Abstract
This study evaluated the structural, mechanical, and cytocompatibility changes of three-dimensional (3D) printed porous polymer scaffolds during degradation. Three porous scaffold designs were fabricated from a poly(propylene fumarate) (PPF) resin. PPF is a hydrolytically degradable polymer that has been well characterized for applications in bone tissue engineering. Over a 224 day period, scaffolds were hydrolytically degraded and changes in scaffold parameters, such as porosity and pore size, were measured nondestructively using micro-computed tomography. In addition, changes in scaffold mechanical properties were also measured during degradation. Scaffold degradation was verified through decreasing pH and increasing mass loss as well as the formation of micropores and surface channels. Current methods to evaluate polymer cytotoxicity have been well established; however, the ability to evaluate toxicity of an absorbable polymer as it degrades has not been well explored. This study, therefore, also proposes a novel method to evaluate the cytotoxicity of the absorbable scaffolds using a combination of degradation extract, phosphate-buffered saline, and cell culture media. Fibroblasts were incubated with this combination media, and cytotoxicity was evaluated using XTT assay and fluorescence imaging. Cell culture testing demonstrated that the 3D-printed scaffold extracts did not induce significant cell death. In addition, results showed that over a 224 day time period, porous PPF scaffolds provided mechanical stability while degrading. Overall, these results show that degradable, 3D-printed PPF scaffolds are suitable for bone tissue engineering through the use of a novel toxicity during degradation assay.
Introduction
Understanding changes in structural properties, mechanical properties, and tissue response of absorbable polymer scaffolds during degradation is critical for designing a successful bone tissue repair product. Ideal repair of a bone defect allows for mechanical support while native bone replaces the void. To facilitate rapid repair, bone tissue engineering strategies suggest a combination approach of a biomaterial scaffold and a biologically active component. In this study, we propose using three-dimensional (3D) printed porous poly(propylene fumarate) (PPF) as the biomaterial for these scaffolds. Our scaffolds would provide mechanical stability during degradation while the bioactive material, housed in the lumen of the scaffold, would promote host tissue ingrowth and eventual repair of the bone defect. In addition, our use of 3D printed scaffolds allows for the precise fabrication of computationally designed scaffolds with controlled shapes, porosity, and interconnectivity. Use of these 3D printed scaffolds for bone tissue applications represents a huge opportunity for the community, particularly if the scaffolds allow for tissue ingrowth while the scaffolds degrade.
Another key property of a successful biomaterial is a reparative or regenerative tissue response at the implantation site. As PPF is an absorbable polymer, the tissue response to PPF depends on both the polymer and its degradation byproducts. PPF has already been shown to be noncytotoxic; however, the cytotoxicity of PPF during degradation has not been evaluated.1 Therefore, we investigated the potential for a cytotoxic response to the byproducts of degradation.
PPF is an aliphatic polyester consisting of a carbon–carbon double bond, flanked by two ester groups, that has been determined to be well suited for bone tissue engineering.2,3 PPF degrades through the hydrolysis of the ester groups on the repeating unit (Fig. 1). The byproducts of degradation are fumaric acid and propylene glycol.4 Fumaric acid is produced during the citric acid cycle; it is, therefore, expected that in vivo some of the fumaric acid would naturally be taken up by the cells in the immediate environment.5 It was established as “practically non-toxic” by the European Commission Report of the Scientific Committee on Animal Nutrition on the Safety of Fumaric Acid.6 In addition, fumaric acid esters have been used for the treatment of severe psoriasis.7 The other main degradation byproduct, propylene glycol is a common food additive.8
FIG. 1.
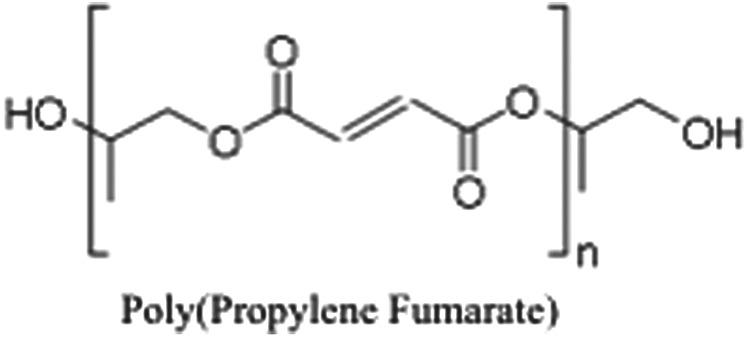
A schematic of poly(propylene fumarate) (PPF). PPF contains a repeating unit of two ester groups flanking a carbon–carbon double bond.
We hypothesize that the degradation extract of the 3D-printed PPF scaffolds will have a low cytotoxic response, as its degradation byproducts are nontoxic. The scaffolds investigated were 3D printed with a resin containing PPF, diethyl fumarate (DEF), vitamin E, and oxybenzone (2-hydroxy-4-methoxybenzophenone, HMB). The individual resin components have also been shown to be noncytotoxic; however, the cytotoxicity of the combined materials as well as during polymer absorption has not been evaluated.1,7,9,10 In addition, we hypothesize that due to the slow degradation of PPF, the reduction in pH from the formation of the degradation byproduct fumaric acid would have a little to no detrimental impact as the cells would metabolize the fumaric acid. To test this hypothesis, we modified testing recommendations in ISO Standard 10993-5, “Tests for In vitro Cytotoxicity”11 to evaluate polymer degradation cytotoxicity. The modifications represent a novel method for absorbable scaffold cytotoxicity, as the standards for evaluating the cytotoxicity during degradation are not well defined for in vitro analysis. To evaluate the appropriateness of these scaffolds as a bone tissue engineering strategy, changes in scaffold structure, mechanical properties, and cytotoxicity were evaluated during degradation using simulated physiological conditions.
Materials and Methods
PPF synthesis
PPF was synthesized in a two-step process as previously described.12 Briefly, propylene glycol and DEF were combined in a 3:1 molar ratio. Zinc chloride and hydroquinone were added to act as catalyst and radical inhibitor, respectively. The solution was reacted under a flow of nitrogen gas, producing ethanol as a byproduct and bis(hydroxypropyl) fumarate as the intermediate. The second step is a transesterification of the intermediate, performed under a vacuum, to produce PPF with propylene glycol as a byproduct. Gel permeation chromatography was used to calculate the number average molecular weight (Mn) and polydispersity index (PDI) of the purified PPF. The number average molecular weight of 3D-printed PPF scaffolds with variable designs ranged from 1078 Da (PDI=1.67) to 1157 Da (PDI=1.15).
Scaffold design
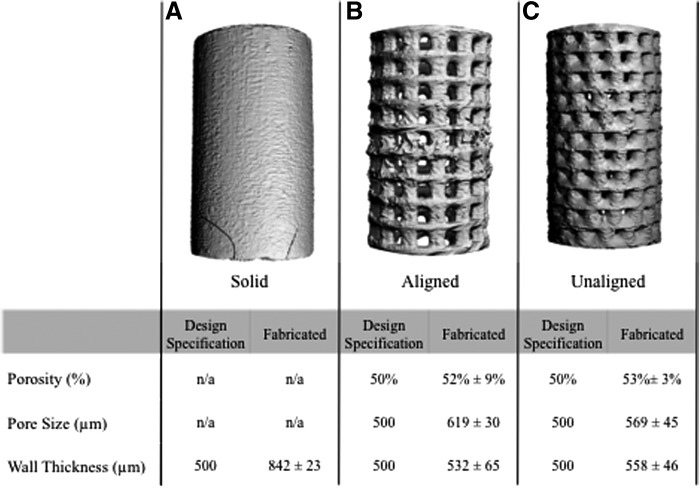
Scaffolds for 3D printing were designed (SolidWorks, Waltham, MA) for repeating units of base rings connected by uniformly distributed cylindrical posts (Fig. 2A). These repeating units were stacked on the first unit until the desired height of the scaffold was reached. The scaffolds were designed to be 12 mm in height and 6 mm in diameter, which would allow for compressive testing at the proper ratio of length to diameter for cylinders per ASTM D0695-10.13 This cylindrical structure has a lumen, with the idea that it is used to house a biologically active component, such as bone marrow or hydrogel encapsulated cells. The pores would then allow for angiogenesis to occur through the scaffold and interact with the bioactive component.14 Two of the scaffold designs were porous: one with the pores from each modular unit aligned (Fig. 2C), and the other based on the same modular unit but with each unit rotated when stacked on the previous unit to create an unaligned structure (Fig. 2D). The third design was a solid wall design (Fig. 2B).
FIG. 2.
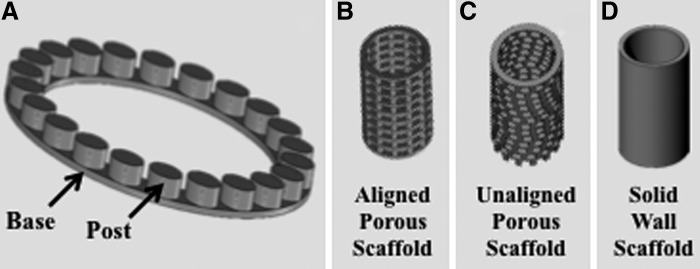
Design of standardized modular scaffolds. (A) Repeating unit of the modular scaffold design, where the base ring and post structure are used as the repeating unit to create the porous scaffold designs. (B) Solid wall scaffold, where the scaffold has the same wall thickness as the porous scaffolds, but without pores. (C) Aligned porous scaffold design, where the scaffold is formed through the stacking of the repeating unit in a uniform manner to create a structure with pores one on top of another. (D) Unaligned porous scaffold design, where the scaffold is formed by the repeating unit shifted around the central axis during stacking so that the pores are not aligned.
3D fabrication and post curing
The scaffold designs were 3D printed using the EnvisionTEC Perfactory® P4 per previously described methods.15 The EnvisionTEC Perfactory P4 uses continuous Digital Light Processing (cDLP) technology, which is similar to stereolithography (SLA), as it allows for the precise spatiotemporal control of photopolymerization. However, as SLA typically uses a laser to initiate photopolymerization, the EnvisionTEC Perfactory P4 uses the exposure of ultraviolet (UV) light projected through a cDLP chip. Specifically, scaffolds were built with an exposure to the UV light of 275 mW/dm2 for 120 s (burn-in) or 100 s per layer. Fabrication occurred under ambient temperature and pressure. The printing resolution was ∼25 μm in the x–y directions and 50 μm in the z direction. Briefly, the polymer resin used s comprised PPF and DEF in a 1:0.8 ratio along with dyes and initiator. Bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO, 1%) was used as the photoinitiator, while HMB (1%), and α-tocopherol (0.01%) were used as photoinhibiting dyes. Immediately after printing, any uncured resin was removed with an ethanol wash, and then the scaffolds were postcured in a 3D systems UV-box for 2000 flashes. After this postprinting curing, the scaffolds were then rinsed for 15 min in phosphate-buffered saline (PBS) (0.01 M) to eliminate any extraneous debris. Next, the scaffolds were washed for 30 min in a 50% acetone solution to remove any unreacted resin. Finally, the scaffolds were rinsed for 15 min in a PBS solution to remove all traces of acetone. Scaffolds were vacuum dried, and their initial mass (Mi) was recorded. Scaffolds were sterilized with UV irradiation overnight before initiating the degradation study.
Sol fraction
To assess photoinitiated PPF crosslinking, the cured network sol fraction was measured as per the previously described method.2 Samples of photocrosslinked films were weighed (Wi) before incubation in acetone, the extraction solvent. The samples were then submerged in the solvent for 24 h. After incubation, samples were dried overnight and weighed again (Wd) (n=3). Sol fraction was calculated using the equation (Equation 1):
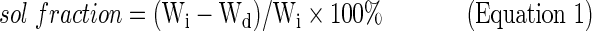 |
In vitro degradation
Scaffolds were placed into 20 mL of PBS (0.01 M, pH 7.4) and ascorbic acid (0.01 M) for in vitro degradation as previously described.4 The ascorbic acid was added to stabilize the scaffold degradation byproducts. Vials were stored at 37°C on a shaker table (75 rpm). At each timepoint (days 0, 1, 7, 14, 28, 56, 112, and 224), pH was measured, PBS was replaced, and scaffolds were analyzed for mass loss (Equation 2) based on the initial (Mi) and dry weight (Md) after vacuum drying.
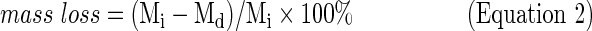 |
To evaluate mechanical and structural attributes of the scaffolds during degradation, at each timepoint, wet scaffold mechanical properties were evaluated in compression (n=5) and scaffold mean pore size, porosity, and wall thickness were evaluated nondestructively using micro-computed tomography (μCT) (n=3).
Micro-computed tomography
μCT was used to noninvasively image and characterize changes in scaffolds. Scanning was performed on a μCT 100 (SCANCO Medical, Brüttisellen, Switzerland) operated at 70 kVp, 9 μm voxels and 200 mA. The resulting 3D datasets were segmented using thresholds (lower: 35, upper: 188, maximum: 1000), with gauss sigma (0.8) and support (1) values to separate pores from polymer. Threshold segmentation values were determined using a peak histogram approach and confirmed visually. The 3D images were evaluated for pore size, porosity, and wall thickness using Scanco's Image Processing Language (IPL).
Compressive mechanical testing
Compressive mechanical testing was performed at each timepoint using an Instron mechanical testing system (33R/4465). At each timepoint, fully hydrated scaffolds were removed from PBS and immediately tested in air with the exclusion of day 0 (n=5). Day 0 testing was performed before exposure to PBS. The ends of each sample were placed into stainless steel fixtures, which ensured that compression was applied homogeneously. Each fixture had a radius of 7.5 mm, an outer ring of 4 mm, an inner post of 3 mm, and a channel for the sample that was 2 mm in width. All tests were performed using a high-capacity load cell (5000 N). Samples were compressed at a displacement rate of 10 mm/min, and experimental values were recorded every 10 ms. Compression was maintained until a drop of at least 10% in force was measured. Engineering stress and strain were calculated based on original cross-sectional area and height, respectively, which were then used to compute compressive modulus, 1% offset yield stress, and ultimate compressive stress. Compressive modulus was calculated using Matlab® (Natick, MA) to determine the slope of the linear region of the stress-strain curve. The program evaluated the slope after a preload of 2 N. The linear region was then calculated using linear line fit command for the first 10 data points. The program then continues to add data points in steps of 10 until the R2 >0.97. The slope of this region is representative of the modulus of the sample. Yield stress was calculated as the intersection of the stress-strain curve with a line, drawn parallel to the initial slope, whose x-axis intercept is shifted 0.01 mm/mm strain.
Cytotoxicity of degradation byproducts
PPF scaffolds were placed in PBS (0.01 M and pH=7.4), with 0.01 M of ascorbic acid, in a ratio of 6:100 of scaffold for in vitro degradation following the principles outlined in ASTM F1635.16 An aliquot of PBS from degrading PPF scaffolds (aligned, unaligned, or solid wall) was removed at days 1, 7, 14, 28, 56, and 112 to generate an extract for cytotoxicity evaluation of the degradation byproducts. The cellular response to the extract PBS was evaluated using a fibroblast cell line (L929) (ATCC, Manassas, VA) as recommended by the ISO standard 10933-5.11 In previous studies, the L929 cell line was found to be the most sensitive for cytotoxicity testing with PPF and therefore was chosen to be used as a representative cell line for the cells that would be exposed to PPF in vivo.1 Cells were cultured as per the manufacturer's specifications with Minimum Essential Medium (Life Technologies, Frederick, MD) and 10% horse serum (Life Technologies). Cells were plated and grown to ∼80% confluency before initiating the assays. Once at confluence, the extract PBS was combined with L929 cell culture media (in one of three ratios, 1:99, 10:90, and 50:50) and added to the L929 cells that were cultured on tissue culture polystyrene well plates. Further modifications of the PBS extract and cell culture media solutions for pH or osmolality adjustment were not completed. Cultured cells were exposed to extract and media solution at 37°C and 5% CO2 for 24 h (Fig. 3), after which the cytotoxicity was evaluated qualitatively with fluorescence microscopy and quantitatively with the XTT cell metabolic activity assay. For the cytotoxic control, cultured cells were incubated with 70% methanol in the absence of culture media for 30 min before evaluation of cytotoxicity. Blank culture media was used as a negative control (i.e., cell viability should be maintained at a high level).
FIG. 3.
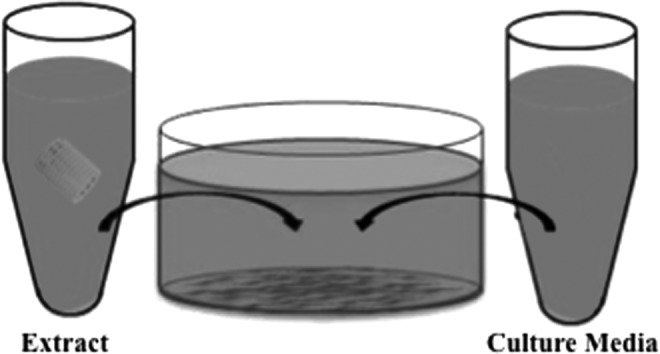
Schematic of cytotoxicity test. To evaluate the cytotoxicity of the degradation extract, cell culture media and the extract were incubated with fibroblasts. The degradation extract combined with cell culture media to achieve one of three concentrations (1%, 10%, or 50% extract) and was incubated with L929 fibroblasts for 24 h.
XTT assay
The Cell Proliferation Kit II (XTT) (Roche, Mainheim, Germany) was used to quantitatively evaluate cell metabolic activity. XTT [2,3-bis-(2- methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] was used according to the manufacturer's protocols. The electron coupling and XTT labeling reagents were thawed and immediately combined in a 1:50 ratio. Then, 250 μL of the XTT solution was added to the cell culture wells. Absorbance was measured after 4 h of incubation at 37°C with a M5 SpectraMax plate reader (Molecular Devices, Sunnyvale, CA). Net absorbance was calculated (A492-A650) for each sample of the biological replicates. Relative cell metabolic activity was normalized to the mean of the blank culture media. The mean cell metabolic activity and standard deviations are reported (n=3).
Fluorescence imaging
Qualitative evaluation of cell viability was performed using live/dead imaging (4 μM of calcein AM and 2 μM of ethidium homodimer in 0.01 M PBS) (Invitrogen, Carlsbad, CA) as previously described.17 Before the addition of the live/dead stain, cells were washed with PBS to remove any remaining culture media and extract PBS. Cells were incubated with the live/dead solution in dark conditions at room temperature for 30 min before imaging. Images were obtained with a fluorescence microscope (Axiovert 40CFL, filter set 23; Zeiss, Thornwood, NY) fitted with a digital camera (SPOT Insight 1120, or SPOT Idea 2920; Diagnostics Instruments, Sterling Heights, MI).
Statistical analyses
Statistical analysis was performed using ANOVA and Tukey's multiple pairwise comparison (p<0.05). All tests were performed in triplicate (n=3) unless otherwise specified. Values provided are mean±standard deviation. Please note that only relevant statistical relationships are denoted in figures.
Results
Scaffolds were 3D printed with a high degree of accuracy compared with the design specifications (Fig. 4). The aligned and unaligned porous scaffolds had an average porosity of 52±9% and 53±3%, respectively, close to the designed porosity of 50%. The scaffold mean wall thickness was fabricated at 532±65 and 558±46 μm for the aligned and unaligned scaffolds, respectively, compared with the 500 μm design specification. In contrast, the wall thickness for the solid wall scaffolds was fabricated at 842 μm, which is much larger than the 500 μm design specification. The printed pores were larger than the desired 500 μm size, with an average pore size of 619±30 μm for the aligned scaffolds and 569±45 μm for the unaligned scaffolds.
FIG. 4.
Micro-computed tomography (μCT) three-dimensional (3D) rendering and evaluation of porosity, pore size, and wall thickness. All three scaffold designs, (A) solid wall, (B) aligned pore, and (C) unaligned pore scaffolds were successfully fabricated using 3D printing. Most parameters of the porous scaffold, aligned and unaligned pore, were fabricated similar to the design specifications. Wall thickness of the solid wall scaffolds was found to be larger than the wall thickness of either of the porous scaffolds (n=10).
Sol fraction was performed to determine whether the different designs had any impact on the crosslinking efficiency of the printed PPF scaffolds (Table 1). The scaffolds were found to have sol fractions of 12.6±1.7% (solid), 13.3±8.4% (aligned), and 9.6±3.3% (unaligned) (Table 1). The sol fraction for all three groups was found to be statistically similar.
Table 1.
Sol Fraction of 3D Printed Scaffolds
| Scaffold design | Sol fraction (%) |
|---|---|
| Solid wall scaffold | 12.6±1.7 |
| Porous scaffold with aligned pores | 13.3±8.4 |
| Porous scaffold with unaligned pores | 9.6±3.3 |
Results show that the sol fractions of the three classes of scaffolds (solid, porous with aligned pores, and porous with unaligned pores) were similar, implying the photocrosslinking efficiency of the 3D printing process was similar regardless of scaffold design.
3D, three dimensional.
All three scaffold designs showed degradation during the 224 day study as seen through increasing mass loss (Fig. 5A). Mass loss increased throughout the study, with the biggest increase in mass loss seen in the first 28 days. After 224 days, the solid wall scaffolds had lost 17±7% of the mass, compared with 12±1% mass loss of the aligned scaffolds and 17±2% of the unaligned scaffolds. At each timepoint, the solid wall scaffolds had the same or greater percentage of mass loss compared with the porous scaffolds. Another indication of scaffold degradation was demonstrated by the decreasing pH (Fig. 5B). As one of the degradation byproducts of PPF is fumaric acid, this decrease in pH was expected. The lowest pH was seen at day 224, with a range of values from 5.62±0.29 (unaligned), 6.05±0.55 (solid) to 6.21±0.40 (aligned).
FIG. 5.
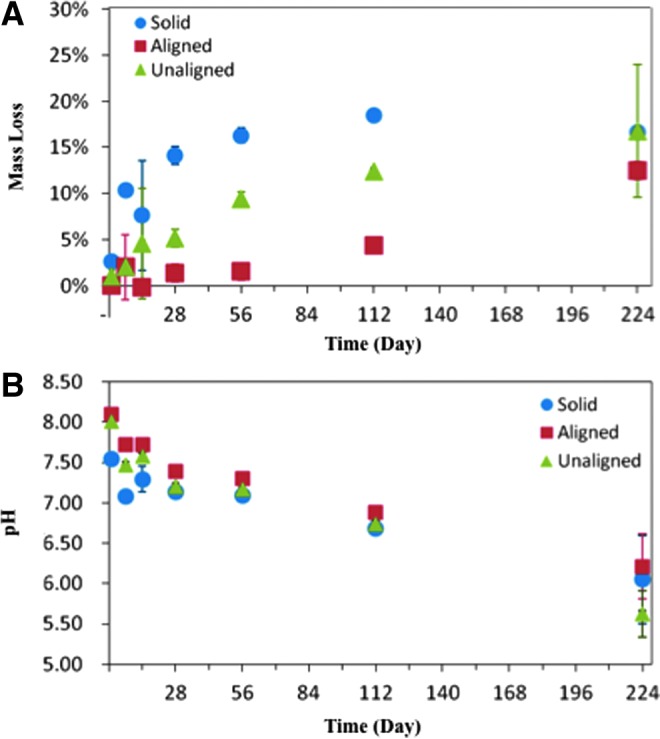
Physical changes during scaffold degradation. (A) Mass loss increases through the 224 day study, indicating that degradation is occurring. (B) Decreasing pH is also indicative of scaffold degradation. The decrease in pH is due to the formation of fumaric acid, one of the degradation byproducts of PPF. The mean and standard deviation are reported; some standard deviations are too small for the error bars to appear (n=3).
As degradation was measured through changes in mass loss and pH, μCT was used to elucidate which features (i.e., pore size, porosity, and wall thickness) of the scaffolds were changing. Scaffold porosity showed an increasing trend throughout the study, while the mean pore size remained statistically equivalent for the porous scaffolds (Fig. 6). The average porosity increased from 52±9% and 53±3% at day 0 to 64±2% and 57±4% at day 224, for the aligned and unaligned scaffolds, respectively (Fig. 6A). Average pore size was 619±30 and 569±45 μm at day 0 compared with 634±25 and 573±13 μm at day 224 for the aligned and unaligned scaffolds, respectively (Fig. 6B). A decreasing trend in wall thickness was observed as the study progressed for the porous scaffolds (Fig. 6C). The solid wall scaffolds also showed a decrease in wall thickness, with the measurement at day 0 being statistically larger than all other timepoints (Fig. 6C, p<0.05).
FIG. 6.
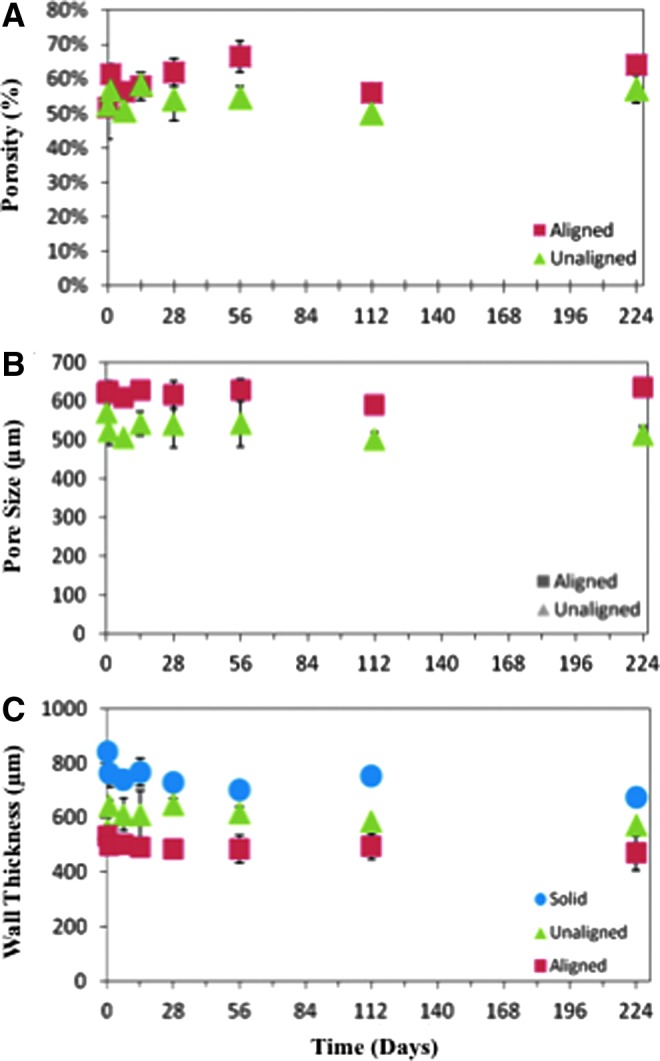
Scaffold pore changes during degradation. μCT was used to calculate the porosity, pore size, and wall thickness of the scaffolds during the degradation study. Porosity (A) is seen to increase slightly from days 0 to 224, while mean pore size remains constant (B). Similarly, there is no statistical difference in wall thickness for the aligned or unaligned scaffolds. The solid wall scaffolds (C) wall thickness decreases over the study, with a statistically larger wall thickness on day 0 compared with all other timepoints. The mean and standard deviation are reported, and some standard deviations are too small for the error bars to appear (n=3). *Denotes p<0.05 from all other timepoints within the same group for the solid wall scaffolds.
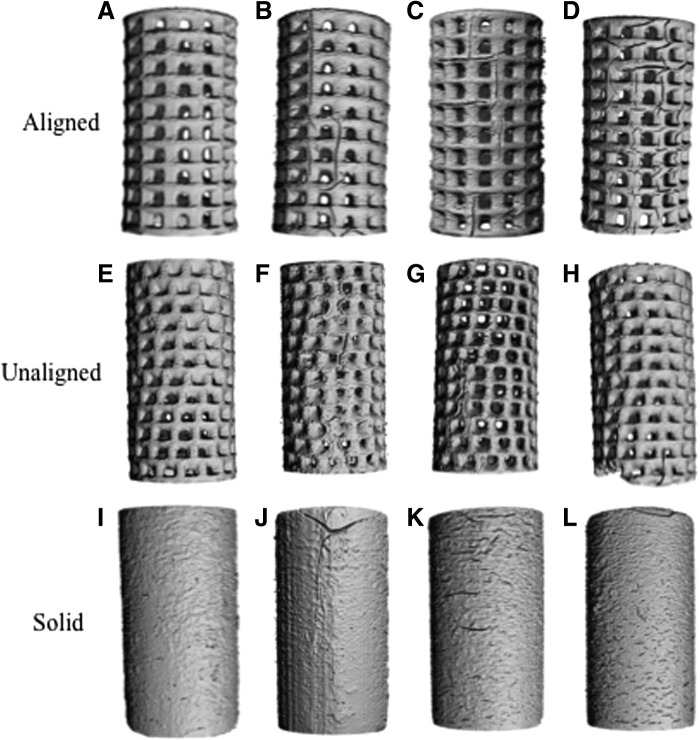
μCT was used to produce 3D renderings at each timepoint of the study to visualize the degrading scaffolds (Fig. 7). As the scaffolds degrade, we can see both the emergence of surface channels and an increase in surface texture. This is highlighted as changes in the surface of the solid wall scaffolds as they degrade (Fig. 7A–D). Figure 7E–H are representative images of the aligned scaffolds during degradation. In comparison, the aligned pore scaffolds have the greatest number and length of surface channels of the two porous scaffold designs by day 224 compared with the unaligned pore scaffolds (Fig. 7I–L).
FIG. 7.
μCT 3D rendering of scaffolds during degradation. (A–D) Solid wall scaffolds at day 1 (A), day 28 (B), day 56 (C), and day 224 (D). Degradation can be seen with the formation of smaller surface channels at day 224 compared with the previous timepoints during the study. (E–H) Aligned scaffolds at day 1 (E), day 28 (F), day 56 (G), and day 224 (H). Surface channel formation was seen with increasing number and length as the study progressed. (I–L) Unaligned scaffolds at day 1 (I), day 28 (J), day 56 (K), and day 224 (L). Small number of surface channels can be seen over the course of the study.
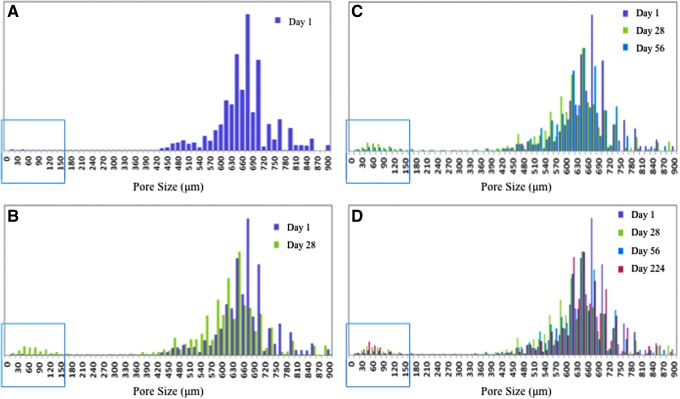
Histograms of pore sizes were produced for each of the porous scaffolds. Figure 8 is an example of these histograms of pore sizes at four different times during the study, where day 1 (Fig. 8A), day 28 (Fig. 8B), day 56 (Fig. 8C), and day 224 (Fig. 8D) data are presented for the aligned pore scaffolds. The green box highlights that as the scaffolds degrade, a population of small pores with a thickness of less than 150 μm emerges. These are referred to as surface channels and micropores.
FIG. 8.
Histogram of aligned pore scaffold pore sizes during degradation. (A) On day 1, there are very few pores <150 μm (population highlighted in the green box). (B) By day 28, a small population of pores <150 μm begin to emerge. (C) At day 56, the trend continues with additional small pores, along with some larger small pores, showing the propagation of surface channels. (D) At day 224, the population of small pores continues to grow as scaffold degradation occurs.
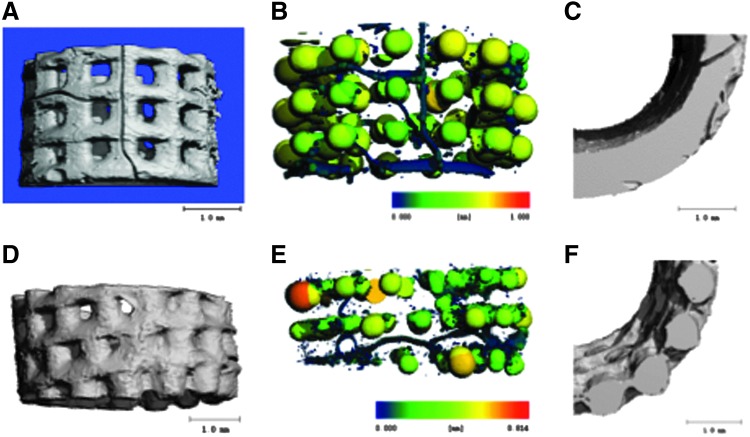
The emergence of micropores could also be visualized on the μCT pore map (Fig. 9B, E). As seen in the figure, the smallest pores or micropores are shown in dark blue. Also seen in dark blue are the surface channels (Fig. 9B), some of which span the scaffold. Fewer surface channels are seen on the unaligned scaffolds compared with the aligned pores. As seen in Figure 9A–C, the majority of dark blue pixels for aligned scaffolds are formed by surface channels. In contrast, in the unaligned pore scaffolds, the majority of the dark blue pixels are in the form of micropores as seen in Figure 9D–F. The micropores are unable to be visualized from the exterior scaffold surface but can be seen when the 3D μCT renderings are bisected (Fig. 9E).
FIG. 9.
μCT 3D renderings and pore maps. (A) 3D rendering of the bottom section of an aligned pore scaffold. (B) Pore map of (A) used to visualize pores in scaffold. (C) Bisected cross-section of 3D rendering, segmented to allow for visualization of channels that span the scaffold; channels are visible in the scaffold. (D) 3D rendering of bottom section of unaligned pore scaffold. (E) Pore map of (D). Color on pore maps represents thickness. Dark blue pores are present in the aligned scaffold mainly as surface channels, whereas in the unaligned pore scaffold the dark blue pores are seen as micropores on the interior scaffold and are not visible on the surface. (F) Bisected cross-section of rendering unaligned 3D scaffold; micropores are seen within the interior of the scaffold.
All three scaffold designs generally maintained their compressive mechanical properties during degradation in vitro (Fig. 10). In both the aligned and unaligned scaffolds, the maximum compressive loads were found to be statistically equivalent at all timepoints (Fig. 10A). Similarly, there was no statistical difference in the yield strength for the porous scaffolds throughout the degradation study (Fig. 10B). Similar to the maximum compressive load and the yield strength, the compressive moduli of the porous scaffolds were found to be statistically equivalent throughout the study (Fig. 10C). In contrast, the solid wall scaffolds were found to exhibit statistical differences in yield strength and maximum compressive load during degradation. The yield strength was found to increase over the study with a statistically higher yield at day 224. Unlike the yield strength, the maximum compressive load was found to be statistically higher at day 0 compared with all other timepoints. In comparison, the modulus of solid wall scaffolds was found to be statistically different between day 0 and days 7, 14, 28, 56, 112, and 224 (Fig. 10C, p<0.05).
FIG. 10.
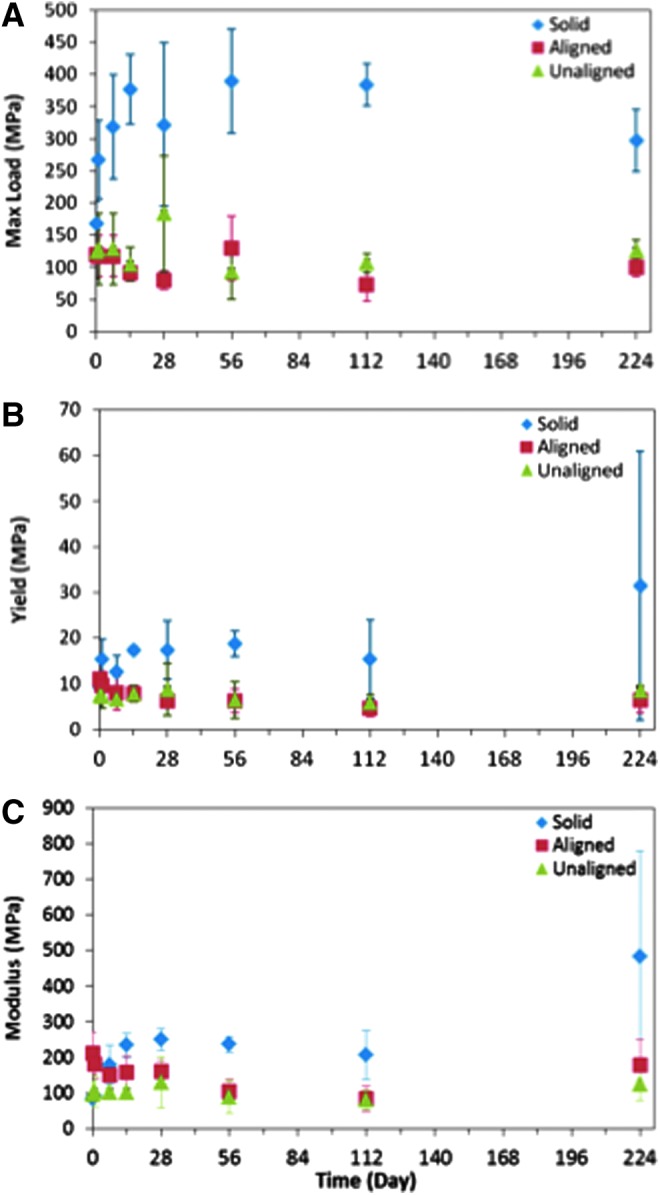
Wet compressive mechanical testing. Maximum load (A), 1% offset yield (B), and compressive modulus (C) are presented. Porous scaffolds do not demonstrate any statistical differences in mechanical properties during the study. Solid wall scaffolds were seen to have a statistically higher yield at day 224 (p<0.05, n=5 per timepoint) and a statistically lower maximum compressive load when dry, at day 0 (p<0.05, n=5 per timepoint). For the aligned scaffolds, days 56 and 112 had statistically lower moduli than the day 0 timepoint; however, by day 224, the moduli returned to being statistically similar to day 0. For the unaligned scaffold, there was no statistical difference between timepoints. *Denotes p<0.05 from all other timepoints within the same group for the solid wall scaffolds.
Cytotoxicity evaluation by XTT demonstrated that cell metabolic activity, when exposed to the extract of degrading PPF scaffolds, generally exceeds 80% and is statistically similar to cell metabolic levels of cells cultured with blank culture media at all timepoints, with the exception of day 56 (Fig. 11). On day 56, the highest extract exposure condition of 50% was found to be statistically lower than that of the blank media control (p<0.05). However, it still was statistically greater than the positive dead control (70% methanol). In addition, at day 56, the remaining two concentrations of extract (1% and 10%) resulted in cell metabolic activities that were statistically similar to that of blank culture media.
FIG. 11.
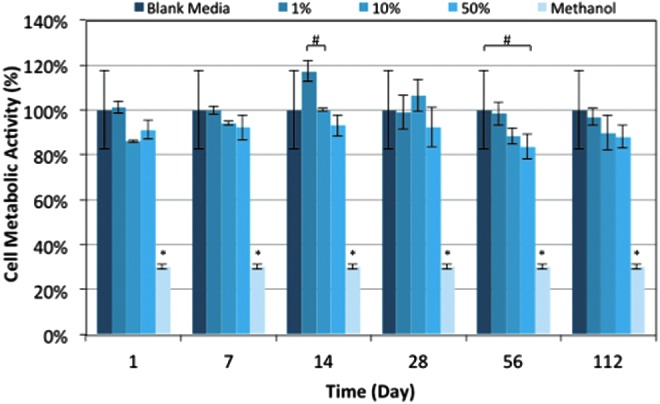
Cell metabolic activity levels. Fibroblast cells (L929) were cultured with a mixture of degradation extract and cell culture media in one of three ratios of extract: 1% extract:99% media (1%), 10% extract:90% media (10%), or 50% extract:50% media (50%). Cell metabolic levels were compared with metabolic activities from cells cultured with blank media, or a toxic control, 70% methanol. The * symbol denotes statistical difference within the timepoint (p<0.05), and #denotes statistical difference between the two groups within the timepoint (p<0.05) (n=3).
At all timepoints with the exception of day 14, the three concentrations of extract had statistically similar cell metabolic activity levels. At day 14, the middle concentration (10%) had a statistically lower cell metabolic activity level than that generated by the 50% extract exposure (p<0.05); however, the results still indicated statistically greater metabolic activity than the dead control. These results were qualitatively confirmed with fluorescent imaging.
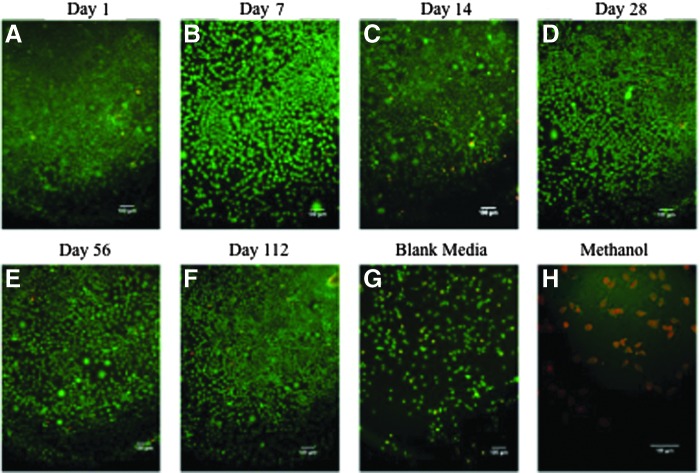
Fluorescence imaging was used to verify the cell viability. For all timepoints, no significant morphological changes were observed in cell populations that were incubated with the three different ratios of extract media (Fig. 12A–F), when compared with the cells incubated with blank culture media (Fig. 12G). Cells exposed to 70% methanol appeared to be red, indicating a significant decrease in viability of the entire population per cell type (Fig. 12H).
FIG. 12.
Cytotoxicity of degradation byproducts. Fluorescent images of L929 fibroblast cells after 24 h of incubation with media and degradation extract, where calcein AM (green) represents live cells, and ethidium homodimer (red) represents dead cells. Images are of those cells cultured with 50% extract and 50% culture media at day 1 (A), day 7 (B), day 14 (C), day 28 (D), day 56 (E), and day 112 (F). No significant changes in morphology are present after exposure (A–F) when compared with the blank media control (G). Cells were incubated with 70% methanol as the toxic control (H). Scale bars represent 100 μm.
Discussion
Absorbable polymers that are used for bone tissue engineering must provide mechanical support during degradation to allow for defect site stability during bone repair. In addition, this scaffold must not elicit a cytotoxic response in vivo, as this would deter the natural assimilation process required for native tissue integration. PPF scaffolds were evaluated for changes in their structure, mechanical properties, and cytotoxicity during absorption. Three scaffold designs (aligned pore, unaligned pore, and solid wall) were successfully fabricated using 3D printing (Fig. 4). 3D printing represents the ability to fabricate complicated computational scaffold designs with varying interconnectivity, porosity, and pore shape that was difficult to be accomplished earlier, and these are necessary to fabricate successful scaffolds.18,19 Our previous work identifies a set of tools to evaluate 3D printed scaffolds for use as basis for vascularized musculoskeletal tissues; however, we identified that there is a lack of standards that can provide the methodology to evaluate the long-term degradation of absorbable polymers scaffolds.20 In this work, our motivation was to investigate novel methodology to determine which 3D-printed porous scaffold design would provide long-term mechanical stability during degradation. This long-term mechanical stability would allow for the scaffold to function as a delivery vehicle for a bioactive material that is loaded into the lumen of the porous-walled scaffold. This scaffold would ideally provide the mechanical support to a bone defect while the bioactive material, such as stem cells encapsulated in a hydrogel, would facilitate new bone and vessel ingrowth into the defect. Finally, in this work, we have developed a novel strategy to assess the effects of degradation on cytotoxicity for 3D-printed scaffolds.
Two of the porous designs, aligned and unaligned pores, were formed using a modular design. The modular design is based on a repeating unit comprising a ring for the base with cylindrical posts extending from it. The pores were formed as squares and were designed to be 500 μm on each side, with the expectation that the printed pores would be slightly smaller. Initial studies demonstrated that the optimal pore size of ∼100 μm is based on cell size; however, currently many studies have shown that pore sizes of 100–350 μm promote greater cell migration and vascularization.21,22 In addition, previous studies have shown that for scaffolds with pores larger than 270 μm, it is as if there is no scaffold to hinder the vascularization process.23 Studies have also shown that interconnected pores above 300 μm may improve nutrient flow.24 As this scaffold is expected to function as a delivery method for bioactive materials for the bone tissue applications, it is anticipated that the cells that will interact with the pores would primarily be mesenchymal stem cells or osteoblasts. Previous studies have shown that these cells can easily migrate through pores within the average pore sizes that were obtained.21,25 Therefore, our porous scaffolds would be expected to allow for cellular migration and nutrient flow to the inner lumen when implanted in vivo, as the pores of the unaligned and the aligned scaffolds were 569±45 and 619±30 μm, respectively.
Sufficient nutrient flow was expected to occur into the inner lumen from the large pores. The 3D-printed scaffolds were found to have larger wall thickness and pores than the designs (Fig. 4). We believe that the larger wall thicknesses and pores may be attributed to the characteristics of the PPF polymer resin. Previous studies using similar resin compositions have shown that achieving a high accuracy of small features during 3D printing may be difficult due to inadvertent dark curing or the viscosity of the polymer resin.15,26
Degradation was observed through mass loss and changes in extract pH (Fig. 5). Samples were tested at increasing time intervals to allow for the cytotoxic evaluation of the immediate release of byproducts as well as the long degradation byproducts. Increasing the duration between timepoints allowed for the evaluation of the structural changes during the expected period of greatest mass loss as shown in previous studies.4 Previous studies have demonstrated that porous PPF scaffolds may have mass losses ranging from 18% to 30% over a 32 week period.4 During the current 224 day (32 week) study, the scaffolds lost 12±1% (aligned), 17±2% (unaligned), or 17±7% (solid wall) of their initial mass (Fig. 5). These mass loss values are moderately smaller than those in previous in vitro PPF degradation studies but are within the range of expected values and indicated that degradation through mass loss was occurring.4
One key parameter that impacts degradation time is PPF network crosslinking, where a highly crosslinked network generally degrades more slowly than a modestly crosslinked network. This concept has been shown in previous studies with polymer networks containing PPF.27 To examine this parameter, we measured scaffold sol fraction which describes the amount of the polymer network that is not included in the crosslinking reaction, so that high network crosslinking is associated with low sol fraction. Results from our studies here (Table 1) show that sol fraction was statistically similar among the three groups, demonstrating that the fabrication methods were consistent between the different designs and therefore the scaffold designs would not be expected to have different degradation time frames. Interestingly, our scaffolds degraded slower than previously published studies using similar PPF-based polymer scaffolds in which 18–30% mass loss was seen over 224 days.4 We suggest that this may have occurred due to greater crosslinking compared with the previously evaluated scaffolds.
To elucidate which scaffold parameters were altered during degradation, μCT was used to evaluate changes in scaffold wall thickness, porosity, and pore size (Fig. 6). Interestingly, there were no statistically significant changes in mean pore size or wall thickness; however, there was a significant increase in porosity from the initial printing (day 0) to day 224 (Fig. 6A). This is believed to occur due to the emergence of a population of small pores. As the population of micropores and surface channels began emerging in pore thickness counts, visual alterations of the scaffold during degradation occurred as depicted through μCT 3D renderings. The 3D renderings showed that surface channel formation occurred at each timepoint, with increasing number and length as the degradation study progressed (Fig. 8). This was especially prevalent with the aligned pore scaffold group (Fig. 8A–D) compared with the unaligned (Fig. 8E–H). For the unaligned scaffolds, the small pore formation occurred through the formation of micropores throughout the scaffold as seen in the μCT pore maps (Fig. 9). We hypothesize that it is the irregular architecture of the unaligned scaffolds that prevents the surface channel formation and propagation along the struts of the scaffold.
As mentioned earlier, degradation is expected to occur faster in locations where there is reduced crosslinking. In addition, we expect that scaffold design may impact crosslinking due to local areas of higher or lower crosslinking as seen in previous studies using similar methods of 3D printing.15,20,26 For example, as the aligned scaffolds have a symmetrical geometry, if there was an area of the modular design that was associated with decreased or increased crosslinking, then this area would be repeated layer after layer in the same X-Y location, thus creating regions of higher or lower crosslinking. Alternatively, the irregular scaffold design uses the modular design unit as the aligned scaffold but it is stacked randomly. The random stacking creates a nonsymmetrical scaffold, such that as the region of decreased or increased crosslinking occurs it would not occur in the same X-Y location as the scaffold is built. Therefore, we suggest that as the degradation was initiated, it would be easier for these pockets of decreased crosslinking to link together and form the surface channels on the aligned scaffolds.
Interestingly, neither the emergence of surface channels nor the micropores were found to have an impact on the mechanical properties over the 224 day degradation study as demonstrated by the statistical equivalence of the maximum compressive load and the yield strength throughout the study (Fig. 10). Throughout the degradation study, the porous scaffolds demonstrated average compressive strength and moduli larger or within the range of average trabecular bone.4 Specifically, the average compressive strength of trabecular bone has been reported as 1.4–79,28 1–13,29 0.52–11,30 0.98–22.5,31 and 0.45–15.6 MPa.32 To make a comparison, our aligned pore scaffolds had compressive strengths ranging from 73.3±26.2 MPa (day 112) to 130.1±49.8 MPa (day 56). The unaligned pore scaffolds' compressive strength ranged from 93.0±42.6 MPa (day 56) to 128.0±55.7 MPa (day 1). For the average compressive modulus, studies have estimated it as 1.4–79,28 7.6–800,30 and 49–572.30 Our scaffolds had an average compressive moduli ranging from 84.1±36.6 MPa (day 112) to 211.6±57.8 MPa (day 0) and 80.32±26.2 MPa (day 112) to 125.6±46.9 MPa (day 224) for the aligned and the unaligned porous scaffolds, respectively. This is a key finding, as to treat bone defects, the scaffolds must provide long-term mechanical support during degradation and this result suggests that the stiffness of our PPF scaffolds throughout degradation may be sufficient for this purpose.
Finally, scaffold and host tissue compatibility is a critical parameter, and with a degradable material it is necessary to ensure that byproducts of degradation do not elicit a cytotoxic response. Currently, only a few standards (e.g., ASTM F1635, ASTM F2902, ASTM F1983) evaluate absorbing polymers16,33,34 and tend to focus on degradation testing guidelines (F1635 and F2902) or evaluations with animal tissue (F1983). These standards provide little guidance on how to evaluate cytotoxicity during long-term degradation. To address this, we developed a novel method to evaluate cytotoxicity of degradation byproducts over an extended time frame. This method is based on two standards: the ISO standard 10993-5 for extract testing and the ASTM standard F1635.11,16
As PPF hydrolytically degrades, it produces propylene glycol and fumaric acid.35 This is important, as one indication of degradation of polyesters is a decrease in pH as seen in the study (Fig. 5). However, this decrease in pH did not impact cell viability. Cell metabolic activity levels and live/dead fluorescent imaging confirmed that the extract of PPF as it is absorbed does not elicit a significant cytotoxic response (Figs. 10 and 11). Cell metabolic activity levels after incubation with the degradation extract were statistically greater than the cell metabolic activity levels of the cells exposed to methanol and generally similar to blank culture media except for day 56. These results are in line with our previous work that showed good cellular responses to highly crosslinked PPF thin films.1
The novelty of this work is that we evaluated the potential cytotoxicity elicited during the absorption of PPF scaffolds. PPF is absorbed slower than other absorbable polyesters, such as the saturated aliphatic polyesters poly(glycolic) acid (PGA) or poly(l-lactic) acid (PLA).12,36–38 During absorption, cytotoxicity has been shown to be tied to the acidic byproducts as seen with the many forms of PLA, PGA, and its co-polymer poly(lactic-co-glycolic acid), which degrade through hydrolysis and produce lactic and glycolic acid as products.36,39 PPFs slow absorption allows for prolonged mechanical stability and reduced concerns of cytotoxicity from the slow release of acid. The slow absorption and the body's ability to metabolize fumaric acid are instrumental to a low cytotoxicity response in vivo. Previous studies have shown that the rate of degradation increases in vivo compared with that in vitro due to the accumulation of acidic degradation byproducts, local cellular activity.40,41 For PPF in vivo, the acidic byproduct is fumaric acid and it would be metabolized as a part of the Kreb's cycle; whereas in vitro we are unable mimic this process. Therefore, although in vivo degradation rates are believed to be faster than in vitro rates, we believe that any potential increased rate of mass loss from the PPF scaffolds would not have any additional cytotoxic effect. Overall, our study demonstrated that the degradation byproducts of PPF elicited little to no cytotoxic response and had the same cytotoxic response as a known noncytotoxic solution, cell culture media, when cultured with fibroblasts.
Conclusions
PPF porous scaffolds degrade slowly and provide mechanical properties that may be sufficient for the treatment of cancellous bone defects. This slow degradation and stable mechanical properties would allow for the delivery of a bioactive material to enhance the treatment of bone defects. Degradation is seen through both bulk mass loss and the formation of surface channels and micropores. These surface channels and micropores are best seen nondestructively with μCT evaluation. The degradation byproducts from the hydrolysis of the polymer network were shown to not elicit a negative cellular response when cultured with fibroblasts. These results indicate that porous degradable PPF scaffolds, whether with aligned or unaligned scaffolds, would be suitable for bone tissue engineering.
Acknowledgments
This research was supported, in part, by the National Institutes of Health (R01-DE013740) and a Food and Drug Administration Center of Excellence in Regulatory Science and Innovation (CERSI) grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wang M.O., et al. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 14,1321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher J.P., Dean D., and Mikos A.G.Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials 23,4333, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Kim K., et al. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules 10,1810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher J.P., et al. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules 4,1335, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Krebs H.A., and Johnson W.A.The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett 117,K2, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Directorate-General E.C.H.C.P. Report of the Scientific Committee on Animal Nutrition on the Safety of Fumaric Acid, M.o.s.c.s.c.-o.a.n. Directorate C—Scientific Opinions, Editor 22 January 2013. http://ec.europa.eu/food/fs/sc/scan/out112_en.pdf
- 7.Altmeyer P.J., et al. Antipsoriatic effect of fumaric acid derivatives: results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol 30,977, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Zar T., Graeber C., and Perazella M.A.Reviews: recognition, treatment, and prevention of propylene glycol toxicity. Semin Dial 20,217, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Shi X., et al. , Poly(Propylene Fumarate). Boca Raton, FL: CRC Press, 2006 [Google Scholar]
- 10.Singh U., Devaraj S., and Jialal I.Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 25,151, 2005 [DOI] [PubMed] [Google Scholar]
- 11.International Organization for Standardization. Biological Evaluation of Medical Devices, in ISO 10993: Parts 1–12 Various. Geneva, Switzerland: ISO, 2009 [Google Scholar]
- 12.Kasper F.K., et al. Synthesis of poly(propylene fumarate). Nat Protoc 4,518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ASTM. Standard Test Method for Compressive Properties of Rigid Plastics. West Conshohocken, PA: ASTM International, 2012 [Google Scholar]
- 14.Wang M.O., et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater 27,138, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean D., et al. Continuous digital light processing (cDLP): highly accurate additive manufacturing of tissue engineered bone scaffolds. Virtual Phys Prototyp 7,13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ASTM. Standard test method for in vitro degradation testing of hydrolytically degradable polymer resins and fabricated forms for surgical implants. In: F1635. West Conshohocken, PA: ASTM International, 2011 [Google Scholar]
- 17.Betz M.W., et al. Cyclic acetal hydrogel system for bone marrow stromal cell encapsulation and osteodifferentiation. J Biomed Mater Res A 86,662, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Adachi T., et al. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 27,3964, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hollister S.J.Scaffold design and manufacturing: from concept to clinic. Adv Mater 21,3330, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wang M.O., et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater 27,138, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karageorgiou V., and Kaplan D.Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26,5474, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Yang S., et al. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 7,679, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Artel A., et al. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng Part A 17,2133, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hutmacher D.W.Scaffolds in tissue engineering bone and cartilage. Biomaterials 21,2529, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Ishaug-Riley S.L., et al. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 19,1405, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Wallace J., et al. Validating continuous digital light processing (cDLP) additive manufacturing accuracy and tissue engineering utility of a dye-initiator package. Biofabrication 6,015003, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Timmer M.D., Ambrose C.G., and Mikos A.G.In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials 24,571, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Carter D.R., and Hayes W.C.Bone compressive strength: the influence of density and strain rate. Science 194,1174, 1976 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein S.A., et al. The mechanical properties of human tibial trabecular bone as a function of metaphyseal location. J Biomech 16,965, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Ciarelli M., et al. Experimental determination of the orthogonal mechanical properties, density, and distribution of human trabecular bone from the major metaphyseal regions utilizing materials testing and computed tomography. Trans Orth Res Soc 42,238, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Ducheyne P., et al. The mechanical behaviour of intracondylar cancellous bone of the femur at different loading rates. J Biomech 10,747, 1977 [DOI] [PubMed] [Google Scholar]
- 32.Martens M., et al. The mechanical characteristics of cancellous bone at the upper femoral region. J Biomech 16,971, 1983 [DOI] [PubMed] [Google Scholar]
- 33.ASTM. Standard Practice for assessment of compatibility of absorbable/resorbable biomaterials for implant applications. In: F1983. West Conshohocken, PA: ASTM International, 2008 [Google Scholar]
- 34.ASTM. Standard guide for characterization and testing of biomaterial scaffolds used in tissue-engineered medical products. In: F2150-07. West Conshohocken, PA: ASTM International, 2007 [Google Scholar]
- 35.He S., et al. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)-diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 42,1251, 2001 [Google Scholar]
- 36.Rezwan K., et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27,3413, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Törmälä P., et al. Ultra high strength absorbable self reinforced polyglycolide (SR PGA) composite rods for internal fixation of bone fractures: in vitro and in vivo study. J Biomed Mater Res 25,1, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Hollinger J.O., and Battistone G.C.Biodegradable bone repair materials synthetic polymers and ceramics. Clin Orthop Relat Res 207,290, 1986 [PubMed] [Google Scholar]
- 39.Taylor M.S., et al. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater 5,151, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Sung H.-J., et al. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 25,5735, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Lu L., et al. In vitro and in vivo degradation of porous poly (dl-lactic-co-glycolic acid) foams. Biomaterials 21,1837, 2000 [DOI] [PubMed] [Google Scholar]