the median level for boxers was 1.6 ng/mL (range, 0.303–10), compared to controls (1.2 ng/mL; range, 0.29–10; p>0.05). - For h-FABP, the median level for boxers was 1.5 ng/mL (range, 0.39–2.9), compared to controls (1.8 ng/mL; range, 0.94–13; p>0.05). - For NSE, the median level for boxers was 11 ng/mL (range, 2.3–41), compared to controls (4.8 ng/mL; range, 0.78–27; p=0.014). - For GFAP, levels were below the detection limit of the assay in all samples (<0.78 ng/mL). - Serum levels of NSE did not correlate with age, body mass index, age at boxing debut, boxing duration, or total number of bouts. |
| 2011 Graham | - There were significant increases in NSE, S100B, and cortisol in those with the punches to the head (PTH) group, but not the punches to the body (PTB) group. CK significantly increased in both PTH and PTB groups. - S100B: PTH group: pre, 0.35±0.61; post, 0.54±0.73 PTB group: pre, 0.42±0.19, post, 0.43±0.2 - CK: PTH group: pre, 207±107; post, 244±118 PTB group: pre, 50±43; post, 195±63 - NSE: PTH group: pre, 19.7±14; post, 31.1±26.6 PTB group: pre, 16.4±13; post, 17.5±14 - Cortisol: PTH group: pre, 373±202; post, 756±93 PTB group: pre, 416±140; post, 417±135 |
| 2013 Neselius | - Plasma tau concentrations significantly increased after a bout, compared to control levels (2.46±5.10 vs. 0.79±0.961 ng/L; p=0.038). - The other biomarkers were not significantly elevated. - Tau decreased significantly after a rest period to 1.43±2.5 ng/L (p=0.030). - There were no differences in concentrations of BDNF, Aβ1-41, and S100B between boxers and controls. Additionally, there were no differences for these biomarkers between the two time points (1–6 vs. 14 days). - For GFAP, all samples were below detection. - For BDNF (mean±SD) Controls=29,146±5419 ng/L Boxers (1–6 days)=28,353±7170 Boxers (14 days)=27, 836±7621 - For Aβ42: Controls=11.6±4.4 ng/L (range, 0.7–18.9) Boxers (1–6 days)=12.1±4.8 (range, 4.0–26.9) Boxers (14 days)=11.2±.2 (range, 0.0–20.1) - For S100B: Controls=0.041±0.025 ng/L (range, 0.011–0.137) Boxers (1–6 days)=0.037±0.018 (range, 0.015–0.088) Boxers (14 days)=0.043±0.024 (range, 0.014–0.118) - For tau: Controls=0.79±0.96 ng/L (range, 0.02–4.76) Boxers (1–6 days)=2.46±5.1 (range, 0.13–26.73) Boxers (14 days)=1.43±2.51 (range, 0.02–11.60) |
| 2006 Zetterberg | - After a bout, there was a marked increase in the CSF levels of NFL, T-tau, and GFAP, compared to after a 3-month rest from boxing (mean±SD). - NFL=845±1140 vs. 208±108 ng/L (p=0.008) - T-tau=449±176 vs. 306±78 ng/L (p=0.006) - GFAP=541±199 vs. 405±138 ng/L (p=0.003). - Levels of NFL and GFAP, but not T-tau, were significantly higher in boxers after a bout than in controls. - For GFAP and T-tau, there were no significant differences in biomarker levels in boxers after the 3-month rest period and controls. However, NFL remained significantly elevated in boxers after 3 months, compared to controls. - NFL, T-tau, and GFAP concentrations were higher in boxers who had received many hits (>15) or high-impact hits to the head, compared with boxers who reported few hits. - Levels of P-tau, Aβ(1–40), and Aβ(1–42) were not significantly altered in boxers after a bout, compared with after rest or levels detected in controls. |
| 2007 Zetterberg | - There were no significant differences in biomarker levels (CSF or serum) in soccer players who performed either 10 or 20 approved headings and no significant differences in biomarker levels (CSF or serum) in soccer players (either 10 or 20 headings) versus controls. - Surprisingly, S-100B concentrations were higher in the control group compared to players with 10 or 20 approved headings (p=0.049 and p=0.008). - There were no correlations between the number of approved headings and any of the biomarker levels. - For albumin ratio (median/range): Controls=4.1 (range, 2.5–6.3) Players (10 headings)=4.1 (range, 2.4–9.3) Players (20 headings)=3.9 (range, 2.0–8.7) - For NF-L, all levels were less than 125 ng/L. - For T-tau: Controls=320 ng/L (range, 120–540) Players (10 headings)=315 ng/L (range, 170–400) Players (20 headings)=250 ng/L (range, 190–420) - For GFAP: Controls=280 ng/L (range, 190–460) Players (10 headings)=265 ng/L (range, 180–510) Players (20 headings)=260 ng/L (range, 190–330) - For S100B in CSF: Controls=1.1 μg/L (range, 0.77–1.2) Players (10 headings)=0.87μg/L (range, 0.71–1.2) Players (20 headings)=0.82 μg/L (range, 0.48–1.3) - For S100B in serum: Controls=0.040 μg/L (range, 0.030–0.060) Players (10 headings)=0.06 μg/L (range, 0.03–0.12) Players (20 headings)=0.04 μg/L (range, 0.01–0.07) |
| 2012 Neselius | - Levels of NFL (p=0.001), GFAP (p=0.001), T-tau (p=0.025), and S-100B (p=0.03) were significantly increased after boxing, compared to controls. - Levels of NFL (p=0.004) and GFAP (p=0.001) remained elevated after the 14-day rest period. - For NFL (mean±SD) Controls=135±51 ng/L (range, 125–380) Boxers (1–6 days)=532±553 (range, 125–2480) Boxing (14 days)=402±220 (range, 125–1780) - For GFAP: Controls=244±145 ng/L (range, 90–820)] Boxing (1–6 days)=496±238 (range, 70–1020) Boxing (14 days)=367±113 (range, 170–600) - For FABP: Controls=458±271 ng/L (range, 67–1383) Boxing (1–6 days)=407±208 (range, 108–1089) Boxing (14 days)=334±195 (range, 40–769) - For Aβ1-42: Controls=297±39 ng/L (range, 231–362) Boxing (1–6 days)=306±52 (range, 191–411) Boxing (14 days)=294±54 (range, 178–423) - For S100B: Controls=0.60±0.23 ng/L (range, 0.30–1.16) Boxing (1–6 days)=0.76±0.29 (range, 0.34–1.68) Boxing (14 days)=0.63±0.16 (range, 0.33–0.99) - For T-tau: Controls=45±17 ng/L (range, 24–95) Boxing (1–6 days)=58±25 (range, 25–132) Boxing (14 days)=49±21 (range, 19–121) - For P-tau: Controls=23±6 ng/L (range, 14–40) Boxing (1–6 days)=21±7 (range, 9–38) Boxing (14 days)=22±8 (range, 9–43) |
Aβ1-42, amyloid β1-42; Aβ1-40, amyloid β1-40; BDNF, brain-derived neurotrophic factor; CK, creatine kinase; CSF, cerebrospinal fluid; GFAP, glial fibrillary acidic protein; NFL(P), neurofilament light protein; h-FABP, heart-type fatty acid binding protein; NSE, neuron-specific enolase; P-tau, phosphorylated tau at threonine; RPSQ, Rivermead Post-Concussion Symptom Questionnaire; SD, standard deviation; T-tau, total tau; TBI, traumatic brain injury; TNF, tumor necrosis factor.
The association between headers in soccer players and biomarkers were assessed in five studies.30,32,35,36,41 In the article by Otto and colleagues, 12 soccer players performed 20 controlled headers and showed no rise in S100β protein levels. Similarly, controlled headers in studies by Mussack and Zetterberg produced insignificant elevations in S100β.32,41 However, these headers were performed in a controlled setting where the ball was dropped from a specified height and always impacted the forehead. This is in contrast to a competitive match in which the ball may be traveling faster and with greater force, and may not impact the head on the forehead. Accordingly, Stalnacke and colleagues measured S100β during actual soccer matches and found that S100β levels increased significantly after a game and also correlated with the number of headers.35,36 There were no significant changes in levels of NSE.
There was only one study that compared biomarker levels in athletes participating in sports versus trauma patients with head injury presenting to the emergency department.32 A single biomarker (S100β) was examined in TBI patients versus soccer players. Levels were significantly higher in trauma patients with lesions on CT than trauma patients without lesions. Further, trauma patients with lesions on CT had significantly higher levels than the athletes regardless of whether or not there was heading.
Discussion
This systematic review of the literature provides a comprehensive summary of the status of brain injury biomarker research in sports and concussion. The study of biomarkers in sports concussion is in its infancy, with the earliest published study in this review in the year 2000. Although there are a number of interesting candidate biomarkers for determining severity of concussion, validation of these markers is lacking. There are a handful of biomarkers showing correlation with number of hits to the head (e.g., headers in soccer), acceleration/deceleration forces (e.g., jumps, collisions, and falls with and without head injury), postconcussive symptoms (PCS), trauma to the body versus the head (e.g., boxing), and the effects/dynamics of play. Unfortunately, the studies are difficult to combine and compare because the included sports are so different (some have contact, others do not), sample collection times are variable, and the assays used to measure the biomarkers are not uniform.
The most frequently examined biomarker among the studies in this review, and in the TBI literature as a whole, is S100β. S100β is the major low-affinity calcium-binding protein in astrocytes,43 which helps to regulate intracellular levels of calcium; however, its brain specificity has been questioned. Findings of elevated levels of S100β in athletes participating in noncontact sports without head trauma support the concern about its potential release from other cells, such as chondrocytes and adipocytes.44,45 In the study by Otto and colleagues, S100β protein rose after running, with no significant difference in levels of S100β between jogging, running, a 25-km race, and boxing, suggesting that S100β was derived from extracranial sources.30 Similarly, in Dietrich and colleagues' study, swimming increased S100β levels independent of any head trauma.31In the study by Hasselblatt and colleagues, both serum S100β and CK concentrations increased significantly after a marathon race and were correlated. As such, S100β levels exceeded those studied in other sports, such as joggers, short-distance runners, basketball players, and ice hockey players observed in other studies.30,33 This issue has also been observed in the trauma literature in patients with mTBI.19,46,47
Tau is an intracellular, microtubule-associated protein that is highly enriched in axons and is involved with assembling axonal microtubule bundles.48 In 2013, when Neselius and colleagues measured tau in plasma, levels were significantly increased after a bout of Olympic boxing, compared to control levels, and decreased significantly after a rest period. These elevations were in boxers who had no symptoms of concussion. Moreover, in 2012, when CSF levels of tau were examined in this same group of boxers, there was also a significant increase in tau, but there was no correlation between plasma and CSF-tau.42 There are inconsistencies in the performance of tau (in the form of cleaved-tau, total-tau, and phosphorylated-tau) that are echoed in the trauma literature. Studies assessing tau in CSF in severe TBI have correlated with clinical outcome.49–54 However, these findings have not held true when measured in peripheral blood50,55 or in mTBI, where tau is a poor predictor of CT lesions and postconcussion syndrome.56–59 These inconsistencies could be a result of many factors, including the type, variability, sensitivity, and specificity of the tau assays used, variability in the measurement of outcomes, and the timing of the sample collection.
Permanent neurological impairment is a serious concern for athletes who experience repetitive head traumas given that both concussive and subconcussive blows can be significantly damaging.16,60,61 Accordingly, we have observed, through this review, that biomarkers can remain elevated even after resting from their sport. Zetterberg and colleagues measured S100β and NSE (found in neuronal cell bodies) after 2 months of nonparticipation in boxing and found that NSE showed a prolonged decay in boxers who were exposed to very frequent, repetitive head trauma during most of the year.37,40 Similarly, Neselius and colleagues showed that the repetitive head trauma occurring in olympic boxing induced increases in CSF levels of NFL (from neuron cytoskeleton), GFAP (glial origin), T-tau, and S-100B acutely and subacutely (after 14 days without boxing), even without anamnestic or clinical symptoms of a concussion or TBI.42 A recent study by Shahim and colleagues assessed T-tau, S100B, and NSE in professional ice hockey players preseason and postconcussion at 1, 12, 36, and 144 h. T-tau levels peaked during the first hour after concussion and were significantly higher in postconcussion samples at all times, compared with preseason samples. S100B also peaked within the first hour, but was only significantly higher at 1 h after concussion, compared with preseason. NSE remained at preseason levels and was not significantly elevated at any time point. Interestingly, T-tau after concussion remained significantly elevated in players with PCS lasting more than 6 days versus players with PCS for less than 6 days.62
GFAP is a monomeric intermediate protein found in astroglial skeleton that was first isolated by Eng and colleagues in 1971.63 GFAP is found in white and gray brain matter and is strongly up-regulated during astrogliosis.64 Although GFAP has been studied in brain injury since the 1990s, it is not until recently that it has been assessed in serum following trauma3,46,65,66 and, specifically, in sports.34,37,39 The performance of GFAP has been much better (more accurate in detecting injury) in trauma patients presenting with mTBI to the emergency department, compared with the studies performed in athletes to date. This discrepancy appears to stem from the timing of the blood draws relative to head injury. In studies with trauma patients, samples have been drawn within 446,65 and 24 h after injury.3,66 However, in athletes, the samples have been drawn after several days39 or months.37 This underscores the importance of understanding the temporal profile of the biomarkers being applied in studies.18,67
There are a number of new serum biomarkers on the horizon for mTBI, including ubiquitin C-terminal hydrolase (UCH-L1)66,68 and alpha-II spectrin breakdown products (SBDP150).69,70 The UCH-L1 protein is involved in the addition and removal of ubiquitin from proteins that are destined for metabolism.71 Alpha-II-spectrin (280 kDa) is the major structural component of the cortical membrane cytoskeleton and is particularly abundant in axons and presynaptic terminals.72,73 Cytoskeletal αII-spectrin is cleaved by caspase-3 and calpain-2 activation into spectrin breakdown products (SBDPs),74,75 which are detectable after TBI. Both have shown a significant association with acute measures of injury severity in mTBI, such as Glasgow Coma Score score, intracranial injuries on CT, and neurosurgical intervention.66,68–70
Over the last decade, research in the field of sports concussion biomarkers has led to a greater understanding of the effects of head injury from sports. Moving forward, there are many challenges to consider and overcome as we continue to pursue the clinical application of brain-related biofluid biomarkers in sports. First, biomarkers are being compared to variable definitions of concussion and to subjective outcome measures. It is difficult to rate the predictive power of serum biomarkers if the clinical measures they are being compared against are inconsistent and lack sensitivity and/or specificity. Second, common clinical and biomarker-related data elements need to be consistently applied to future studies on sports concussion, given that they are currently being employed for all severities of TBI.76,77 Third, timing of outcome measures relative to the biomarkers need to be carefully considered in the design of future studies. Finally, sample collection for biomarker measurement will need to span longitudinally over multiple time points in order to assess their temporal profiles. This, in turn, will be useful for determining optimal times to measure levels of these markers after concussion and for guiding return-to-play decisions.
Conclusion
In an effort to prevent CTE and long-term consequences of concussion, early diagnostic and prognostic tools are becoming increasingly important, particularly in sports and in military personnel, where concussions are common occurrences. The study of TBI biomarkers is rapidly evolving, and should these biomarkers be validated and become widely available, they could have many roles. They could help with clinical decision making by clarifying injury severity and help to monitor progression of injury and/or recovery. Biomarkers could have a role in managing patients at high risk of repeated injury and could be incorporated into guidelines for return to duty, work, or sports activities. They could also be combined with neuroimaging to improve diagnostic and prognostic accuracy as injuries evolve over time. Future studies will require more uniform research methodology, common data elements, and consistent performance measures.
Appendix 1
Articles that were screened but not included in the systematic review
1. Barkhoudarian, G., Hovda, D.A., and Giza, C.C. (2011). The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 30, 33–48, vii–iii.
2. Baugh, C.M., Stamm, J.M., Riley, D.O., Gavett, B.E., Shenton, M.E., Lin, A., Nowinski, C.J., Cantu, R.C., McKee, A.C., and Stern, R.A. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 6, 244–254.
3. Bernick, C., Banks, S., Phillips, M., Lowe, M., Shin, W., Obuchowski, N., Jones, S., and Modic, M. (2013). Professional fighters brain health study: rationale and methods. Am. J. Epidemiol. 178, 280–286.
4. Biasca, N., and Maxwell, W.L. (2007). Minor traumatic brain injury in sports: a review in order to prevent neurological sequelae. Prog. Brain Res. 161, 263–291.
5. Blennow, K., Hardy, J., and Zetterberg, H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899.
6. Casson, I.R., Viano, D.C., and Pellman, E.J. (2008). Synopsis of the National Football League Player Health and Safety Meeting: Chicago, Illinois, June 19, 2007. Neurosurgery 62, 204–209; discussion, 9–10.
7. Chan, C.P., Wan, T.S., Watkins, K.L., Pelsers, M.M., Van der Voort, D., Tang, F.P., Lam, K.H., Mill, J., Yuan, Y., Lehmann, M., Hempel, A., Sanderson, J.E., Glatz, J.F., and Renneberg, R. (2005). Rapid analysis of fatty acid-binding proteins with immunosensors and immunotests for early monitoring of tissue injury. Biosens. Bioelectron. 20, 2566–2580.
8. Czirják, S., Rácz, K., and Góth, M. (2012). Neuroendocrine dysfunctions and their consequences following traumatic brain injury. [Article in Hungarian]. Orv. Hetil. 153, 927–933.
9. Davis, G.A., Iverson, G.L., Guskiewicz, K.M., Ptito, A., and Johnston, K.M. (2009). Contributions of neuroimaging, balance testing, electrophysiology and blood markers to the assessment of sport-related concussion. Br. J. Sports Med. 43, Suppl. 1, i36–i45.
10. DeKosky, S.T., Blennow, K., Ikonomovic, M.D., and Gandy, S. (2013). Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200.
11. Dziemianowicz, M.S., Kirschen, M.P., Pukenas, B.A., Laudano, E., Balcer, L.J., and Galetta, S.L. (2012). Sports-related concussion testing. Curr. Neurol. Neurosci. Rep. 12, 547–559.
12. Esopenko, C., and Levine, B. (2014). Aging, neurodegenerative disease and traumatic brain injury: the role of neuroimaging. J. Neurotrauma Sep 5. [Epub ahead of print]
13. Faviou, E., Zachari, A., Nounopoulos, C., Agrafiotis, E., Vourli, G., Dionyssiou-and Asteriou, A. (2008). Elevation of serum N-terminal pro-brain natriuretic peptide after exercise is an index of myocardial damage or a cytoprotective reflection? J. Sports Med. Phys. Fitness 48, 90–96.
14. Finnoff, J.T., Jelsing, E.J., and Smith, J. (2011). Biomarkers, genetics, and risk factors for concussion. PM R. 3, 10 Suppl. 2, S452–S459.
15. Gordon, K.E. (2010). Apolipoprotein E genotyping and concussion: time to fish or cut bait. Clin. J. Sport Med. 20, 405–406.
16. Harmon, K.G., Drezner, J., Gammons, M., Guskiewicz, K., Halstead, M., Herring, S., Kutcher, J., Pana, A., Putukian, M., and Roberts, W. (2013). American Medical Society for Sports Medicine position statement: concussion in sport. Clin. J. Sport Med. 23, 1–18.
17. Johnson, V.E., Stewart, W., Graham, D.I., Stewart, J.E., Praestgaard, A.H., and Smith, D.H. (2009). A neprilysin polymorphism and amyloid-beta plaques after traumatic brain injury. J. Neurotrauma 26, 1197–1202.
18. Jordan, B.D. (2013). The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 9, 222–230.
19. Kilbourne, M., Kuehn, R., Tosun, C., Caridi, J., Keledjian, K., Bochicchio, G., Scalea, T., Gerzanich, V., and Simard, J.M. (2009). Novel model of frontal impact closed head injury in the rat. J. Neurotrauma 26, 2233–2243.
20. Kirchhoff, C., Leidel, B.A., Kirchhoff, S., Braunstein, V., Bogner, V., Kreimeier, U., Mutschler, W., and Biberthaler, P. (2008). Analysis of N-terminal pro-B-type natriuretic peptide and cardiac index in multiple injured patients: a prospective cohort study. Crit. Care 12, R118.
21. Konig, D., Neubauer, O., Nics, L., Kern, N., Berg, A., Bisse, E., and Wagner, K.H. (2007). Biomarkers of exercise-induced myocardial stress in relation to inflammatory and oxidative stress. Exerc. Immunol. Rev. 13, 15–36.
22. Kristman, V.L., Tator, C.H., Kreiger, N., Richards, D., Mainwaring, L., Jaglal, S., Tomlinson, G., and Comper, P. (2008). Does the apolipoprotein epsilon 4 allele predispose varsity athletes to concussion? A prospective cohort study. Clin. J. Sport Med. 18, 322–328.1
23. McKee, A.C., Cantu, R.C., Nowinski, C.J., Hedley-Whyte, E.T., Gavett, B.E., Budson, A.E., Santini, V.E., Lee, H.S., Kubilus, C.A., and Stern, R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735.
24. Melanson, S.E., Green, S.M., Wood, M.J., Neilan, T.G., and Lewandrowski, E.L. (2006). Elevation of myeloperoxidase in conjunction with cardiac-specific markers after marathon running. Am. J. Clin. Pathol. 126, 888–893.
25. Mez, J., Stern, R.A., and McKee, A.C. (2013). Chronic traumatic encephalopathy: where are we and where are we going? Curr. Neurol. Neurosci. Rep. 13, 407.
26. Neumayr, G., Pfister, R., Mitterbauer, G., Eibl, G., and Hoertnagl, H. (2005). Effect of competitive marathon cycling on plasma N-terminal pro-brain natriuretic peptide and cardiac troponin T in healthy recreational cyclists. Am. J. Cardiol. 96, 732–735.
27. Ohba, H., Takada, H., Musha, H., Nagashima, J., Mori, N., Awaya, T., Omiya, K., Murayama, M. (2001). Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am. Heart J. 141, 751–758.
28. Ojo, J.O., Mouzon, B., Greenberg, M.B., Bachmeier, C., Mullan, M., and Crawford, F. (2013). Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 72, 137–151.
29. Papa, L., Ramia, M.M., Kelly, J.M., Burks, S.S., Pawlowicz, A., and Berger, R.P. (2013). Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J. Neurotrauma 30, 324–338.
30. Papa, L., Robinson, G., Oli, M., Pineda, J., Demery, J., Brophy, G., Robicsek, S.A., Gabrielli, A., Robertson, C.S., Wang, K.W., and Hayes, R.L. (2008). Use of biomarkers for diagnosis and management of traumatic brain injury patients. Exp. Opin. Med. Diagn. 2, 937–945.
31. Pelsers, M.M., Hermens, W.T., and Glatz, J.F. (2005). Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta 352, 15–35.
32. Pontier, J.M., Gempp, E., and Ignatescu, M. (2012). Blood platelet-derived microparticles release and bubble formation after an open-sea air dive. Appl. Physiol. Nutr. Metab. 37, 888–892.
33. Salvagno, G.L., Schena, F., Gelati, M., Danese, E., Cervellin, G., Guidi, G.C., Lippi, G. (2014). The concentration of high-sensitivity troponin I, galectin-3 and NT-proBNP substantially increase after a 60-km ultramarathon. Clin. Chem. Lab. Med. 52, 267–272.
34. Scharhag, J., George, K., Shave, R., Urhausen, A., and Kindermann, W. (2008). Exercise-associated increases in cardiac biomarkers. Med. Sci. Sports Exerc. 40, 1408–1415.
35. Serrano-Ostariz, E., Legaz-Arrese, A., Terreros-Blanco, J.L., Lopez-Ramon, M., Cremades-Arroyos, D., Carranza-Garcia, L.E., Izquierdo-Alvarez, S., and Bocos-Terraz, P. (2009). Cardiac biomarkers and exercise duration and intensity during a cycle-touring event. Clin. J. Sport Med. 19, 293–299.
36. Stavrinou, L.C., Kalamatianos, T., Stavrinou, P., Papasilekas, T., Psachoulia, C., Tzavara, C., and Stranjalis, G. (2011). Serum levels of S-100B after recreational scuba diving. Int. J. Sports Med. 32, 912–915.
37. Sun, D., McGinn, M.J., Zhou, Z., Harvey, H.B., Bullock, M.R., and Colello, R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272.
38. Tanriverdi, F., Unluhizarci, K., Coksevim, B., Selcuklu, A., Casanueva, F.F., and Kelestimur, F. (2007). Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin. Endocrinol. (Oxf.) 66, 360–366.
39. Tate, C.M., Wang, K.K., Eonta, S., Zhang, Y., Carr, W., Tortella, F.C., Hayes, R.L., and Kamimori, G.H. (2013). Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J. Neurotrauma 30, 1620–1630.
40. Turner, R.C., Lucke-Wold, B.P., Robson, M.J., Omalu, B.I., Petraglia, A.L., and Bailes, J.E. (2013). Repetitive traumatic brain injury and development of chronic traumatic encephalopathy: a potential role for biomarkers in diagnosis, prognosis, and treatment? Front. Neurol. 3, 186.
41. Vagnozzi, R., Signoretti, S., Floris, R., Marziali, S., Manara, M., Amorini, A.M., Belli, A., Di Pietro, V., D'Urso, S., Pastore, F.S., Lazzarino, G., and Tavazzi, B. (2013). Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. J. Head Trauma Rehabil. 28, 284–292.
42. Vanitallie, T.B. (2013). Preclinical sporadic Alzheimer's disease: target for personalized diagnosis and preventive intervention. Metabolism 62, Suppl 1, S30–S33.
43. Zetterberg, H., Smith, D.H., and Blennow, K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210.
Acknowledgments
The project described was supported, in part, by Award Number R01NS057676 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the National Institutes of Health.
Author Disclosure Statement
Dr. Papa is a consultant of Banyan Biomarkers, Inc., but receives no stocks or royalties from the company and will not benefit financially from this publication.
References
- 1.Kesler S.R., Adams H.F., and Bigler E.D. (2000). SPECT, MR and quantitative MR imaging: correlates with neuropsychological and psychological outcome in traumatic brain injury. Brain Inj. 14, 851–857 [DOI] [PubMed] [Google Scholar]
- 2.Jagoda A.S., Bazarian J.J., Bruns J.J., Jr., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R. (2008). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 3.Metting Z., Wilczak N., Rodiger L.A., Schaaf J.M., and van der Naalt J. (2012). GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 4.Gajawelli N., Lao Y., Apuzzo M.L., Romano R., Liu C., Tsao S., Hwang D., Wilkins B., Lepore N., and Law M. (2013). Neuroimaging changes in the brain in contact versus noncontact sport athletes using diffusion tensor imaging. World Neurosurg. 80, 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazarian J.J., Zhu T., Blyth B., Borrino A., and Zhong J. (2012). Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 30, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAllister T.W., Sparling M.B., Flashman L.A., and Saykin A.J. (2001). Neuroimaging findings in mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 23, 775–791 [DOI] [PubMed] [Google Scholar]
- 7.McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., and Saykin AJ. (2001). Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 8.Ptito A., Chen J.K., and Johnston K.M. (2007). Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation 22, 217–227 [PubMed] [Google Scholar]
- 9.Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Bello J.A., and Branch C.A. (2008). Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 10.Gasparovic C., Yeo R., Mannell M., Ling J., Elgie R., Phillips J., Doezema D., and Mayer A.R. (2009). Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J. Neurotrauma 26, 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slobounov S.M., Gay M., Zhang K., Johnson B., Pennell D., Sebastianelli W., Horovitz S., and Hallett M. (2011). Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. Neuroimage 55, 1716–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K., Johnson B., Gay M., Horovitz S.G., Hallett M., Sebastianelli W., and Slobounov S. (2012). Default mode network in concussed individuals in response to the YMCA physical stress test. J. Neurotrauma 29, 756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slobounov S.M., Zhang K., Pennell D., Ray W., Johnson B., and Sebastianelli W. (2010). Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp. Brain Res. 202, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., and Peterson D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 [DOI] [PubMed] [Google Scholar]
- 15.Huang M.X., Theilmann R.J., Robb A., Angeles A., Nichols S., Drake A., D'Andrea J., Levy M., Holland M., Song T., Ge S., Hwang E., Yoo K., Cui L., Baker D.G., Trauner D., Coimbra R., and Lee R.R. (2009). Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 16.Dashnaw M.L., Petraglia A.L., and Bailes J.E. (2013). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus 33, E5, 1–9 [DOI] [PubMed] [Google Scholar]
- 17.Kochanek P.M., Berger R.P., Bayr H., Wagner A.K., Jenkins L.W., and Clark R.S. (2008). Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care 14, 135–1141 [DOI] [PubMed] [Google Scholar]
- 18.Papa L. (2012). Exploring the role of biomarkers for the diagnosis and management of traumatic brain injury patients, in: Poteomics—Human Diseases and Protein Functions. Man T.K., and Flores R.J. (eds). ISBN:978-953-307-832-8. InTech Open Access Publisher. doi: 10.5772/31776 [DOI] [Google Scholar]
- 19.Papa L, Ramia M.M., Kelly J.M., Burks S.S., Pawlowicz A., and Berger R.P. (2013). Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J. Neurotrauma 30, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papa L. (2014). Biomarkers for concussion, in: Concussions in Athletics: From Brain to Behavior. Randolph J., and Sebastianelli W. (eds). Springer: New York [Google Scholar]
- 21.Benson R.R., Gattu R., Sewick B., Kou Z., Zakariah N., Cavanaugh J.M., and Haacke E.M. (2013). Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: implications for neurorehabilitation. NeuroRehabilitation 31, 261–279 [DOI] [PubMed] [Google Scholar]
- 22.Govind V., Gold S., Kaliannan K., Saigal G., Falcone S., Arheart K.L., Harris L., Jagid J., and Maudsley A.A. (2010). Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma 27, 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millis S.R., Rosenthal M., Novack T.A., Sherer M., Nick T.G., Kreutzer J.S., High W.M., Jr., and Ricker J.H. (2001). Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 16, 343–355 [DOI] [PubMed] [Google Scholar]
- 24.Alves W., Macciocchi S., and Barth J.T. (1993). Postconcussive symptoms after uncomplicated mild head injury. J. Head Trauma Rehabil. 8, 48–59 [Google Scholar]
- 25.Rimel R.W, Giordani B., Barth J.T., Boll T.J., and Jane J.A. (1981). Disability caused by minor head injury. Neurosurgery 9, 221–228 [PubMed] [Google Scholar]
- 26.Alexander M.P. (1995). Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 27.Barth J.T., Macciocchi S.N., Giordani B., Rimel R., Jane J.A., and Boll T.J. (1983). Neuropsychological sequelae of minor head injury. Neurosurgery 13, 529–533 [DOI] [PubMed] [Google Scholar]
- 28.Gavett B.E., Stern R.A., and McKee A.C. (2010). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med.30, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavett B.E., Cantu R.C., Shenton M., Lin A.P., Nowinski C.J., McKee A.C., and Stern RA. (2012). Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr. Opin. Neurol. 24, 525–531 [DOI] [PubMed] [Google Scholar]
- 30.Otto M., Holthusen S., Bahn E., Sohnchen N., Wiltfang J., Geese R., Fischer A., and Reimers C.D. (2000). Boxing and running lead to a rise in serum levels of S-100B protein. Int. J. Sports Med. 21, 551–555 [DOI] [PubMed] [Google Scholar]
- 31.Dietrich M.O., Tort A.B., Schaf D.V., Farina M., Goncalves C.A., Souza D.O., and Portela L.V. (2003). Increase in serum S100B protein level after a swimming race. Can. J. Appl. Physiol. 28, 710–716 [DOI] [PubMed] [Google Scholar]
- 32.Mussack T., Dvorak J., Graf-Baumann T., and Jochum M. (2003). Serum S-100B protein levels in young amateur soccer players after controlled heading and normal exercise. Eur. J. Med. Res. 8, 457–464 [PubMed] [Google Scholar]
- 33.Stalnacke B.M., Tegner Y., and Sojka P. (2003). Playing ice hockey and basketball increases serum levels of S-100B in elite players: a pilot study. Clin. J. Sport Med. 13, 292–302 [DOI] [PubMed] [Google Scholar]
- 34.Hasselblatt M., Mooren F.C., von Ahsen N., Keyvani K., Fromme A., Schwarze-Eicker K., Senner V., and Paulus W. (2004). Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology 62, 1634–1636 [DOI] [PubMed] [Google Scholar]
- 35.Stalnacke B.M., Tegner Y., and Sojka P. (2004). Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players: a pilot study. Brain Inj. 18, 899–909 [DOI] [PubMed] [Google Scholar]
- 36.Stalnacke B.M., Ohlsson A., Tegner Y., and Sojka P. (2006). Serum concentrations of two biochemical markers of brain tissue damage S-100B and neurone specific enolase are increased in elite female soccer players after a competitive game. Br. J. Sports Med. 40, 313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zetterberg H., Tanriverdi F., Unluhizarci K., Selcuklu A., Kelestimur F., and Blennow K. (2009). Sustained release of neuron-specific enolase to serum in amateur boxers. Brain Inj. 23, 723–726 [DOI] [PubMed] [Google Scholar]
- 38.Graham M.R., Myers T., Evans P., Davies B., Cooper S.M., Bhattacharya K., Grace F.M., and Baker J.S. (2011). Direct hits to the head during amateur boxing is associated with a rise in serum biomarkers for brain injury. Int. J. Immunopathol. Pharmacol. 24, 119–125 [DOI] [PubMed] [Google Scholar]
- 39.Neselius S., Zetterberg H., Blennow K., Randall J., Wilson D., Marcusson J., and Brisby H. (2013). Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 27, 425–433 [DOI] [PubMed] [Google Scholar]
- 40.Zetterberg H., Hietala M.A., Jonsson M., Andreasen N., Styrud E., Karlsson I., Edman A., Popa C., Rasulzada A., Wahlund L.O., Mehta P.D., Rosengren L., Blennow K., and Wallin A. (2006). Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 [DOI] [PubMed] [Google Scholar]
- 41.Zetterberg H., Jonsson M., Rasulzada A., Popa C., Styrud E., Hietala M.A., Rosengren L., Wallin A., and Blennow K. (2007). No neurochemical evidence for brain injury caused by heading in soccer. Br. J. Sports Med. 41, 574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neselius S., Brisby H., Theodorsson A., Blennow K., Zetterberg H., and Marcusson J. (2012). CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One 7, e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong H., Liang W.L., Wu X.R. (2000). Pathophysiological alterations in cultured astrocytes exposed to hypoxia/reoxygenation. [Article in Chinese]. Sheng Li Ke Xue Jin Zhan 31, 217–221 [PubMed] [Google Scholar]
- 44.Haimoto H., Hosoda S., and Kato K. (1987). Differential distribution of immunoreactive S100-a and S100-b proteins in normal nonnervous human tissues. Lab. Invest. 57, 489–498 [PubMed] [Google Scholar]
- 45.Donato R. (1999). Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450, 191–231 [DOI] [PubMed] [Google Scholar]
- 46.Papa L., Silvestri S., Brophy G.M., Giordano P., Falk J.L., Braga C.F., Tan C.N., Ameli N.J., Demery J.A., Dixit N.K., Mendes M.E., Hayes R.L., Wang K.K., and Robertson C.S. (2014). GFAP out-performs S100B In detecting traumatic intracranial lesions On CT In trauma patients with mild traumatic brain injury and those with extracranial lesions. J. Neurotrauma 31, 1815–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippidis A.S., Papadopoulos D.C., Kapsalaki E.Z., and Fountas K.N. (2011). Role of the S100B serum biomarker in the treatment of children suffering from mild traumatic brain injury. Neurosurg. Focus 29, E2. [DOI] [PubMed] [Google Scholar]
- 48.Teunissen C.E., Dijkstra C., and Polman C. (2005). Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 4, 32–41 [DOI] [PubMed] [Google Scholar]
- 49.Shaw G.J., Jauch E.C., and Zemlan F.P. (2002). Serum cleaved tau protein levels and clinical outcome in adult patients with closed head injury. Ann. Emerg. Med. 39, 254–257 [DOI] [PubMed] [Google Scholar]
- 50.Zemlan F.P., Jauch E.C., Mulchahey J.J., Gabbita S.P., Rosenberg W.S., Speciale S.G., and Zuccarello M. (2002). C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 947, 131–139 [DOI] [PubMed] [Google Scholar]
- 51.Franz G., Beer R., Kampfl A., Engelhardt K., Schmutzhard E., Ulmer H., and Deisenhammer F. (2003). Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 60, 1457–1461 [DOI] [PubMed] [Google Scholar]
- 52.Marklund N., Blennow K., Zetterberg H., Ronne-Engstrom E., Enblad P., and Hillered L. (2009). Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J. Neurosurg. 110, 1227–1237 [DOI] [PubMed] [Google Scholar]
- 53.Ost M., Nylen K., Csajbok L., Ohrfelt A.O., Tullberg M., Wikkelso C., Nellgard P., Rosengren L., Blennow K., and Nellgard B. (2006). Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67, 1600–1604 [DOI] [PubMed] [Google Scholar]
- 54.Sjogren M., Blomberg M., Jonsson M., Wahlund L.O., Edman A., Lind K., Rosengren L., Blennow K., and Wallin A. (2001). Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J. Neurosci. Res. 66, 510–516 [DOI] [PubMed] [Google Scholar]
- 55.Chatfield D.A., Zemlan F.P., Day D.J., and Menon D.K. (2002). Discordant temporal patterns of S100beta and cleaved tau protein elevation after head injury: a pilot study. Br. J. Neurosurg. 16, 471–476 [DOI] [PubMed] [Google Scholar]
- 56.Ma M, Lindsell C.J., Rosenberry C.M., Shaw G.J., and Zemlan F.P. (2008). Serum cleaved tau does not predict postconcussion syndrome after mild traumatic brain injury. Am. J. Emerg. Med. 26, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bazarian J.J., Zemlan F.P., Mookerjee S., and Stigbrand T. (2006). Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 20, 759–765 [DOI] [PubMed] [Google Scholar]
- 58.Bulut M., Koksal O., Dogan S., Bolca N., Ozguc H., Korfali E., Ilcol Y.O., and Parklak M. (2006). Tau protein as a serum marker of brain damage in mild traumatic brain injury: preliminary results. Adv. Ther. 23, 12–22 [DOI] [PubMed] [Google Scholar]
- 59.Kavalci C., Pekdemir M., Durukan P., Ilhan N., Yildiz M., Serhatlioglu S., and Seckin D. (2007). The value of serum tau protein for the diagnosis of intracranial injury in minor head trauma. Am. J. Emerg. Med. 25, 391–395 [DOI] [PubMed] [Google Scholar]
- 60.Matser E.J., Kessels A.G., Lezak M.D., Jordan B.D., and Troost J. (1999). Neuropsychological impairment in amateur soccer players. JAMA 282:971-3 [DOI] [PubMed] [Google Scholar]
- 61.Echemendia R.J., Putukian M., Mackin R.S., Julian L., and Shoss N. (2001). Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin. J. Sport Med. 11, 23–31 [DOI] [PubMed] [Google Scholar]
- 62.Shahim P., Tegner Y., Wilson D.H., Randall J., Skillback T., Pazooki D., Kallberg B., Blennow K., and Zetterberg H. (2014). Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 71, 684–692 [DOI] [PubMed] [Google Scholar]
- 63.Eng L.F., Vanderhaeghen J.J., Bignami A., and Gerstl B. (1971). An acidic protein isolated from fibrous astrocytes. Brain Res. 28, 351–354 [DOI] [PubMed] [Google Scholar]
- 64.Duchen L.W. (1984). General pathology of neurons and neuroglia, in: Greenfield's Neuropathology, Adams J.A., Corsellis J.A.N., and Duchen L.W. (eds). Edward Arnold: London, pp. 1–52 [Google Scholar]
- 65.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz-Arrastia R., Wang K.K., Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., Okonkwo D.O., and Manley G.T.; TRACK-TBI Investigators. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-l1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papa L., Robinson G., Oli M., Pineda J., Demery J., Brophy G., Robicsek S.A., Gabrielli A., Robertson C.S., Wang K.W., and Hayes R.L. (2008). Use of biomarkers for diagnosis and management of traumatic brain injury patients. Exp. Opin. Med. Diagn. 2, 937–945 [DOI] [PubMed] [Google Scholar]
- 68.Papa L., Lewis L.M., Silvestri S., Falk J.L., Giordano P., Brophy G.M., Demery J.A., Liu M.C., Mo J., Akinyi L., Mondello S., Schmid K., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 72, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papa L., Braga C.F., Tan C.N., Ameli N.J., Peterson S.A., Burks S.S., Dixit N.K., and Demery J.A. (2013). Elevated levels of serum SBDP150 in the emergency department are associated with poor outcome at one month from mild and moderate traumatic brain injury. Acad. Emerg. Med. 20(abst) [Google Scholar]
- 70.Papa L, Wang K.W., Brophy G.B., Demery J.A., Silvestri S., Giordano P., Falk J.L., Schmid K., Tortella F.C., Hayes R.L., and Robertson C.S. (2012). Serum levels of spectrin breakdown product 150 (SBDP150) distinguish mild traumatic brain injury from trauma and uninjured controls and predict intracranial injuries on CT and neurosurgical intervention. J. Neurotrauma 29, A39–A40 [Google Scholar]
- 71.Tongaonkar P., Chen L., Lambertson D., Ko B., and Madura K. (2000). Evidence for an interaction between ubiquitin-conjugating enzymes and the 26S proteasome. Mol. Cell. Biol. 20, 4691–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodman S.R., Zimmer W.E., Clark M.B., Zagon I.S., Barker J.E., and Bloom M.L. (1995). Brain spectrin: of mice and men. Brain Res. Bull. 36, 593–606 [DOI] [PubMed] [Google Scholar]
- 73.Riederer B.M., Zagon I.S., and Goodman S.R. (1986). Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J. Cell Biol. 102, 2088–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pike B.R., Flint J., Dave J.R., Lu X.C., Wang K.K., Tortella F.C., and Hayes R.L. (2004). Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb. Blood Flow Metab. 24, 98–106 [DOI] [PubMed] [Google Scholar]
- 75.Ringger N.C., O'Steen B.E., Brabham J.G., Silver X., Pineda J., Wang K.K., Hayes R.L., and Papa L. (2004). A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J. Neurotrauma 21, 1443–1456 [DOI] [PubMed] [Google Scholar]
- 76.Hicks R., Giacino J., Harrison-Felix C., Manley G., Valadka A., and Wilde E.A. (2013). Progress in developing common data elements for traumatic brain injury research: version two—the end of the beginning. J. Neurotrauma 30, 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maas A.I., Harrison-Felix C.L., Menon D., Adelson P.D., Balkin T., Bullock R., Engel D.C., Gordon W., Langlois-Orman J., Lew H.L., Robertson C., Temkin N., Valadka A., Verfaellie M., Wainwright M., Wright D.W., and Schwab K. (2010). Standardizing data collection in traumatic brain injury. J. Neurotrauma 28, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]


