Abstract
ISG15 conjugation (ISGylation) to proteins is a multistep process involving interferon (IFN)-inducible UBE1L (E1), UbcH8 (E2), and ISG15 E3 ligases (E3s). Studies performed over the past several years have shown that ISGylation plays a pivotal role in the host antiviral response against certain viruses. Recent in vitro studies revealed that human Herc5 and mouse Herc6 are major ISG15 E3 ligases, respectively. However, the global function of Herc5/6 proteins in vivo still remains unclear. Here, we report generation and initial characterization of Herc6 knockout mice. Substantial reductions of ISGylation were observed in Herc6-deficient cells after polyinosinic-polycytidylic acid double-stranded RNA injection of mice or IFN treatment of cells. On the other hand, Herc6-deficient cells and wild-type (WT) cells had similar responses to IFN stimulation, Sendai virus (Z strain) infection, and vesicular stomatitis virus infection. These results indicate that Herc6 does not play a critical role in antiviral defense of these viral infections in mice. Interestingly, male Herc6-deficient mice showed seminal vesicle hypertrophy. No such problem was detected in WT and ISG15 activating enzyme Ube1L-deficient mice. These results suggest that in addition to promoting protein ISGylation, Herc6 has a novel and protein ISGylation-independent function in the male reproductive system.
Introduction
ISG15 is a 17 kDa ubiquitin-like modifier. Its expression is rapidly induced by type I interferon (IFN) (Bedford and others 2011). Similar to ubiquitin, ISG15 is conjugated to lysines on broad target proteins through the reaction of specific E1-activating (UBE1L), E2-conjugating (UbcH8), and E3-ligase enzymes (Yuan and Krug 2001; Kim and others 2004; Dastur and others 2006; Wong and others 2006). The deconjugation of ISG15 from cellular proteins is carried out by USP18 (Burkart and others 2013). Previous in vitro knockdown studies suggested that human Herc5 and mouse Herc6 are the main ISG15 E3 ligases to mediate global conjugation in human cells and mouse cells, respectively (Wong and others 2006; Oudshoorn and others 2012). Mice do not possess the Herc5 gene among the Herc family genes. Human Herc6, which is the closest relative to human Herc5, was devoid of any ISG15 E3 ligase activity (Hochrainer and others 2005; Dastur and others 2006; Oudshoorn and others 2012).
ISG15-mediated antiviral activity against influenza, herpes, and Sindbis virus has been shown in vivo by means of infections in ISG15−/− and UBE1L−/− mice (Lenschow and others 2005, 2007; Lai and others 2009; Lenschow 2010). In addition, in vitro studies for either the overexpression of ISG15 or knockdown of ISG15 using siRNA have implicated ISGylation in the regulation of influenza B virus, vaccinia virus, Sindbis virus, herpes simplex-1 virus, Sendai virus, and Japanese encephalitis virus, as well as in the release of virus-like particles derived from HIV-1 and avian sarcoma leukosis virus (Okumura and others 2006, 2008; Guerra and others 2008; Malakhova and Zhang 2008; Hsiang and others 2009; Hsiao and others 2010; Lenschow 2010; Pincetic and others 2010). Although the mechanism by which ISG15 is regulating viral growth is still unknown for the majority of these viruses, it has been reported that ISG15 achieves its antiviral role by conjugating to target proteins, including both host proteins and viral proteins, and altering their functions. For example, ISG15 can be conjugated to host antiviral protein interferon regulatory factor 3 (IRF3) and, thus, stabilize IRF3 by inhibiting its interaction with peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), a protein that promotes IRF3 ubiquitination and degradation (Shi and others 2010). In most overexpression studies, ISG15 and its conjugation-related enzymes have been coexpressed, suggesting that ISG15 conjugation to target proteins is required for these antiviral effects. Despite these observations with either in vivo or in vitro studies, there are some controversial phenotypes: No differences in viral growth of influenza A, herpes simplex virus-1, Sindbis, and wild-type (WT) vaccinia virus in ISG15−/− mouse embryonic fibroblast cells (MEFs) and vesicular stomatitis virus (VSV) in UBE1L−/− MEFs have also been reported (Osiak and others 2005; Kim and others 2006; Lenschow and others 2007; Guerra and others 2008).
A recent report indicated that human Herc5 globally targets de novo synthesized proteins for ISG15 conjugation, thereby making viral proteins major targets for ISGylation (Durfee and others 2010). However, the question remains whether Herc5 and global ISGylation are important for antiviral activity in vivo, as ISGylation-mediated antiviral effects might be due to other minor ISG15 E3 ligases with more narrow specificity, such as estrogen-responsive finger protein (EFP, also called TRIM25) (Park and others 2014). Since there is no mouse ortholog of human HERC5, the other members of HERC family proteins have been examined for ISG15 E3 activity. A recent report shows that Herc6 knockdown in mouse L929 cells abolished global ISGylation, whereas its overexpression enhanced ISGylation as well as IFN-β production, and conferred antiviral activity against vesicular stomatitis virus and Newcastle disease virus, which indicated that Herc6 is likely the functional antiviral factor in mouse cells (Oudshoorn and others 2012).
Here, we established the Herc6 null mice to study their role in protein ISGylation in vivo. Herc6 knockout mice lacked protein ISGylation. Herc6-deficient primary MEFs and bone marrow-derived macrophages (BMDM) lost the capacity to conjugate ISG15 to a broad group of proteins. These analyses with our newly generated Herc6 knockout mice confirmed the previous finding in the in vitro system that Herc6 is the major ISG15 E3 ligase in mice. Furthermore, we examined STAT1 phospharylation on IFN-β treatment, production of IFN-β and IL-6 after SV infection, or double-stranded RNA poly I:C treatment of WT and Herc6−/− BMDM. No significant difference was detected. In addition, the virus titers of VSV in WT and Herc6−/− MEFs were similar. These results indicate that Herc6-mediated protein ISGylation has no obvious effect on IFN signaling and antiviral activity against SV and VSV under current experimental conditions. As a critical different phenotype from ISG15 E1 Ube1L knockout mice that also lack protein ISGylation, male Herc6-deficient mice showed severe seminal vesicle hypertrophy. This finding suggests that Herc6 has a role in regulating sperm sac morphology, which is independent of protein ISGylation.
Materials and Methods
Generation of Herc6 knockout mice
The Herc6 mutant mice (accession No. CDB0585K; www.cdb.riken.jp/arg/mutant%20mice%20list.html) were generated by gene targeting in TT2 embryonic stem (ES) cells (Yagi and others 1993), as previously described (www.cdb.riken.jp/arg/Methods.html). Two Herc6 mutant mouse strains (line 1, #29 and line 2, #51) were established from independent homologous recombinant ES cells, and no difference in phenotype was apparent between them. In this study, all of the experiments were carried out with line 1 (#29) mice.
Animal studies
CBA/C57BL6 Mix background Herc6 KO (4 times backcrossed) mice and C57BL6 pure background WT and Herc6 KO mice were maintained at Kobe BM laboratory (Oriental Bio Service, Inc.). C57BL6 pure background WT and UBE1L KO mice were maintained at UCSD Moores cancer center. Animal studies in Kobe BM laboratory were properly conducted in accordance with regulations regarding animal experiments in Japan. Animal experiments in UCSD Moores cancer center were performed in accordance with NIH policies on the use of laboratory animals and approved by the Animal Research Committee of the Office for the Protection of Research Subjects at the University of California, San Diego.
Southern blotting and PCR genotyping
Southern blots were performed using a radioactive label or DIG label (Roche) methods according to the manufacturer's protocols. The probe for Southern blotting was amplified using primers as follows: Fw (5′-TGA AGACAG ACA AGG TGG AAT AAC TTG ATA-3′), Rev (5′-CAG CTG CAG TAC CAC AGG TGA TGT GGT ACT-3′). The genotyping of mice was routinely performed with tail by PCR using a mixture of 3 primers after overnight treatment with proteinase K in PCR buffer. The sizes of the PCR products are WT allele (258 bp) and mutant allele (787 bp). The PCR primers were as follows: P1 (5′-ACA GGA TGT GAT AGG CTG CAT GTG AAA G-3′) and P3 (5′-AAA CAC CTA GTT CCC GAG GCT GTG AAC T-3′) for the Herc6 WT allele; P2 (5′-ATC AGG ATG ATC TGG ACG AAG AGC ATC A-3′) and P3 for the Neo gene. The PCR conditions were 95°C for 2 min, 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by 72°C for 10 min.
Cell culture and transfection
MEFs were prepared from E12.5 embryos and grown in Dulbecco's modified Eagle medium supplemented with glutamine, penicillin/streptomycin, and 10% fetal bovine serum (FBS). BMDM were cultured in RPMI1640 medium supplemented with glutamine, penicillin/streptomycin, 10% FBS, and macrophage colony-stimulating factor. For the knockdown analysis, siRNA was transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. At 72 h after the siRNA transfection, cell lysates were prepared for examination.
Antibodies and reagents
Anti-mouse Herc6 polyclonal antibodies were generated in rabbits by using the mouse Herc6 recombinant protein that was bacterially produced using GST (6P-1) mHerc6 652-1003aa. Antibodies against STAT1, p-STAT1 (Y701), and ISG15 (#2743) were purchased from Cell Signaling. For the detection of protein ISGylation in liver and spleen tissue samples of WT and Herc6 knockout mice with or without poly I:C injection, and BMDM samples of WT and Herc6 knockout mice with or without IFN-β treatment, we used Dong-Er Zhang Lab's anti-mouse ISG15 antibody. Anti-α-tubulin (Sigma) and actin were acquired from Oncogene Research Products. Poly I:C was purchased from Amersham. Mouse IFN-β was purchased from PBL. Sendai virus (Z strain) and VSV were kindly provided by Dr. Masato Nakanishi (Research center for stem cell engineering, AIST, Japan).
Knockdown
For knockdown of Herc6, the following siRNAs were used:
si-Herc6-1: 5′-gaaauaagcuuuaugccuauu-3′ (B-Bridge International, Inc.)
si-Herc6-2: 5′-ggaacaaaguuaaagaacauu-3′ (B-Bridge International, Inc.)
si-Herc6-3: 5′-ccuacagaaugaaggaauauu-3′ (B-Bridge International, Inc.)
Control siRNA (si-GFP); 5′-acuuguacagcucguccauuu-3′ (B-Bridge International, Inc.)
Western blotting
Western blotting was conducted as previously described (Arimoto and others 2010). All samples were denatured in 1× sample buffer [50 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 2-mercaptethanol, 10% glycerol, and 1% bromophenol blue] for 5 min at 100°C. Cells were lysed in RIPA buffer composed of 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 0.1% SDS, 1% Nonidet P-40, and 0.5% sodium deoxycholate. The cell lysates were centrifuged (10,000 g) at 4°C for 5 min. All lysis buffer in this study contains proteinase and phosphatase inhibitors (Roche). For the quantification, the Fujifilm Multi-gauge V3.0 was used.
Enzyme-linked immunosorbent assay
Culture media were collected and analyzed for IFN-β and IL-6 production by using enzyme-linked immunosorbent assays (ELISAs). ELISA kits for mouse IFN-β and IL-6 were purchased from PBL Biomedical Laboratories.
TCID50 assay
50% tissue culture infectious dose (TCID50) assay was conducted as previously described (Arimoto and others 2010). Approximate viral titers were calculated by TCID50 assay. Results of this assay are well in accordance with those of the general plaque assay. After 12 h VSV infection, the culture medium was diluted 3×104 times and then added to the first line of a 96-well plate with 50 μL medium containing 293T cells, making a serial 3-fold dilution. At 1–2 days after infection, more than 50% cell alterations in each dilution step was analyzed with the following formula: TCID50=(rate of dilution at first line)×(dilution rate)Σ−0.5, where Σ=the number of wells observed with more than 50% cell alteration in each dilution step/sample sum.
Histology
Whole body was fixed by 10% formaldehyde solution, and tissues were paraffin embedded into OCT compound (Tissue-Tek). For histochemical analysis, paraffin sections were stained with H&E according to standard protocols.
Results
Disruption of the mouse Herc6 gene
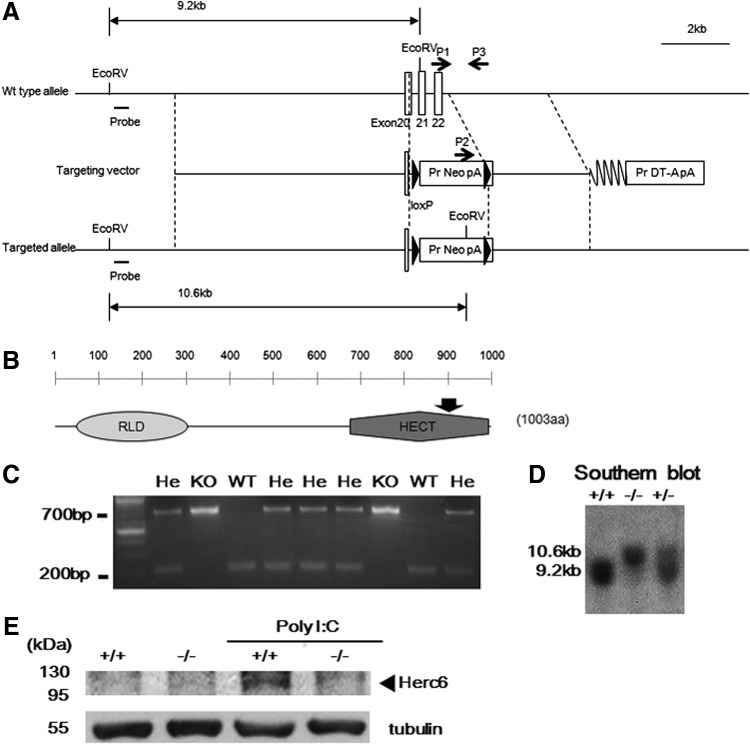
In line with the previous report using L929 cells and siRNA against Herc6 (Oudshoorn and others 2012), we observed the reduction of ISGylation in Herc6 knockdown primary MEFs after IFN treatment (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jir). To investigate the role of protein ISGylation by Herc6 in vivo, we generated Herc6 knockout mice. For the disruption of the Herc6 gene, we added 2 loxP sites to exon 20 and the intron after exon 22 (Fig. 1A). This target region is related to C-terminal HECT domain of Herc6 protein (Fig. 1B). This construct also contains the bacterial neomycin gene as a selection marker in the Herc6 gene. Chimeric mice were produced by an injection of 2 independent clones of heterozygous ES cells into CBA blastocyst-stage embryos, and germ line transmission was determined by breeding with WT C57BL6 mice. We further backcrossed Herc6+/− mice with C57BL6 mice 4 times and 8 times to generate CBA/C57BL6 mix background and C57BL6 background Herc6 null mice, respectively. Mice were genotyped by PCR (Fig. 1C), and confirmed by Southern blot analysis (Fig. 1D). To confirm that Herc6 is no longer expressed in the knockout mice, spleens of WT and Herc6−/− mice that were with or without poly I:C injection were homogenized and subjected to Western blot. Herc6 protein was identified in poly I:C injected WT, but not in Herc6 knockout mouse (Fig. 1E).
FIG. 1.
Generation of Herc6 knockout mice. (A) Schematics of Herc6 knockout strategy. (B) Schematic diagram of Herc6 protein and the target deletion region is indicated with an arrow. (C) PCR genotyping of mouse tail DNA. (D) Southern blot analysis of mouse tail DNA. (E) Western blot analysis of protein ISGylation. Twenty-four hours after poly I:C injection, spleens were harvested. Herc6 protein was detected in the spleen extracts from WT mice, but not from Herc6 knockout mice by Western blotting using anti-mouse Herc6 polyclonal antibody. WT, wild type; He, heterozygous; KO, knockout.
Defective protein ISGylation in Herc6 knockout mice
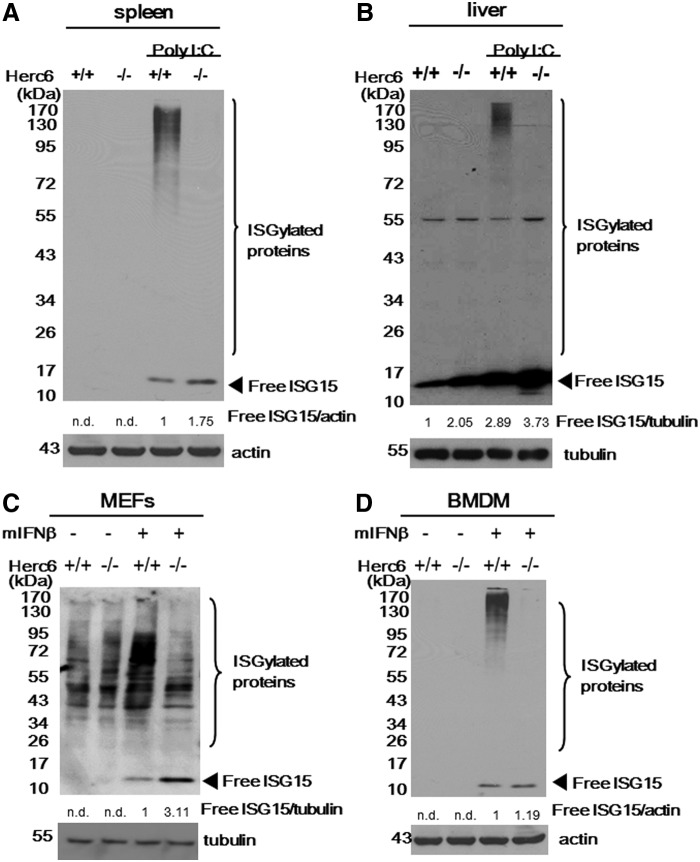
To determine whether lack of Herc6 correlated with decreased ISGylation in vivo, we injected WT and knockout mice with PBS or poly I:C. Twenty-four hours later, spleens and livers were harvested, and protein ISGylation was detected by Western blotting. Protein ISGylation was readily detected in the liver and spleen from WT but was barely detected from Herc6 knockout mice (Fig. 2A and B, respectively). Similar results were observed in IFN-β-treated MEFs and BMDM from WT and Herc6 knockout mice (Fig. 2C and D, respectively). Herc6-deficient tissues or cells showed an increased amount of free ISG15 as a result of the lack of conjugation (Fig. 2A–D).
FIG. 2.
Analysis of protein ISGylation in Herc6 knockout mice. (A, B) Twenty-four hours after PBS or poly I:C injection, spleens and livers were harvested, and ISG15 conjugated protein was detected by Western blotting using anti-mouse ISG15 antibody (Dong-Er Zhang Lab). (C) Mouse embryonic primary fibroblasts from WT and Herc6 knockout mice were treated with mock or 500 U/mL of mIFN-β. Twenty-four hours after treatment, cells were harvested and subjected to Western blotting using anti-ISG15 antibody (CST#2743), the information of which should be in M&M. (D) BMDM from wild-type and Herc6 knockout mice were treated with mock or 100 U/mL of mIFN-β as indicated. Twenty-four hours after treatment, cells were harvested and subjected to Western blotting using anti-mouse ISG15 antibody (Dong-Er Zhang Lab). The levels of free ISG15 were quantified as indicated. n.d. means not detectable. BMDM, bone marrow-derived macrophages; IFN, interferon.
These results indicate that Herc6 knockout mice are defective in protein ISGylation but not in free ISG15 expression.
Normal IFN responses of Herc6-deficient cells and mice
A previous report has shown that human HERC5 positively regulates the IFN-β promoter via enhancing IRF3 function and so confers antiviral activity (Shi and others 2010). In addition, mouse Herc6 also enhanced IFN-β promoter activity similar to its human Herc5 counterpart (Oudshoorn and others 2012). To explore whether Herc6 regulates IFN signal transduction, we examined the IFN-response of Herc6-deficient cells. Macrophages derived from bone marrow cells of WT and Herc6 knockout mice were cultured in vitro and treated with 100 U/mL of IFN-β, and STAT1 phosphorylation was detected as an indication of the activation of the signaling pathway. Increased phosphorylation of STAT1 on IFN treatment was observed. However, there was no difference of STAT1 phosphorylation between WT and Herc6-deficient cells (Fig. 3A).
FIG. 3.
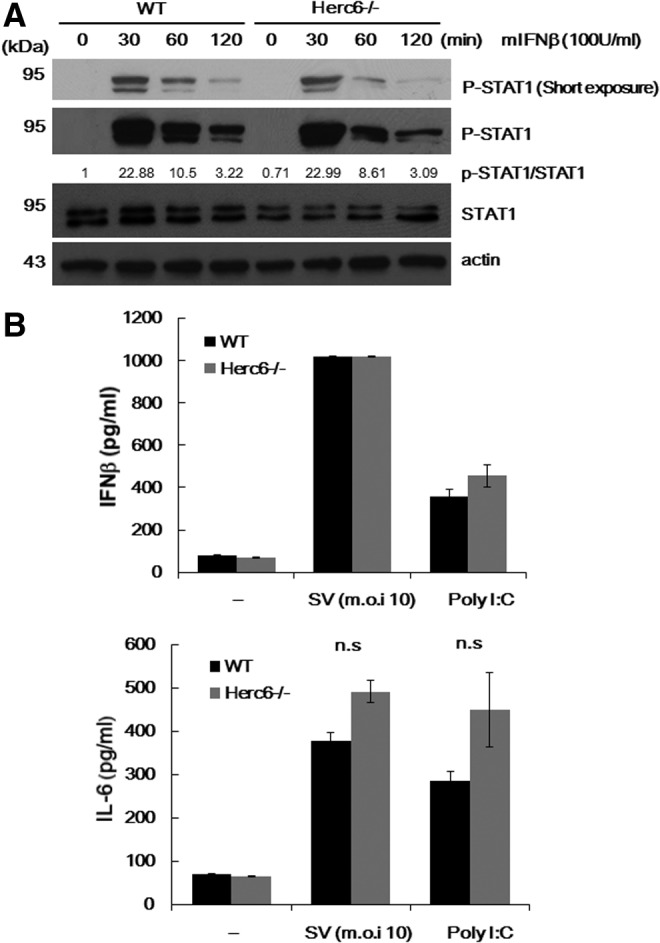
Analysis of IFN and inflammatory response in Herc6 knockout cells. (A) BMDM from WT and Herc6 knockout mice were cultured in the presence of 100 U/mL of mIFN-β for the indicated time periods. Cells were harvested and subjected to Western blotting against pSTAT1, STAT1, and actin. The ratio of p-STAT1/total-STAT1 was also quantified as indicated. (B) Macrophages from WT and Herc6 knockout mice were treated with mock, SV at m.o.i. 10, or poly I:C 10 μg/mL. Twenty-four hours after treatment, cell culture media were harvested and subjected to ELISA for mIFN-β (upper) or mIL-6 (bottom). ELISA, enzyme-linked immunosorbent assay; m.o.i., multiplicity of infection; SV, Sendai virus.
To investigate whether the mouse Herc6 enhances an innate immune signal, we examined the IFN-β and IL-6 production in WT and Herc6−/− MEFs on Sendai virus infection and poly I:C stimulation. No significant differences in WT and Herc6 cells were observed (Fig. 3B top and bottom, respectively). Although it was not significant, a modest increase of IL-6 production in Herc6−/− MEFs with SV infection or poly I:C stimulation was observed (Fig. 3B bottom). This should be investigated with greater detail in the future.
Since lipopolysaccharide (LPS) activates the expression of these genes via the IFN signaling pathway, we also examined the peripheral blood cell count and other blood parameters. However, no significant differences were observed between WT and Herc6 knockout mice with or without LPS stimulation (Table 1).
Table 1.
Blood Analysis With or Without LPS (15 mg/kg Body Weight) Injection
| WBC (103/μL) | RBC (103/μL) | Hb (g/dL) | Ht (%) | MCV (fl) | MCH (pg/cell) | MCHC (g/dL) | PLT (103/μL) | |
|---|---|---|---|---|---|---|---|---|
| Mock | ||||||||
| WT (n=3) | 7.7±1.4 | 8.0±0.2 | 12.3±0.1 | 38.6±1.2 | 48±0.2 | 15.4±0.3 | 32.0±0.8 | 107.9±34.7 |
| KO (n=2) | 6.5±0.3 | 6.8±0.2 | 12±0.9 | 32.9±8.2 | 48.2±2.4 | 18.1±4.3 | 37.3±7.2 | 118.9±13.4 |
| LPS | ||||||||
| WT (n=5) | 3.0±0.5 | 8.1±0.2 | 12.7±0.1 | 39.9±1.3 | 48.9±0.4 | 15.8±0.3 | 31.9±0.7 | 346.0±10.2 |
| KO (n=5) | 3.6±0.8 | 8.1±0.4 | 13.0±0.8 | 40.8±2.1 | 50.1±0.3 | 16.0±0.3 | 31.8±0.7 | 301.0±9.51 |
Blood samples were collected via retro-orbital bleeding from wild-type and Herc6 knockout mice at 1 day after with or without LPS (15 mg/kg body weight) injections. Values are means±standard deviation.
WBC, white blood corpuscles; RBC, red blood corpuscles; Hb, hemoglobin; Ht, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; LPS, lipopolysaccharide; WT, wild type.
In summary, Herc6-deficient cells do not show any detectable differences from WT cells in their responses to IFN-related treatments.
Deletion of Herc6 did not affect the antiviral response against VSV infection
Besides the IFN response (Shi and others 2010), a recent report indicated that Herc5 mainly conjugates ISG15 to newly synthesized proteins in tissue culture and may by this mechanism largely target de novo synthesized viral proteins during infection (Durfee and others 2010).
To investigate whether Herc6 affects the proliferation of virus, we examined the consequences of reduction of protein ISGylation on the antiviral effects of IFN in MEFs. Treatment with increasing concentrations of IFN-β correlated positively with the antiviral stage of both WT and Herc6 knockout MEFs on infection with VSV, with no detectable differences in the response between the 2 genotypes (Fig. 4A). In addition, there was no significant difference of viral titer between WT and Herc6 knockout MEFs after infection with VSV (Fig. 4B). These experiments demonstrate that protein ISGylation via Herc6 in mice is not involved in the antiviral response against VSV.
FIG. 4.
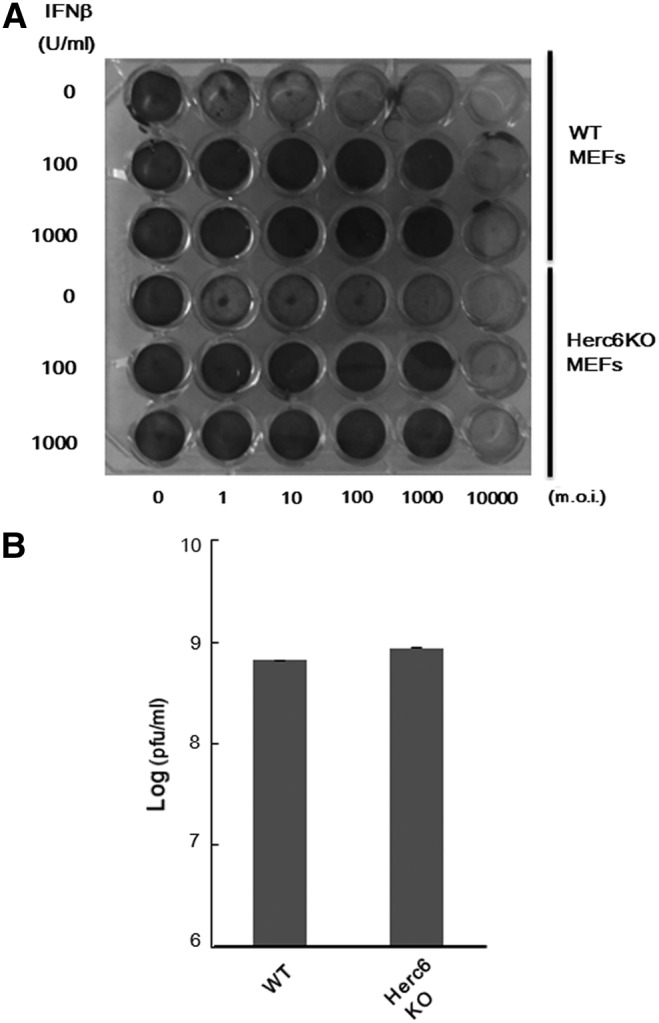
VSV protection assay. (A) WT and Herc6 knockout MEFs were left untreated or treated with 100 or 1,000 U/mL of IFN-β for 24 h, followed by VSV infection at m.o.i. 0–104 per well for an additional 24 h. Cell viability was assessed by crystal violet staining. (B) The VSV (m.o.i. 0.1) was infected with WT and Herc6 knockout MEFs. At 12 h after infection, virus titer was measured according to TCID50 protocol. Data are mean±SD (n=3). MEFs, mouse embryonic fibroblast cells; TCID50, 50% tissue culture infectious dose; VSV, vesicular stomatitis virus.
Male Herc6 null mice showed hypertrophy of seminal vesicle
During the experiments, we noticed that mix-background Herc6 knockout male mice began showing abdominal distension at 28 weeks. Severe enlargement seminal vesicles were observed in Herc6 knockout mice (Fig. 5A). However, only slight epithelial hyperplasia with cystic dilatation of seminal vesicles was observed in Herc6 knockout mice (Fig. 5B), suggesting that the enlargement of seminal vesicles was from increased seminal fluid. Glandular hypoplasia and benign hypertrophy of the prostate, which are commonly found in elderly men, were not observed in Herc6 knockout mice (data not shown). Further examination using C57BL6 background mice showed a higher frequency of enlargement of seminal vesicles in Herc6 knockout mice compared with WT or UBE1L knockout mice (Table 2 and Supplementary Fig. S2). These results demonstrate that mouse Herc6 has an ISG15-independent function in regulating the seminal component.
FIG. 5.
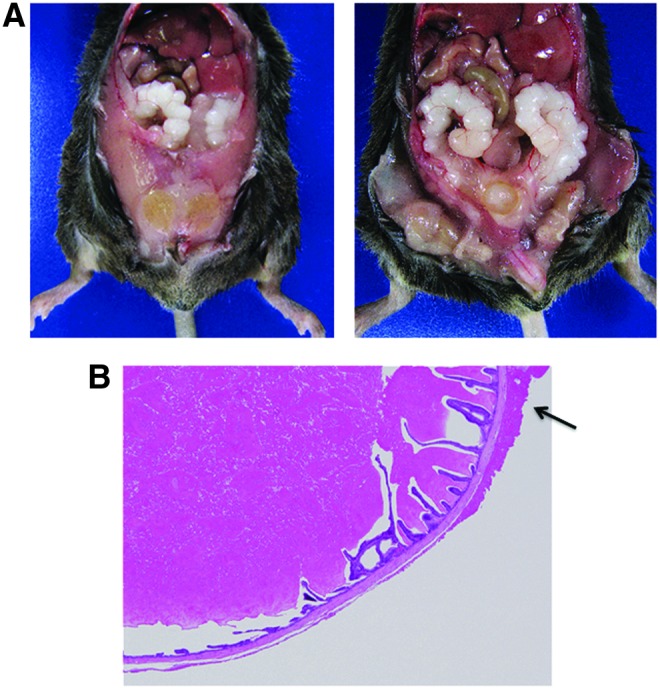
The phenotype of Herc6 knockout mice. (A) The representative enlarged seminal vesicle in Herc6 knockout mouse (30 weeks, mix background). Right panel shows incision of the peritoneum of the left mouse. (B) H&E staining for the seminal vesicle of Herc6 knockout mouse. Arrow shows epithelial hyperplasia of seminal vesicle.
Table 2.
Analysis of Seminal Vesicle of WT, UBE1L−/−, and Herc6−/−, Mice
| Background | Genotype | Population of hypertrophied seminal vesicle | ||
|---|---|---|---|---|
| 30 weeks | 50 weeks | ∼90 weeks | ||
| Mix (CBA/C57BL6) | Herc6−/− (n=5) | 2 | 4 | |
| C57 | WT (n=10) | 0 | 2 | |
| UBE1L−/− (n=5) | 0 | 0 | ||
| Herc6−/− (n=9) | 1a | 8 | ||
One-side anomalistic seminal hypertrophy.
Seminal hypertrophy is defined as above at least 0.04 g seminal vesicle/g body weight.
Discussion
In this article, we described the generation and analysis of the first Herc6 knockout mouse model. The results of our studies demonstrate that (1) Mouse Herc6 is the major E3 ligase for ISG15 conjugation in vivo. (2) ISG15 conjugation via mouse Herc6 does not affect type I IFN response and antiviral response against SV and VSV infection. (3) Mouse Herc6 possesses an ISG15 conjugation-independent role in regulating sperm sac morphology.
Previous reports indicate that human Herc5 knockdown in 293T cells and mouse Herc6 knockdown in L929 cells showed reduced ISG15 conjugation to a broad group of proteins after IFN treatment (Dastur and others 2006; Wong and others 2006; Oudshoorn and others 2012). In line with this result, the level of ISG15 conjugation in tissues of Herc6-deficient mice showed substantial reduction compared with that of WT mice after poly I:C injection. These findings clearly indicate that Herc6 is the major mouse ISG15 E3 ligases in vivo. Furthermore, our report supports that humans and mice developed different Herc proteins to facilitate global ISG15 conjugation during evolution.
A recent report indicates that HERC5 is mainly associated with poly-ribosome, and ISGylation targets newly synthesized proteins in human tissue culture (Durfee and others 2010). Furthermore, in this same report, the authors showed that HPV16 L1 capsid protein was ISGylated and this modification inhibited HPV pseudovirus production in transfection experiments (Durfee and others 2010). In addition, previous reports showed that antiviral effects exerted by human HERC5 (Shi and others 2010) are shared by Herc6 in mouse cells (Oudshoorn and others 2012). ISG15-activating enzyme Ube1L knockout mice also showed no protein ISGylation and no difference in IFN responses and anti-VSV and LCMV defense (Osiak and others 2005; Kim and others 2006). However, further studies of Ube1L knockout mice revealed a critical role of Ube1L in control of influenza B virus infection (Lai and others 2009). These discrepancies of revealed functions of protein ISGylation are likely due to the differences in species (humans and mice), lines, and viruses, and in vitro versus in vivo experiments. These questions need to be addressed in the future.
Herc6 null mice showed hypertrophy of seminal vesicles in both CBA/C56BL6 mixed background and C57BL6 background. However, Ube1L−/− mice did not show similarly enlarged sperm sacs although protein ISGylation is lost in both Herc6 and Ube1L knockout mice (Kim and others 2006). This difference suggests that mouse Herc6 has an ISG15-independent function in seminal fluid production during aging. However, the phenotype of Herc6−/− ISG15−/− also should be investigated in the future to examine whether this seminal hypertrophy is truly Herc6 dependent and ISG15 independent. Since mouse Herc6 belongs to the Herc family of ubiquitin E3 ligases, it is possible that ISG15 conjugation is regulated by the ISG15 E3 ligase activity of Herc6, and seminal component secretion is regulated by its ubiquitin E3 ligase activity. Human Herc5 may have evolved to exclusively function as an ISG15 E3 ligase, while human Herc6 may function as a ubiquitin E3 ligase that is involved in the seminal component secretion.
While we could not see the difference in male fertility of Herc6 knockout mice (∼35 weeks) (data not shown), we cannot rule out this possibility because it could be affected by the conditions associated with older age.
The function of seminal fluid is largely unknown except for the involvement of normal conception. Interestingly, it has recently been reported that paternal seminal fluid composition affects the epigenome of male offspring and that its impact on the periconception environment involves not only sperm protection but also indirect effects on various female factors regulating embryo development, which suggests that offspring of Herc6 null mice may have interesting epigenomic changes by the hypertrophied seminal vesicles of Herc6 male mice (Bromfield and others 2014). Future studies using this mouse model may facilitate to address this question.
In humans, symptoms of enlarged seminal vesicles are frequently seen in patients with ejaculatory duct obstructions (EDO) (Pryor and Hendry 1991). EDO is a congenital or acquired pathological condition that is characterized by the obstruction of one or both ejaculatory ducts, and causes 1%–5% of male infertility (Philip and others 2007). In addition to the congenital form that is often caused by cysts of the müllerian duct, the obstruction can be acquired due to an inflammation caused by chlamydia, prostatitis, tuberculosis of the prostate, and other pathogens (Philip and others 2007). However, in many patients, there is no history of inflammation and the underlying cause simply remains unknown (Philip and others 2007). The finding that the suppression of Herc6 caused the hypertrophied seminal vesicles in this report may be involved in the acquired enlarged seminal vesicle symptoms, and this should be investigated in the future.
Supplementary Material
Acknowledgments
The authors thank all lab members for helpful discussion. They especially thank Dr. Kentson Lam (School of Medicine, University of California San Diego) and Samuel Stoner (Moores Cancer Center, University of California San Diego) for discussion and a critical reading of this article. They also thank Shuichiro Ogawa (Kyoto University) for several experiments and discussion. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (22114004 & 22249012), funding from National Institutes of Health USA (R01CA177305 and R01HL091549), and JSPS KAKENHI Grant (No. 10J00577); Kei-ichiro Arimoto is a JSPS Postdoctoral Fellow for Research Abroad.
Author Disclosure Statement
There is no financial interest to disclose.
References
- Arimoto K, Funami K, Saeki Y, Tanaka K, Okawa K, Takeuchi O, Akira S, Murakami Y, Shimotohno K. 2010. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc Natl Acad Sci U S A 107(36):15856–15861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. 2011. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nature reviews. Drug Discov 10(1):29–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. 2014. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci U S A 111(6):2200–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart C, Arimoto K, Tang T, Cong X, Xiao N, Liu YC, Kotenko SV, Ellies LG, Zhang DE. 2013. Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-lambda and elevated secretion of Cxcl10. EMBO Mol Med 5(7):967–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM. 2006. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem 281(7):4334–4338 [DOI] [PubMed] [Google Scholar]
- Durfee LA, Lyon N, Seo K, Huibregtse JM. 2010. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol Cell 38(5):722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra S, Caceres A, Knobeloch KP, Horak I, Esteban M. 2008. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog 4(7):e1000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrainer K, Mayer H, Baranyi U, Binder B, Lipp J, Kroismayr R. 2005. The human HERC family of ubiquitin ligases: novel members, genomic organization, expression profiling, and evolutionary aspects. Genomics 85(2):153–164 [DOI] [PubMed] [Google Scholar]
- Hsiang TY, Zhao C, Krug RM. 2009. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J Virol 83(12):5971–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao NW, Chen JW, Yang TC, Orloff GM, Wu YY, Lai CH, Lan YC, Lin CW. 2010. ISG15 over-expression inhibits replication of the Japanese encephalitis virus in human medulloblastoma cells. Antiviral Res 85(3):504–511 [DOI] [PubMed] [Google Scholar]
- Kim KI, Giannakopoulos NV, Virgin HW, Zhang DE. 2004. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol Cell Biol 24(21):9592–9600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KI, Yan M, Malakhova O, Luo JK, Shen MF, Zou W, de la Torre JC, Zhang DE. 2006. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol Cell Biol 26(2):472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Struckhoff JJ, Schneider J, Martinez-Sobrido L, Wolff T, Garcia-Sastre A, Zhang DE, Lenschow DJ. 2009. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J Virol 83(2):1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ. 2010. Antiviral properties of ISG15. Viruses 2(10):2154–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Giannakopoulos NV, Gunn LJ, Johnston C, O'Guin AK, Schmidt RE, Levine B, Virgin HWt. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol 79(22):13974–13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, Garcia-Sastre A, Leib DA, Pekosz A, Knobeloch KP, Horak I, Virgin HWt. 2007. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci U S A 104(4):1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhova OA, Zhang DE. 2008. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J Biol Chem 283(14):8783–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura A, Lu G, Pitha-Rowe I, Pitha PM. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A 103(5):1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura A, Pitha PM, Harty RN. 2008. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc Natl Acad Sci U S A 105(10):3974–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiak A, Utermohlen O, Niendorf S, Horak I, Knobeloch KP. 2005. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol 25(15):6338–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudshoorn D, van Boheemen S, Sanchez-Aparicio MT, Rajsbaum R, Garcia-Sastre A, Versteeg GA. 2012. HERC6 is the main E3 ligase for global ISG15 conjugation in mouse cells. PLoS One 7(1):e29870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Yang SW, Yu KR, Ka SH, Lee SW, Seol JH, Jeon YJ, Chung CH. 2014. Modification of PCNA by ISG15 plays a crucial role in termination of error-prone translesion DNA synthesis. Mol Cell 54(4):626–638 [DOI] [PubMed] [Google Scholar]
- Philip J, Manikandan R, Lamb GH, Desmond AD. 2007. Ejaculatory-duct calculus causing secondary obstruction and infertility. Fertil Steril 88(3):706 e9–e11 [DOI] [PubMed] [Google Scholar]
- Pincetic A, Kuang Z, Seo EJ, Leis J. 2010. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol 84(9):4725–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor JP, Hendry WF. 1991. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertil Steril 56(4):725–730 [DOI] [PubMed] [Google Scholar]
- Shi HX, Yang K, Liu X, Liu XY, Wei B, Shan YF, Zhu LH, Wang C. 2010. Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification. Mol Cell Biol 30(10):2424–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JJ, Pung YF, Sze NS, Chin KC. 2006. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci U S A 103(28):10735–10740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, Saga Y, Takeda N, Ikawa Y, Aizawa S. 1993. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem 214(1):70–76 [DOI] [PubMed] [Google Scholar]
- Yuan W, Krug RM. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J 20(3):362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.