Abstract
Study Objective
To identify predictors of medication-related problems (MRPs) among Medicaid patients participating in a telephonic medication therapy management (MTM) program.
Design
Retrospective analysis of data from patients enrolled in a previously published study
Data Sources
Two Medicaid administrative claims file databases (for healthcare utilization and prescription dispensing information) and one pharmacy organization file for MTM program information.
Patients
Seven hundred twelve adult Medicaid patients who participated in a statewide pharmacist-provided telephone-based MTM program and who received an initial medication therapy review.
Measurements and Main Results
The primary dependent variable was the number of MRPs detected during the initial medication therapy review. Secondary dependent variables were the detection of one or more MRPs related to indication, effectiveness, safety, and adherence. Predictor variables were selected a priori that, from the literature and our own practice experiences, were hypothesized as being potentially associated with MRPs: demographics, comorbidities, medication use, and healthcare utilization. Bivariate analyses were performed, and multivariable models were constructed. All predictor variables with significant associations (defined a priori as p<0.1) with the median number of MRPs detected were then entered into a three-block multiple linear regression model. The overall model was significant (p<0.001, R2= 0.064). Significant predictors of any MRPs (p<0.05) were total number of medications, obesity, dyslipidemia, and one or more emergency department visits in the past 3 months. For indication-related MRPs, the model was significant (p<0.001, R2= 0.049), and predictors included female sex, obesity, dyslipidemia, and total number of medications (p<0.05). For effectiveness-related MRPs, the model was significant (p<0.001, R2= 0.054), and predictors included bone disease and dyslipidemia (p<0.05). For safety-related MRPs, the model was significant (p<0.001, R2= 0.046), and dyslipidemia was a predictor (p<0.05). No significant predictors of adherence-related MRPs were identified.
Conclusion
This analysis supports the relative importance of number of medications as a predictor of MRPs in the Medicaid population and identifies other predictors. However, given the models’ low R2 values, these findings indicate that other unknown factors are clearly important and that criteria commonly used for determining MTM eligibility may be inadequate in identifying appropriate patients for MTM in a Medicaid population.
Keywords: administration, outcomes, pharmacy practice
Introduction
The 2006 implementation of the Medicare Prescription Drug, Improvement, and Modernization Act introduced the concept of medication therapy management (MTM) services into the Medicare program as part of the new Medicare Part D benefit providing outpatient prescription drug insurance to Medicare beneficiaries.1 Since then, stakeholders have engaged in numerous conversations regarding delivery models and standards of care for conducting MTM. Among other milestones, this has resulted in the development of a pharmacy professionwide consensus definition for MTM,2 a description of MTM service core elements,3 ongoing modifications to Medicare Part D MTM requirements, and a notable increase in MTM-related published literature.4
Since its inception, one of the “hot topics” surrounding MTM has been the criteria used to determine patient eligibility for these services. With the implementation of the Medicare Part D MTM requirement in 2006, prescription drug plans selected the following eligibility criteria for their plan’s MTM program within the broad Medicare requirements: multiple chronic diseases, taking multiple covered Part D drugs, and likely to incur annual drug costs for covered Part D drugs exceeding approximately $4000.1 As more information about MTM has emerged over the last several years, these criteria have been further refined. As of 2014, these criteria specify that plans can require that patients have two or three comorbid conditions to be eligible for MTM, and if they choose to target specific conditions, they must target at least five out of nine specific comorbidities. Furthermore, plans can require between two and eight Part D–covered medications, and the cost threshold for eligibility of MTM services has been lowered to an approximate annual expenditure of at least $3000.5
Results of MTM program evaluations have been mixed.6–17 Carefully examining patient eligibility is an important consideration in optimizing the outcomes of MTM services and maximizing the return on investment realized. One strategy is to prospectively identify what characteristics predict medication-related problems (MRPs) and then target individuals with those characteristics for MTM services. Previous studies have examined predictors of MRPs and have used these predictors in the development of a variety of risk assessment tools designed to predict a patient’s risk for MRPs.18–27 Examples of previously identified predictors include number of medications, number of doses needed/day, number of comorbidities, and number of providers prescribing medication.18,23 Although MTM is a required benefit for Medicare Part D plans, the provision of MTM by state Medicaid programs is becoming more common.28 In previous studies specifically examining MRPs in a Medicaid population, the number of medications taken by the patient was found to be an important predictor of problems.29–31
Stakeholders, however, have continued to question the value of current Medicare Part D eligibility criteria,32–35 and the Centers for Medicare and Medicaid Services is currently considering significant changes to these criteria.36 Further evaluation of predictors of MRPs and, subsequently, MTM eligibility criteria is warranted among both Medicare beneficiaries and other populations.
In this study, we analyzed data from a previously published study on the implementation of a statewide telephonic MTM program for Medicaid patients,37 as that study provided an opportunity to evaluate MRP predictors in the Medicaid population. Therefore, our objective was to identify predictors of MRPs—first by examining MRP predictors across all problem categories and second by examining each category of MRPs separately. This work could further inform the development of MTM service eligibility, particularly among Medicaid beneficiaries.
Methods
Study Design, Patient Population, and Data Sources
This was a retrospective analysis of data collected from adult (aged 18–65) Medicaid patients who participated in a statewide pharmacist-provided telephonic MTM program.37 This MTM program was offered to patients who were enrolled in a specific Medicaid program that focused on providing disease management services for chronic conditions among the aged, blind, and disabled. The MTM program was optional and offered at no additional charge to the member; eligibility criteria included the following: enrollment in the Medicaid disease management program, continuous eligibility for Medicaid through the start of the MTM program, and receiving at least five Medicaid-covered medications.
In addition to MTM services, the program included medication synchronization and home delivery of medications (28-day supply at a time) using specialized packaging to promote adherence. As part of the MTM service, patients were telephoned by the pharmacist for an initial medication therapy review (MTR) within the first two months of receiving their medications. Follow-up MTM telephone calls occurred as needed. During the initial MTR, all medications were reviewed with the patient and caregiver to identify MRPs within four broad categories—indication, effectiveness, safety, and adherence, based on the taxonomy described by Cipolle et al.38 The pharmacist also prepared a personal medication record and a medication-related action plan for the patient. To evaluate the impact of the program on medication adherence and healthcare utilization, patients eligible for MTM who opted to participate in the service were compared with those who were eligible but opted not to participate, and both groups were followed for 12 months.
For this analysis, all patients who received the initial MTR were included. Data were extracted from two Medicaid administrative claims files for healthcare utilization and prescription dispensing information, and one pharmacy organization file for MTM program information. This project was approved by the Indiana University Institutional Review Board.
Outcome Measures and Data Analysis
Our primary objective was to identify associations between certain patient characteristics and the presence of MRPs. For this analysis, the dependent variable was the number of MRPs detected by the pharmacist during the initial MTR. Our secondary objective was to identify associations between certain patient characteristics and the presence of MRPs in select categories. The dependent variables for this analysis included the detection of one or more MRPs (Table 1) related to each type of broad medication problem: indication, effectiveness, safety, and adherence. From the available databases, predictor variables were selected a priori that, from the literature18,23 and our own practice experiences, were hypothesized as being potentially associated with MRPs. Predictor variables (Table 1) included patient demographics, comorbidities, medication use data, and healthcare utilization. The list of narrow therapeutic index (NTI) drugs was used as it has been referenced in previous studies.18 When applicable, comorbidities were defined by using the Elixhauser criteria,39 and an overall Elixhauser score was calculated for each patient. In addition to select comorbidities included from the Elixhauser criteria, we included conditions targeted by the Centers for Medicare and Medicaid Services for eligibility for Medicare Part D MTM services.5 However, we excluded Alzheimer’s disease because it is not prevalent in this population. The three-month time frame for medication and healthcare utilization variables was selected in an effort to characterize medications currently being used by the patient. Post hoc predictor variables considered were marital status and location of residence. Analyses were conducted by using SPSS, version 20.01 (IBM, Somers, NY), and SAS, version 9.2 (SAS Institute Inc., Cary, NC).
Table 1.
Definitions of Variables
| Variable | Definition/Comments |
|---|---|
| Predictors | |
| Patient Demographics | |
| Age | Continuous, from 18–65 yrs |
| Sex | |
| Race | Dichotomized to Caucasian vs other |
| Urban vs rural residence | Dichotomized by using patient’s ZIP code of residence |
| Marital status (married vs not married) | Dichotomized as married or not married |
| Comorbidities | |
| Hypertension | Determined by all ICD-9 codes in the Medicaid database 1 year prior to first MTM telephone call using Elixhauser criteria39 |
| Chronic lung disease | |
| Diabetes mellitus | |
| Obesity | |
| Depression | |
| Heart Failure | |
| Renal failure | |
| Liver disease | |
| Alcohol abuse | |
| Drug abuse | |
| Psychoses | |
| Bone disease (includes osteoporosis, osteoarthrosis, allied disorders, and rheumatoid arthritis) | Determined by all ICD-9 codes in the Medicaid database 1 year prior to first MTM telephone call using ICD-9 codes recognized as codes for disease states targeted for MTM by CMS. Specific codes included: V17.81 Osteoporosis; V82.81 Osteoporosis; 733.00 Osteoporosis, unspecified; 715 Osteoarthrosis and allied disorders (arthritis or polyarthritis, degenerative, hypertrophic, degenerative joint disease, osteoarthritis); 714.0 Rheumatoid arthritis V82.1 Rheumatoid arthritis |
| Dyslipidemia | Determined by all ICD-9 codes in the Medicaid database 1 year prior to first MTM telephone call using ICD-9 codes recognized as codes for disease states targeted for MTM by CMS. Specific codes included: 272.0 pure hypercholesterolemia; 272.2 hyperlipidemia, mixed; 272.4 hyperlipidemia, other |
| Total no. of comorbidities | Sum of each comorbidity present using Elixhauser criteria39 |
| Medication Use Data | |
| No. of pharmacies | Calculated from Medicaid medication reimbursement records 3 months prior to MTR |
| No. of prescribers | |
| No. of chronic medications | Calculated as no. of unique scheduled oral medications for 3 months prior to MTR |
| No. of pills/day | |
| Total no. of medications | Calculated from all medication records 3 months prior to MTR; all medications and dosage forms included |
| No. of narrow therapeutic index drugs | Calculated from all medication records 3 months prior to MTR; all medications and dosage forms included Narrow therapeutic index drugs included carbamezepine, lithium, phenytoin, quinidine, warfarin, phenobarbital, procainamide, theophylline, digoxin, and insulin |
| Healthcare Utilization | |
| No. of outpatient visits | Calculated by using Medicaid claims data for 3 months prior to MTR |
| No. of patients with ≥ 1 hospitalization | |
| No. of patients with ≥ 1 ED visit | |
| Outcomes | |
| Primary | |
| No. of MRPs detected | Data from initial MTR only; includes all problem categories |
| Secondary | |
| At least one MRP in indication category | Data from initial MTR only. Problems included were untreated condition, synergistic therapy, preventative therapy (collapsed into “needs additional therapy”); no medical indication, recreational drug, nondrug therapy, duplicate, treating avoidable adverse reaction (collapsed into “unnecessary drug therapy”) |
| At least one MRP in effectiveness category | Data from initial MTR only. Problems included dosage form inappropriate, contraindication, condition refractory to drug, not indicated, more effective drug (collapsed into “different drug needed”); ineffective, inappropriate frequency, duration, storage, administration (collapsed into “dosage too low”) |
| At least one MRP in safety category | Data from initial MTR only. Problems included unsafe, allergic reaction, undesired effect, interaction, dosage changed too fast (collapsed into “adverse drug reaction”); dose too high, frequency too short, duration too long (collapsed into “dosage too high”); drug interaction resulting in dose too high/low, needs additional monitoring (collapsed into “dosage too high/low”) |
| At least one MRP in adherence category | Data from initial MTR only. Problems included not available, cannot afford, cannot administer, forgets, does not understand, prefers not to take (collapsed into “noncompliance”) |
ICD = International Classification of Diseases, Ninth Revision; MRP = medication-related problem.
We examined each predictor variable independently by using bivariate tests for associations with the median number of MRPs detected by the pharmacist during the initial MTR: Spearman correlations were used for continuous predictor variables, and Wilcoxon rank sum tests were used for categorical predictor variables. The median, rather than mean, number of problems was used as our outcome because the problem count was not normally distributed. For completeness, we also examined associations between predictor variables and MRPs dichotomized as one or more MRPs were detected by using Student’s t tests or Wilcoxon rank sum tests (for nonnormally distributed data) or the Fisher exact analysis for continuous and categorical predictor variables, respectively (Table 2.)
Table 2.
Demographic and Clinical Characteristics of the Study Patients
| Characteristic | All Patients (n=712) |
Patients without MRPs (n=277) |
Patients with ≥ 1 MRP (n=435) |
p- valuea |
|---|---|---|---|---|
| Age (yrs) | 50.2 +/− 8.6 | 50 +/− 9.7 | 50.4 +/− 7.9 | 0.601 |
| Female sex | 520 (73) | 191 (69) | 329 (75.6) | 0.057 |
| Caucasian | 577 (81) | 233 (84.1) | 344 (79.1) | 0.097 |
| Married | 141 (19.8) | 52 (18.8) | 89 (20.5) | 0.630 |
| Urban residence | 379 (53.2) | 138 (49.8) | 241 (55.4) | 0.166 |
| Hypertension | 358 (50.3) | 139 (50.2) | 219 (50.3) | 1.0 |
| Chronic lung disease | 230 (32.3) | 86 (31) | 144 (33.1) | 0.622 |
| Diabetes mellitus | 278 (39) | 107 (38.6) | 171 (39.1) | 0.935 |
| Depression | 80 (11.2) | 40 (14.4) | 40 (9.2) | 0.038 |
| Psychoses | 64 (9) | 25 (9) | 39 (9) | 1.0 |
| Bone disease | 232 (32.6) | 91 (32.9) | 141 (32.4) | 0.935 |
| Dyslipidemia | 371 (52.1) | 133 (48) | 238 (54.7) | 0.091 |
| End-stage renal disease | 25 (3.5) | 8 (2.9) | 17 (3.9) | 0.536 |
| Heart failure | 69 (9.7) | 28 (10.1) | 41 (9.4) | 0.796 |
| Obesity | 89 (13) | 25 (9) | 64 (14.7) | 0.027 |
| Liver disease | 27 (3.8) | 9 (3.2) | 18 (4.1) | 0.688 |
| Alcohol abuse | 19 (2.7) | 8 (2.9) | 11 (2.5) | 0.814 |
| Drug abuse | 36 (5.1) | 22 (7.9) | 14 (3.2) | 0.108 |
| Total no. of comorbiditiesb | 2.5 +/− 2.1 | 2.5 (2.1) | 2.5 (2.0) | 0.675 |
| No. of pharmacies | 2.9 +/− 1.9 | 2.8 (1.8) | 3 (1.9) | 0.134 |
| No. of prescribers | 1.2 +/− 0.6 | 1.2 (0.7) | 1.3 (0.6) | 0.568 |
| No. of chronic medications | 5.6 +/− 3.0 | 5.4 (3) | 5.7 (3) | 0.268 |
| Total no. of medications | 10.6 +/−5.4 | 10.2 (5.4) | 10.8 (5.4) | 0.128 |
| No. of narrow therapeutic index medications | 0 (0–2) | 0 (0–0) | 0 (0–0) | 0.702 |
| No. of pills/day | 17.3 (10–28.5) | 18 (9–28) | 17 (10–29.9) | 0.582 |
| No. of outpatient visits in the past 3 mo | 2 (0–2) | 1 (0–2) | 2 (0–3) | 0.396 |
| Had ≥ 1 hospitalization in the past 3 mo | 120 (16.8) | 49 (17.7) | 71 (16.1) | 0.607 |
| Had ≥ 1 emergency department visit in the past 3 mo | 479 (67.3) | 174 (62.8) | 305 (70.1) | 0.049 |
Data are mean ± SD, no. (%) of patients, or median (interquartile range [25th–75th percentile]).
: For the comparison between patients with one or more MRPs vs patients without MRPs; data were analyzed by using Student’s t tests or Wilcoxon rank sum tests (for nonnormally distributed data) or the Fisher exact analysis for continuous and categorical predictor variables, respectively.
: Calculated by using the Elixhauser criteria39
All predictor variables with significant associations (defined a-priori as p<0.1) with the median number of MRPs detected were then entered into a three-block multiple linear regression model. First, the total number of medications was entered, as this variable was previously reported to predict MRPs among Medicaid patients.29–31 Then, the other a priori variables with significant associations from the bivariate tests were entered to evaluate the change in R2. Finally, post hoc variables with significant associations from the bivariate tests were entered.
We also conducted sensitivity analyses for the primary outcome by examining predictor variables for associations with the number of MRPs at different thresholds (≥ 10, ≥ 20, ≥ 30, or ≥ 40 MRPs). Predictor variables with a resulting p value of < 0.1 on the bivariate tests described above were entered into a logistic regression model. The dependent categorical variable was the presence or absence of the defined threshold level of MRPs.
For the secondary objective, we examined each predictor variable independently for associations with whether one or more MRP was present for each broad category of MRPs (indication, effectiveness, safety, adherence).38 All predictor variables with p values of < 0.1, as identified by using Student’s t-tests or Wilcoxon rank sum tests (for nonnormally distributed data) or the Fisher exact analysis for continuous and categorical predictor variables, respectively, were entered into four separate logistic regression models. For each regression model, the dependent categorical variable was the presence of at least one MRP in the category under consideration. No cases were deleted from any of the regression analyses, and no data were missing for any of the variables.
Results
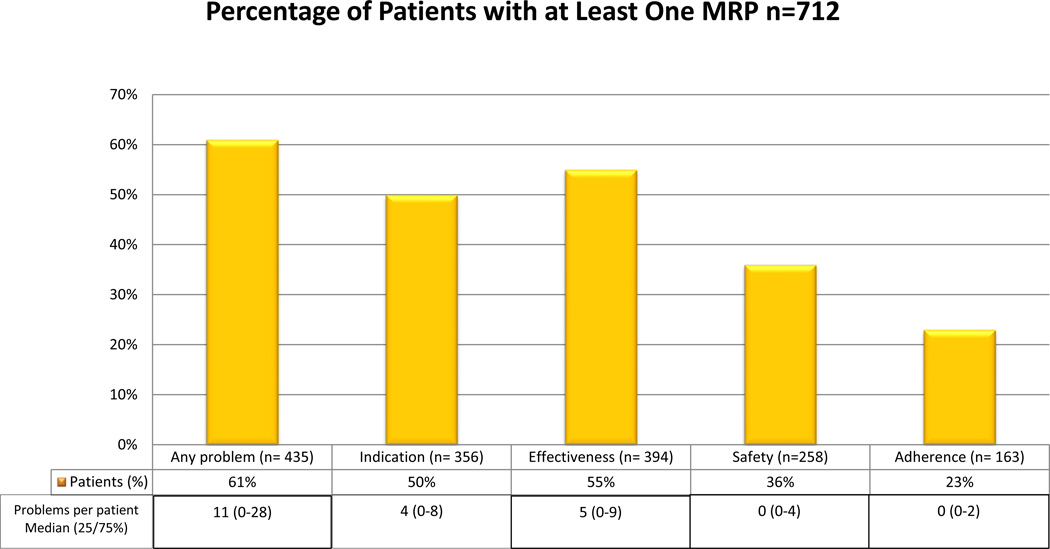
A total of 712 patients received an initial MTR and were included in this analysis. The sample consisted primarily of Caucasian women, approximately 50 years of age, with an average of two comorbidities, and using an average of 11 medications (Table 2). Sixty-one percent of patients (Figure 1) had one or more MRPs identified (median 11, interquartile range [25th–75th percentile] 0–28 MRPs). Patients with one or more MRPs were more likely to be obese and have one or more visits to emergency department (ED), and less likely to be diagnosed with depression, compared to those patients without an MRP. The following predictor variables were significant (p<0.1) on bivariate tests and entered into the multivariate linear regression model for the primary outcome: female sex, race, dyslipidemia, obesity, one or more ED visits, number of chronic medications, and total number of medications. The overall model was significant (p < 0.001, R2= 0.064). Significant predictors of MRPs (p < 0.05, Table 3) included dyslipidemia, obesity, one or more ED visits, and total number of medications. Post hoc predictor variables were evaluated as bivariate tests to determine whether social support (i.e., marital status and access to services) could explain these findings. Location of residence was significant (p= 0.041) and was entered as a third block to the regression model. The adjusted R2 value was unchanged. Collinearity statistics in the stepwise multiple regression were satisfactory (i.e., tolerance statistics > 0.1), although there was a trend toward collinearity for number of chronic medications (tolerance statistic= 0.297.) In sensitivity analyses for 10, 20, 30, and 40 or more MRPs/patient (Table 4), all variables remained significant predictors in the models except for ED visits.
Figure 1.
Prevalence of medication-related problems (MRPs) detected in the 172 patients.
Table 3.
Predictors of Medication-Related Problems Overall
| Dependent Variable |
Significant Predictors | P-value | Parameter Estimate of B (95% Confidence Interval) |
Beta | R2 | Change in R2 |
|
|---|---|---|---|---|---|---|---|
| Total no. of MRPs detecteda | Step 1 | Total no. of medications | 0.001 | 0.663 (0.280– 1.046) | 0.127 | 0.016 | NA |
| Step 2b | Dyslipidemia ≥ 1 ED visit Obesity |
0.001 0.01 0.001 |
7.558 (3.290–11.827) 5.827 (1.393–10.260) 10.878 (4.645–17.111) |
0.134 0.097 0.128 |
0.064 | 0.048 | |
| Step 3 | None identifiedc | NS | 0.066 | 0.002 | |||
: Overall model, p < 0.001.
: Predictor variables that were also included but were not significant were sex, race, and no. of chronic medications.
: Step 3: examined the addition of rural vs urban residence (p= 0.228) as a post hoc predictor variable.
Table 4.
Sensitivity Analyses for the Primary Outcome
| Dependent Variable |
Significant Predictorse | P-value | Parameter Estimate of B |
Odds Ratio (95% Confidence Interval) |
|---|---|---|---|---|
| ≥ 10 MRPsa | Female sex | 0.017 | −0.445 | 0.641 (0.444–0.924) |
| Dyslipidemia | 0.001 | 0.546 | 1.726 (1.243–2.397) | |
| Obesity | 0.011 | 0.605 | 1.831 (1.150–2.918) | |
| ≥ 20 MRPsb | Female sex | 0.017 | −0.445 | 0.641 (0.444–0.924) |
| Dyslipidemia | 0.001 | 0.546 | 1.726 (1.243–2.397) | |
| Obesity | 0.011 | 0.605 | 1.831 (1.150–2.918) | |
| ≥ 30 MRPsc | Total no. of medications | 0.005 | 0.078 | 1.081 (1.024–1.141) |
| No. of chronic medications | 0.020 | −0.118 | 0.889 (0.805–0.981) | |
| Dyslipidemia | 0.001 | 0.573 | 1.773 (1.252–2.512) | |
| Obesity | 0.006 | 0.664 | 1.942 (1.209–3.119) | |
| ≥ 40 MRPsd | Total no. of medications | 0.014 | 0.074 | 1.077 (1.015–1.142) |
| Rural residence | 0.018 | 0.450 | 1.568 (1.080–2.275) | |
| Dyslipidemia | < 0.001 | 0.836 | 2.308 (1.555–3.426) | |
| Obesity | 0.036 | 0.547 | 1.728 (1.035–2.884) | |
: Overall model, p < 0.001, R2= 0.068
: Overall model, p < 0.001, R2= 0.068
: Overall model, p < 0.001, R2= 0.073
: Overall model, p < 0.001, R2= 0.088
: Variables entered in all models: total no. of medications, sex, race, obesity, dyslipidemia, no. of chronic medications, one or more ED visits, and place of residence (rural vs urban)
With regard to specific problem types, predictors of indication, effectiveness, and safety-related problems are summarized in Table 5. No significant predictors of adherence-related MRPs were identified through bivariate tests; therefore, no multivariable model was evaluated.
Table 5.
Predictors of Medication-Related Problems by Problem Typea
| Dependent Variable | Significant Predictors | P-value | Parameter Estimate of B |
Odds Ratio (95% Confidence Interval) |
|---|---|---|---|---|
| One or more indication-related problemsb | Female sex | 0.008 | –0.341 | 1.641 (1.140– 2.372) |
| Dyslipidemia | 0.001 | 0.491 | 1.724 (1.244– 2.400) | |
| Obesity | 0.004 | 0.541 | 1.643 (1.030– 2.635) | |
| Total no. of medications | 0.015 | 0.017 | 1.071 (1.010– 1.130) | |
| One or more effectiveness-related problemsc | Dyslipidemia | 0.002 | 0.558 | 1.684 (1.211– 2.310) |
| Bone disease | 0.033 | 0.327 | 1.433 (1.211– 2.311) | |
| One or more safety-related problemsd | Dyslipidemia | <0.001 | 0.695 | 2.003 (1.460– 2.747) |
: No significant predictors of adherence-related problems were identified on bivariate analyses; therefore, no model was evaluated.
: Overall model, p < 0.001, R2= 0.049. Variables also included were race, depression, no. of chronic medications, and no. of pills per day. Specific problems included in this category were untreated condition, synergistic therapy, preventative therapy, no medical indication, recreational drug, nondrug therapy, duplicate, and treating avoidable adverse reaction
: Overall model, p < 0.001, R2= 0.054. Variables also included were sex, obesity, no. of pharmacies, no. of chronic medications, no. of pills per day, and total no. of medications. Specific problems included in this category were dosage form inappropriate, contraindication, condition refractory to drug, not indicated, more effective drug, ineffective, inappropriate frequency, duration, storage, and administration
: Overall model, p < 0.001, R2= 0.046 Variables also included were no. of pills per day and no. of outpatient visits. Specific problems included in this category were unsafe, allergic reaction, undesired effect, interaction, dosage changed too fast, dose too high, frequency too short, duration too long, drug Interaction resulting in dose too high or low, and needs additional monitoring
Discussion
This work expands current knowledge of MRP predictors, particularly within the context of MTM services. Although Medicare Part D MTM is provided by pharmacists nationally, it is increasingly common for Medicaid programs to provide these services as a covered benefit, and these findings are especially relevant for those stakeholders.28 We identified significant predictors of MRPs in a Medicaid population receiving MTM, both for MRPs in general and for the categories of MRPs related to indication, effectiveness, and safety. Significant predictors of adherence-specific problems were not identified in this analysis. This may be due to the smaller overall prevalence of these problems in this sample compared to other problem types. This sample may have experienced fewer adherence problems than a typical sample receiving MTM, as the program also included special packaging to promote medication adherence. More research to identify predictors of adherence-related problems identified by pharmacists in the course of MTM, particularly among Medicaid patients, is needed.
Furthermore, more research concerning predictors of any MRPs identified in patients receiving MTM is needed. As the outcomes of MTM program evaluations have varied,6–17 one way in which outcomes may be improved is through better targeting of patients who would be likely to benefit from these services. The provision of high-quality MTM is likely to be time consuming for pharmacists; thus, mechanisms that enable the careful selection of patients who should receive MTM may promote more efficient resource allocation and improved service outcomes.
Although we did identify predictors in statistically significant regression models, our models explained very little of the overall variability (as evidenced by a small R2 values for each model) in MRPs experienced by patients receiving the service. This is concerning, as our models included variables commonly used in determining eligibility for MTM. However, patient medication cost is another variable that is commonly used for determining eligibility, but our models did not evaluate this variable. Clearly, additional variables should be considered as playing an important role in MRP variability. Variables to consider include those measuring social support (other than marital status), transportation barriers other than location of residence based on ZIP code, and patient health literacy, medication beliefs, and knowledge and satisfaction pertaining to their medications.
This work suggests an opportunity for enhancing eligibility criteria in order to optimize the outcomes of pharmacist-provided MTM. A patient history of one or more visits to the emergency department in the past three months was a predictor of overall MRPs. A visit to the emergency department may result in the addition of new medications or changes in medications, prompting a need for a comprehensive medication review to resolve duplication in therapies, drug-drug interactions, or other problems. Using this as a criterion for MTM eligibility could be considered by insurers managing both prescription and medical services.
Considering the presence of specific comorbidities also appears to add value. Dyslipidemia was consistently identified in our models as a predictor. One potential explanation could be that statin medications are commonly used for dyslipidemia and are commonly prescribed overall.40 Although generally well tolerated, adverse effects do occur, as does prescribing inertia.41–44 Because of these challenges with statin therapy, pharmacists may have found opportunities for dose optimization and adverse-effect resolution, resulting in associations between dyslipidemia and specific problem types (i.e., indication, effectiveness, and safety) as well as overall MRPs. Obesity was also a significant predictor of problems overall. In further examining obesity in this sample, we found that obese patients had more comorbidities than nonobese patients, and obesity was associated with diabetes mellitus, hypertension, heart failure, and other comorbidities. Therefore, although the presence of some of these comorbidities alone did not predict problems, obese patients may represent more complex patients overall, resulting in a greater risk for MRPs. Interestingly, we did not find an association between obesity and dyslipidemia even though both were predictors. Therefore, targeting patients with obesity and/or dyslipidemia for MTM could be a useful strategy for optimizing MTM outcomes.
Previous studies of MRP predictors in Medicaid patients have identified the number of medications as an important predictor. Specifically, Alkema et al. found that number of medications was a significant predictor of MRPs in an older, dually eligible, Medicaid sample (odds ratio [OR] 1.183, 95% confidence interval [CI] 1.13–1.24), although MRPs were defined by using a different classification system31. Similarly, Bain et al. found the number of medications to be an important MRP predictor (OR 4.17, 95% CI 2.48–7.00) in an older, frail Medicaid sample.29 Finally, McGhan et al. identified the number of medications as an MRP predictor in Medicaid patients in the 1970s.30 Specifically, for every additional medication that a patient was taking, there was a 0.25-increase in the “need for pharmacist to monitor patient’s drug therapy” as measured on a scale from 1 to 5. In our sample, the number of medications was also a predictor of MRPs overall, and this reflects current Medicare Part D MTM eligibility criteria; however, some of the other predictors identified may warrant greater attention. Specifically, although our model identified number of medications as a significant predictor, the number of medications did not distinguish between patients without MRPs and patients having at least one MRP. Rather, the relationship between number of medications and MRPs appears to be linear, with more medications resulting in more MRPs identified. This finding supports previous literature.45
Other predictors that we identified differ somewhat from those identified previously in Medicaid patients. The Alkema,31 Bain,29 and McGhan30 studies all found age to be a predictor of MRPs; however, the associations were fairly modest (e.g., OR 1.029, 95% CI 1.01–1.05 in the Alkema study31), which may partially explain why age was not identified as a predictor in our models. In contrast, female sex was identified in the McGhan study to be a predictor30 and was also a predictive variable in our analyses.
Nevertheless, our analyses resulted in some unexpected findings. Specifically, our bivariate tests found that depression appears to be associated with fewer MRPs. Although the reason for this is unknown, one possibility is that pharmacists were not able to detect as many MRPs in these patients. Literature suggests that pharmacists are less confident caring for patients with mental health diagnoses, including depression, and that additional training for pharmacists is needed to improve the pharmaceutical care of these patients.46–48
There are several limitations to our work. First, several taxonomies for defining MRPs exist,46–47 and the results of our analysis may have been influenced by the specific taxonomy used by the pharmacists providing the MTM service. The number of MRPs reported by pharmacists in this MTM program was higher than that reported previously among Medicaid patients29,31 and may be due to differences in how MRPs were identified and documented. The pharmacists received one day of training along with supporting manuals on the MTM documentation system and clinical thought processes for MRP identification, using materials developed by the company providing the commercially available MTM documentation platform. The MRP documentation process and MTM approach used by the company is well described.38 However, the extent to which the pharmacists documented problem categories similarly or differently is unknown, particularly given that multiple pharmacists provided the MTM service. In addition, given that our analyses were based on data from a retrospective cohort study that evaluated the MTM program, the extent to which the MTM program conducted quality assurance checks to determine the quality of MRP documentation and authenticity of identified MRPs is unknown. Therefore, the influence of inadequate or inappropriate MRP documentation on our outcomes is unknown. Additionally, our data source included only Medicaid claims for medications; therefore, medications that patients may have received from other sources were not included in our calculations. Furthermore, the definition of our outcome measure (i.e., MRPs as documented by the pharmacist) may have impacted our findings. Our findings could have been different had the service been provided by another provider type (e.g., a nurse) or if the outcome would have been defined differently (e.g., adverse drug event requiring hospitalization with an accompanying diagnosis code.) These findings could change according to the modality used for delivery of the MTM intervention. As pharmacists in this MTM program provided the service and identified MRPs by telephone, different or additional MRPs may have been detected had the service been provided face to face. An evaluation of a different telephonic MTM program found that the program was effective for a subset of, but not all, patients and may be related to the modality used to deliver MTM.51 Other research has found that MTM services provided by community pharmacists (whether by telephone or face to face) resulted in a greater reduction in drug use and associated drug costs compared to MTM provided by call center pharmacists52; however, the role that the telephone versus a preexisting relationship between the pharmacist and patient may play in MRP detection is unclear. It is also important to note the limitations of our sample. As we examined data from individuals participating in an MTM program, we are limited by the eligibility criteria initially used for the service. In this case, the program was available for patients enrolled in the state Medicaid care management program who were taking five or more medications and with one or more specific chronic diseases. Finally, as this was a Medicaid population, our ability to generalize these findings to all patients receiving MTM, particularly Medicare Part D, is limited.
Conclusion
This work supports the relative importance of the number of medications as a predictor of MRPs in the Medicaid population and identifies other MRP predictors such as the number of recent ED visits and comorbid obesity for providers and policymakers to consider when developing MTM eligibility criteria. However, these findings indicate that other unknown factors are clearly important in understanding patient risk for MRPs. Criteria commonly used for determining MTM eligibility may be inadequate in identifying appropriate patients for MTM in a Medicaid population. Future studies examining other types of predictor variables (e.g., transportation barriers, social support variables, patient health literacy, and medication beliefs) may fill a gap in the literature and further explain variability among MRPs.
Acknowledgment
The authors would like to thank Heather Hemmeger, Pharm.D., for her assistance in preparing this manuscript.
Dr. Snyder’s effort was supported in part by a Young Investigator award (KL2RR025760) from the Indiana Clinical and Translational Sciences Institute. Part of Dr. Snyder’s effort was supported by a grant (K08HS022119) from the Agency for Healthcare Research and Quality. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Zillich was supported by a Research Career Development Award (RCD 06-304-1) from Veterans Affairs Health Services Research & Development. This work was supported by a grant from the Indiana Medicaid Office of Policy and Planning.
Footnotes
Presented at the International Society for Pharmacoeconomics and Outcomes Research 15th Annual European Congress, Berlin, Germany, November 3–7, 2012.
Contributor Information
Margie E. Snyder, Department of Pharmacy Practice, Purdue University College of Pharmacy, Indianapolis, Indiana.
Caitlin K. Frail, Department of Pharmaceutical Care and Health Systems, University of Minnesota College of Pharmacy, Minneapolis, Minnesota.
Heather Jaynes, Department of Pharmacy Practice, Purdue University College of Pharmacy, Indianapolis, Indiana.
Karen S. Pater, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, Pittsburgh, Pennsylvania.
Alan J. Zillich, Department of Pharmacy Practice, Purdue University College of Pharmacy, Research Scientist, Center for Implementing Evidence-Based Practices, Roudebush VA Medical Center, Indianapolis, Indiana.
References
- 1.Centers for Medicare and Medicaid Services (CMS) HHS. Medicare program; medicare prescription drug benefit, Final Rule. Federal Register. 2005;70:4193–4585. [PubMed] [Google Scholar]
- 2.Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc. 2005;45:566–572. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- 3.American Pharmacists Association and National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model. version 2.0. J Am Pharm Assoc. 2008;48:341–353. doi: 10.1331/JAPhA.2008.08514. [DOI] [PubMed] [Google Scholar]
- 4.American Pharmacists Association. MTM Digest. [Accessed January 20, 2014];2013 Available at: http://www.pharmacist.com/sites/default/files/files/MTMDigest_2013.pdf. [Google Scholar]
- 5.Tudor CG. CY 2014 medication therapy management program guidance and submission instructions. [Accessed January 20, 2014]; Available at: http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Memo-Contract-Year-2014-Medication-Therapy-Management-MTM-Program-Submission-v040513.pdf.
- 6.Fox D, Ried LD, Klein GE, Myers W, Foli K. A medication therapy management program's impact on low-density lipoprotein cholesterol goal attainment in Medicare Part D patients with diabetes. J Am Pharm Assoc. 2009;49(2):192–199. doi: 10.1331/JAPhA.2009.09016. [DOI] [PubMed] [Google Scholar]
- 7.Pindolia VK, Stebelsky L, Romain TM, Luoma L, Nowak SN, Gillanders F. Mitigation of medication mishaps via medication therapy management. Ann Pharmacother. 2009;43(4):611–620. doi: 10.1345/aph.1L591. [DOI] [PubMed] [Google Scholar]
- 8.Moczygemba LR, Barner JC, Lawson KA, Brown CM, Gabrillo ER, Godley P, Johnsrud M. Impact of telephone medication therapy management on medication and health-related problems, medication adherence, and Medicare Part D drug costs: a 6-month follow up. Am J Geriatr Pharmacother. 2011;9(5):328–338. doi: 10.1016/j.amjopharm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ward MA, Xu Y. Pharmacist-provided telephonic medication therapy management in an MAPD plan. Am J Manag Care. 2011;17(10):e399–e409. [PubMed] [Google Scholar]
- 10.Welch EK, Delate T, Chester EA, Stubbings T. Assessment of the impact of medication therapy management delivered to home-based Medicare beneficiaries. Ann Pharmacother. 2009;43(4):603–610. doi: 10.1345/aph.1L524. [DOI] [PubMed] [Google Scholar]
- 11.Winston S, Lin YS. Impact on drug cost and use of Medicare part D of medication therapy management services delivered in 2007. J Am Pharm Assoc. 2009;49(6):813–820. doi: 10.1331/JAPhA.2009.09066. [DOI] [PubMed] [Google Scholar]
- 12.Zillich AJ, Jaynes HA, Snyder ME, Harrison J, de Moor C, French DD, Hudmon KS. Evaluation of specialized medication packaging combined with medication therapy management: adherence, outcomes, and costs among Medicaid Patients. Med Care. 2012;50:485–493. doi: 10.1097/MLR.0b013e3182549d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cranor CW, Bunting BA, Christensen DB. The Asheville Project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc. 2003;43:173–184. doi: 10.1331/108658003321480713. [DOI] [PubMed] [Google Scholar]
- 14.Fera T, Bluml BM, Ellis WM. Diabetes Ten City Challenge: final economic and clinical results . J Am Pharm Assoc. 2009;49:383–391. doi: 10.1331/JAPhA.2009.09015. [DOI] [PubMed] [Google Scholar]
- 15.Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc. 2008;48:203–211. doi: 10.1331/JAPhA.2008.07108. [DOI] [PubMed] [Google Scholar]
- 16.Planas LG, Crosby KM, Mitchell KD, Farmer KC. Evaluation of a hypertension medication therapy management program in patients with diabetes. J Am Pharm Assoc. 2009;49:164–170. doi: 10.1331/JAPhA.2009.08164. [DOI] [PubMed] [Google Scholar]
- 17.Smith M, Giuliano MR, Starkowski MP. In connecticut: improving patient medication management in primary care. Health Aff. 2011;30:646–654. doi: 10.1377/hlthaff.2011.0002. [DOI] [PubMed] [Google Scholar]
- 18.Koecheler JA, Abramowitz PW, Swim SE, Daniels CE. Indicators for the selection of ambulatory patients who warrant pharmacist monitoring. Am J Health-System Pharm. 1989;46:729–732. [PubMed] [Google Scholar]
- 19.George J, Phun Y, Bailey MJ, Kong DCM, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 20.Levy HB. Self-administered medication-risk questionnaire in an elderly population. Ann Pharmacother. 2003;37:982–987. doi: 10.1345/aph.1C305. [DOI] [PubMed] [Google Scholar]
- 21.Gordon KJ, Smith FJ, Dhillon S. The development and validation of a screening tool for the identification of patients experiencing medication-related problems. Int J Pharm Pract. 2005;13:187–193. [Google Scholar]
- 22.Langford BJ, Jorgenson D, Kwan D, Papoushek C. Implementation of a self-administered questionnaire to identify patients at risk for medication-related problems in a family health center. Pharmacother. 2006;26:260–268. doi: 10.1592/phco.26.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Pit S, Byles JE, Cockburn J. Prevalence of self-reported risk factors for medication misadventure among older people in general practice. Journal of Evaluation in Clinical Practice. 2008;14:203–208. doi: 10.1111/j.1365-2753.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 24.Raehl CL, Bond CA, Woods T, Patry RA, Sleeper RB. Individualized drug use assessment in the elderly. Pharmacother. 2002;22:1239–1248. doi: 10.1592/phco.22.15.1239.33473. [DOI] [PubMed] [Google Scholar]
- 25.Kassam R, Martin LG, Farris KB. Reliability of a modified medication appropriateness index in community pharmacies. Ann Pharmacother. 2003;37:40–46. doi: 10.1345/aph.1c077. [DOI] [PubMed] [Google Scholar]
- 26.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- 27.Rovers J, Hagel H. Self-assessment tool for screening patients at risk for drug therapy problems. J Am Pharm Assoc. 2012;52:646–652. doi: 10.1331/JAPhA.2012.11120. [DOI] [PubMed] [Google Scholar]
- 28.National Conference of State Legislatures. Medication therapy management: pharmaceutical safety and savings. [Accessed January 20, 2014]; Available at: http://www.ncsl.org/research/health/medication-therapy-management.aspx. [Google Scholar]
- 29.Bain KT, Weschules DJ, Tillotson P. Prevalence and predictors of medication-related problems. Medicare Patient Management. 2006;1:14–26. [Google Scholar]
- 30.McGhan WF, Wertheimer AI, Rowland CR. Using Medicaid data to identify patients with drug therapy problems. Inquiry. 1982;19:79–88. [PubMed] [Google Scholar]
- 31.Alkema GE, Wilber KH, Simmons WJ, Enguidanos SM, Frey D. Prevalence of potential medication problems among dually eligible older adults in Medicaid waiver services. Ann Pharmacother. 2007;41:1971–1978. doi: 10.1345/aph.1K270. [DOI] [PubMed] [Google Scholar]
- 32.Stuart B, Loh E, Miller L, et al. Should eligibility for medication therapy management be based on drug adherence? JMCP. 2014;20:66–75. doi: 10.18553/jmcp.2014.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rucker NL. AARP Public Policy Institute; 2012. Jun, [Accessed February 28, 2013]. Medicare Part D’s medication therapy management: shifting from neutral to drive. Insight on the Issues. Available at: http://www.aarp.org/content/dam/aarp/research/pub-lic_policy_institute/health/medicare-part-d-shifting-from-neutral-to-drive-insight-AARP-ppi-health.pdf. [Google Scholar]
- 34.Wang J, Qiao Y, Shih YCT, et al. Potential health implications of racial and ethnic disparities in meeting MTM eligibility criteria. Res Soc Adm Pharm. 2014;10:106–125. doi: 10.1016/j.sapharm.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Mullins CD, Brown LM, et al. Disparity implications of medicare eligibility criteria for medication therapy management services. Health Serv Res. 2010;45:1061–1082. doi: 10.1111/j.1475-6773.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services (CMS) HHS. Medicare program; Contract year 2015 policy and technical changes to the medicare advantage and the medicare prescription drug benefit programs, Proposed Rule. Federal Register. 2014;79:1918–2073. [PubMed] [Google Scholar]
- 37.Zillich AJ, Jaynes HA, Snyder ME, Harrison J, de Moor C, French DD, Hudmon KS. Evaluation of specialized medication packaging combined with medication therapy management: adherence, outcomes, and costs among Medicaid patients. Med Care. 2012;50:485–493. doi: 10.1097/MLR.0b013e3182549d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice: The Clinician’s Guide. 2nd ed. New York: McGraw-Hill; 2004. [Google Scholar]
- 39.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Top 100 Selling Drugs of 2013. Medscape. 2014 Jan 30; [Google Scholar]
- 41.Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol. 2003;92:79–81. doi: 10.1016/s0002-9149(03)00474-0. [DOI] [PubMed] [Google Scholar]
- 42.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why Don’t Diabetes Patients Achieve Recommended Risk Factor Targets? Poor Adherence versus Lack of Treatment Intensification. J Gen Intern Med. 2008;23:588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giugliano D, Esposito K. Clinical Inertia as a Clinical Safeguard. JAMA. 2011;305:1591–1592. doi: 10.1001/jama.2011.490. [DOI] [PubMed] [Google Scholar]
- 44.Silva MA, Swanson AC, Gandhi PJ, et al. Statin-related adverse events: A meta-analysis. Clin Ther. 2006;28:26–35. doi: 10.1016/j.clinthera.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2006;63:187–195. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phokeo V, Sproule B, Raman-Wilms L. Community pharmacists’ attitudes toward and professional interactions with users of psychiatric medication. Psychiatric Services. 2004;55:1434–1436. doi: 10.1176/appi.ps.55.12.1434. [DOI] [PubMed] [Google Scholar]
- 47.Rickles NM, Dube GL, McCarter A, Olshan JS. Relationship between attitudes toward mental illness and provision of pharmacy services. J Am Pharm Assoc. 2010;50:704–713. doi: 10.1331/JAPhA.2010.09042. [DOI] [PubMed] [Google Scholar]
- 48.Scheerder G, De Coster I, Van Audenhove C. Pharmacists’ role in depression care: a survey of attitudes, current practices, and barriers. Psychiatric Services. 2008;59:1155–1161. doi: 10.1176/ps.2008.59.10.1155. [DOI] [PubMed] [Google Scholar]
- 49.van Mil JW, Westerlund T, Hersberger KE, Schaefer MA. Drug-Related problem classification systems. Ann Pharmacother. 2004;38:859–867. doi: 10.1345/aph.1D182. [DOI] [PubMed] [Google Scholar]
- 50.AbuRuz SM, Bulatova NR, Yousef AM. Validation of a comprehensive classification tool for treatment-related problems. Pharm World and Sci. 2006;28:222–232. doi: 10.1007/s11096-006-9048-0. [DOI] [PubMed] [Google Scholar]
- 51.Zillich AJ, Snyder ME, Frail CK, Lewis JL, Deshotels D, Grove S, Dunham P, Jaynes H, Sutherland JM. A randomized controlled pragmatic trial of telephonic medication therapy management to reduce hospitalization in home health patients. Health Services Research. 2014 doi: 10.1111/1475-6773.12176. Published online ahead of print on April 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winston S, Lin YS. Impact of drug cost and use of Medicare Part D of medication therapy management services delivered in 2007. J Am Pharm Assoc. 2009;49:813–820. doi: 10.1331/JAPhA.2009.09066. [DOI] [PubMed] [Google Scholar]