Abstract
Microtubules are essential components of the cytoskeleton and are involved in many aspects of cell responses including cell division, migration, and intracellular signal transduction. Among other factors, post-translational modifications play a significant role in the regulation of microtubule dynamics. Here, we demonstrate that the ubiquitin-editing enzyme UCH L1, abundant expression of which is normally restricted to brain tissue, is also a part of the microtubule network in a variety of transformed cells. Moreover, during mitosis, endogenous UCH L1 is expressed and tightly associated with the mitotic spindle through all stages of M phase, suggesting that UCH L1 is involved in regulation of microtubule dynamics. Indeed, addition of recombinant UCH L1 to the reaction of tubulin polymerization in vitro had an inhibitory effect on microtubule formation. Unexpectedly, western blot analysis of tubulin fractions after polymerization revealed the presence of a specific ∼50 kDa band of UCH L1 (not the normal ∼25 kDa) in association with microtubules, but not with free tubulin. In addition, we show that along with 25 kDa UCH L1, endogenous high molecular weight UCH L1 complexes exist in cells, and that levels of 50 kDa UCH L1 complexes are increasing in cells during mitosis. Finally, we provide evidence that ubiquitination is involved in tubulin polymerization: the presence of ubiquitin during polymerization in vitro by itself inhibited microtubule formation and enhanced the inhibitory effect of added UCH L1. the inhibitory effects of UCH L1 correlate with an increase in ubiquitination of microtubule components. Since besides being a deubiquitinating enzyme, UCH L1 as a dimer has also been shown to exhibit ubiquitin ligase activity, we discuss the possibility that the ∼50 kDa UCH L1 observed is a dimer which prevents microtubule formation through ubiquitination of tubulins and/or microtubule-associated proteins.
Keywords: mitosis, microtubules, ubiquitination, deubiquitination, dimer, tubulin, MAPs
Introduction
The array of microtubules in any cellular activity is a highly dynamic structure, and continuous polymerization and depolymerization of microtubules is regulated by many different processes, including post-transcriptional modification of microtubule components—the tubulin.1-4
Although the role of post-translational modifications of tubulin in microtubule formation and functions has been studied intensively,1,2,4 the impact of ubiquitination in these processes is still unclear. It has been shown that ubiquitination of γ-tubulin by BRCA1 ubiquitin ligase is required for normal centrosome function and tubulin nucleation.5-10 Mono- and multiubiquitination are involved in regulation of microtubule-associated proteins such as tau.11-13 The ubiquitin/proteasome system has been implicated in α/β-tubulin turnover,14 and ubiquitination of α/β-tubulin has been observed during diverse processes,15-17 but the role of regulatory ubiquitination in microtubule functions is still largely unexplored.
Ubiquitin C-terminal Hydrolases—UCHs—are relatively small proteins (25–75 kDa) with approximately 50% sequence homology among the members.18-20 Among them is Ubiquitin C-terminal Hydrolase L1 (UCH L1), a cysteine hydrolase that contains the typical active-site triad of cysteine, histidine, and aspartic acid and catalyzes hydrolysis of C-terminal esters and amides of ubiquitin.21 Normally UCH L1 is expressed abundantly and exclusively in brain and reproductive tissues.20 Mutations in the UCH L1 gene have been associated with Parkinson's and Alzheimer's diseases.22 Recent studies indicate that UCH L1 is a multi-functional protein of the ubiquitin system: besides being a deubiquitinating enzyme,23 UCH L1 dimer has a ubiquitin ligase activity in vitro,24 and stabilizes mono-ubiquitin in neurons.25,26
In addition, elevated levels of UCH L1 have been detected in many cancers of different origin,27-33 indicating potential involvement of this protein in oncogenesis. Recent studies demonstrate that inhibition of UCH L1 expression reduces tumorigenic phenotype of transformed cells,33-35 and oncogenic transcription factors such as B-Myb and β-catenin/TCF upregulate uch l1 gene expression.36,37 Still, the physiological roles of UCH L1 and regulation of its expression in normal and transformed cells need further analysis.38
UCH L1 is abundantly expressed in brain tissue, and abnormal microtubule dynamics and tubulin polymerization are associated with several neurodegenerative diseases.39,40 Recently, a connection between UCH L1 and microtubules has been suggested: UCH L1 was identified as a tubulin-interacting protein by mass spectrometric analysis, and UCH L1 I93M mutant (the mutation connected to Parkinson's disease) as well carbonyl-modified UCH L1 aberrantly promote tubulin polymerization.41
In this study we provide evidence showing that UCH L1 is involved in regulation of microtubule dynamics in vitro and in vivo in transformed cells. Moreover, the association of UCH L1 with mitotic spindle suggests a functional role during mitosis. We hypothezise that ubiquitination of tubulin or/and microtubule-associated proteins during polymerization is mediated by a UCH L1-based complex and inhibits microtubule formation.
Results
Endogenous UCH L1 is associated with microtubules in interphase and mitotic cells of different origin
In analyzing the sub-cellular localization of UCH L1, we found that while some portion of UCH L1 is present in nuclei, cytoplasmic UCH L1 is closely associated with microtubules in lymphoid cells. To determine whether this is a general phenomenon, we performed immunofluorescence co-staining of UCH L1 and β-tubulin in cells of different origin: fibroblasts, lymphoid and epithelial cells. As seen in Figure 1A, UCH L1 intensely stained the microtubule organizing center (MTOC) in interphase cells of different origins. It is interesting to note that localization of UCH L1 in epithelial cells (5th and 6th panels) is more nuclear as compared with fibroblasts (1st and 2nd panels) and lymphoid cells (3rd and 4th panels), where association of UCH L1 with microtubules is greater. These observations led us to infer that UCH L1 may bind to microtubules during mitosis as well. We performed co-immunofluorscence staining for UCH L1 and tubulin in GM00637F cells which are human fibroblasts transformed by SV40. As seen in Figure 1B, UCH L1 was associated with β-tubulin from early prophase until cytokinesis. During early prophase, UCH L1 begins to co-localize with centrioles and during later stages, UCH L1 is associated with the mitotic spindle, including poles and spindle microtubules. During cytokinesis, cytoplasmic microtubules reappear and UCH L1 is distributed along the astral microtubules and concentrated in the mid-body region. To confirm these data, immunofluorescence staining for UCH L1 in mitotic cells was performed with four different UCH L1 antibodies with similar results (data not shown). The association of UCH L1 with microtubules in mitotic spindles suggests that UCH L1 may play a role during mitosis and cytokinesis.
Figure 1.
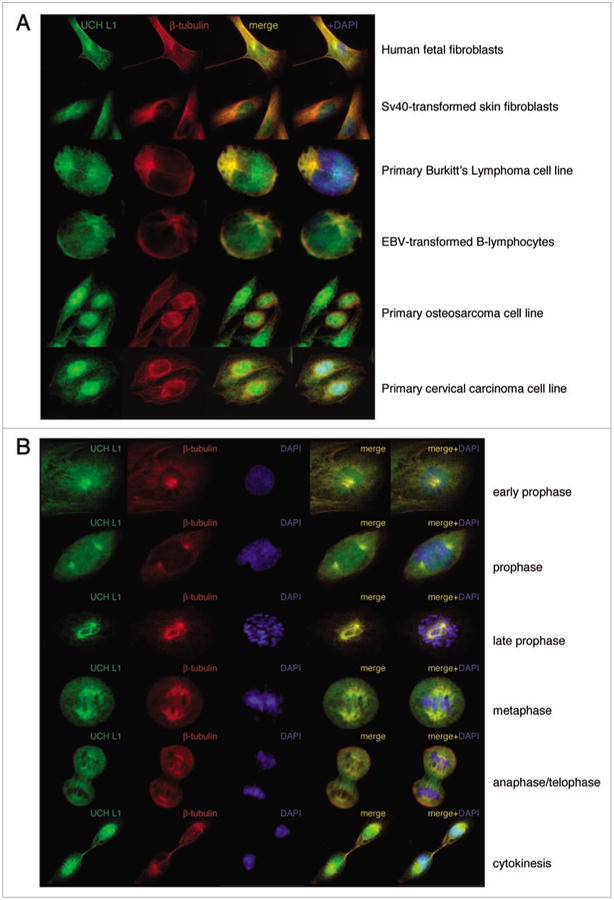
endogenous UCH L1 is associated with microtubules in interphase and mitotic cells. Cells as indicated were fixed in 4% pFA and double-immunostained with UCH L1 and β-tubulin antibodies and green and red fluorescent secondary antibodies. DApI staining was used to visualize nuclei. Images were analyzed with fuorescent light microscopy and openlab software (40× magnification for adherent and 100× for suspension cells).
We observed increased UCH L1 expression during mitosis in lymphoid (EBV-positive KR4), fibroblastic (NIH 3T3) and epithelial (U2OS) cells (Fig. 2A). To further validate these observations, we separated cells from G0/G1 and G2/M phases by Fluorescence-activated cell sorting (FACS) (lymphoid KR4 cells) and mitotic “shake-off” (epithelial U3OS cells) respectively (Fig. 2B). Western blot analysis and RT-PCR showed a significant increase in UCH L1 RNA and protein expression in G2/M-phase cells (Fig. 2B).
Figure 2.
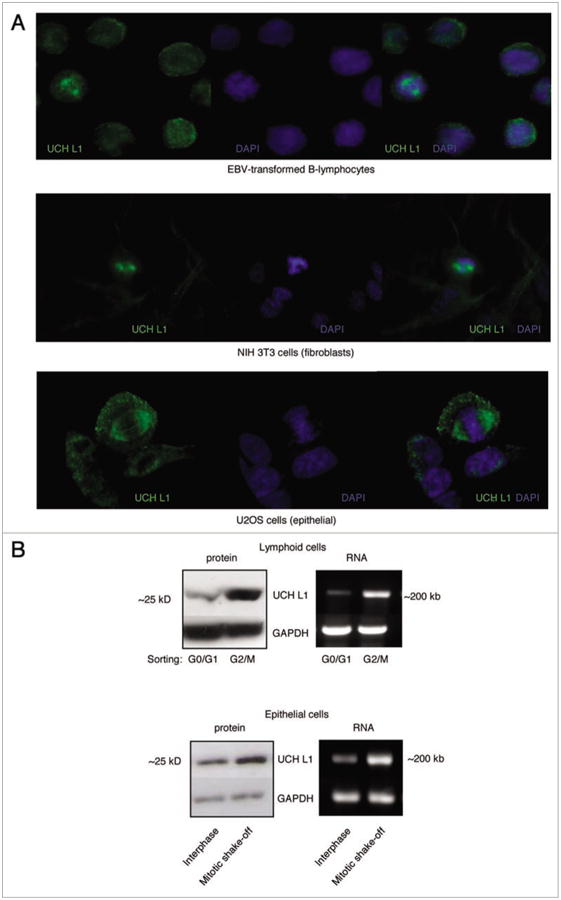
UCH L1 expression is increased in G2/M-phase of the cell cycle. (A) KR4, NIH 3t3 and U2oS cells were fixed in 4% pFA and stained with UCH L1 antibody (green) and DApI. 20× magnification. (B) G0/G1 and G2/M phases sorted from asynchronously growing KR4 cells by FACS. U2OS mitotic cells were collected by shaking of from the monolayer surface. RNA and protein were extracted and analyzed with pCR and western blot analysis for UCH L1 expression (see Materials and Methods).
UCH L1 decreases tubulin's ability to form microtubules in vitro and in vivo
Our finding that UCH L1 is associated with microtubules in interphase and mitotic cells raised the question of whether UCH L1 affects tubulin polymerization. To address this, we performed in vitro tubulin polymerization assay in the presence or absence of recombinant UCH L1 (Fig. 3). The ability of tubulin to polymerize at concentrations of 3 mg/ml (∼30 μM) for pure tubulin and 2 mg/ml (∼20 μM) for MAP-rich tubulin was monitored at OD340 for 30 mins at 37°C in a temperature controlled plate spectrophotometer. Addition of a substoichio-metric amount of exogenous UCH L1 (1 μg/reaction) to 99% pure tubulin did not alter its ability to form microtubules (Fig. 3A, top). However, addition of same amounts of UCH L1 to MAP-rich tubulin (70% tubulin and 30% MAPs) decreased the ability of MAP-rich tubulin to polymerize by approximately 40% (Fig. 3A and bottom). To confirm the absorbance data, western blot analysis was performed for α/β-tubulin on the polymerization assay samples by performing sedimentation assay. Assembled microtubules were separated into supernatants and pellets by centrifugation at 25,000 g for 30 mins at room temperature. The pellet was re-dissolved in PEM buffer containing GTP and glycerol, and the samples were run on PAGE under native (where we observed oligomeric forms of tubulin) (Fig. 3A) or denaturated (Fig. 3B) conditions. Less microtubules were formed in the presence of exogenous UCH L1 for both purified tubulin and MAP-rich tubulin as observed by western blot analysis under native and denatured conditions, with more pronounced differences between results with MAP-rich tubulin in accord with the absorbance data.
Figure 3.
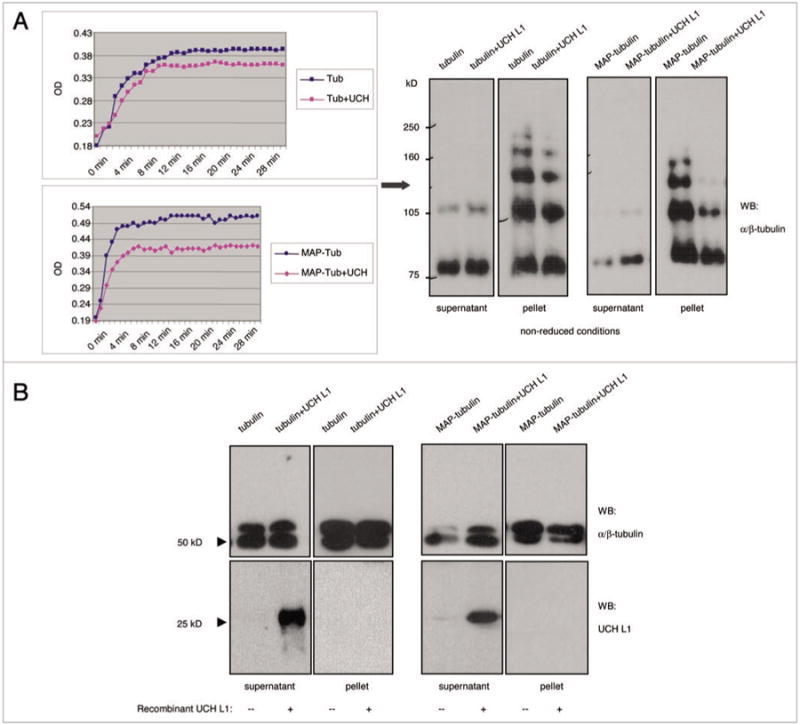
UCH L1 inhibits tubulin polymerization in vitro. (A) pure (99% tubulin) and MAp-rich (70% tubulin + 30% MAps) tubulin were polymerized at the concentration of 3 mg/ml and 2 mg/ml respectively along with all necessary components (see Materials and Methods for details) in presence or absence of 1 μg recombinant UCH L1 in a total volume of 100 μl. the ability of tubulin to polymerize was monitored at oD340 every minute up to 30 mins at 37°C in a temperature controlled plate spectrophotometer. (B) Assembled microtubules were separated into soluble and insoluble tubulin by centrifugation at 25,000 g for 30 mins at room temperature. Upon separation, pellet was re-dissolved in peM buffer containing Gtp and glycerol, and the samples were run on pAGe under native (A, right) or denaturing (B) conditions. After transfer to membrane, the blots were probed with α/β-tubulin antibody.
Since there was strong co-localization of endogenous UCH L1 with microtubules (Fig. 1), we also performed western blot for exogenous UCH L1 localization after co-sedimentation assay: surprisingly, the antibody detected UCH L1 with the expected molecular weight of 25 kDa only in the supernatant, but not in the pellet (Fig. 3B and bottom). According to this result, UCH L1 does not interact with microtubules, which contradicts the results of co-immunostaining of endogenous UCH L1 and tubulin (Figs. 1 and 2).
Nevertheless, to investigate whether UCH L1 effects microtubule formation in vivo, we overexpressed UCH L1 transiently in SV40-transformed fibroblastic cell line GM00637F (Fig. 4A). The reduced tubulin staining in cells transfected with UCH L1 indicates that the overexpression of UCH L1 caused reduction in microtubule assembly (we observed the effect in approximately 30% of transfected cells). Staining of transfected cells undergoing mitosis for tubulin shows less definite spindle formation compared to un-transfected cells; and overexpressed UCH L1 was localized at the spindle poles (Fig. 4A, right). Taken together these results indicate that overexpressed UCH L1 might, at least at some extent, negatively affect microtubule-associated cellular functions.
Figure 4.
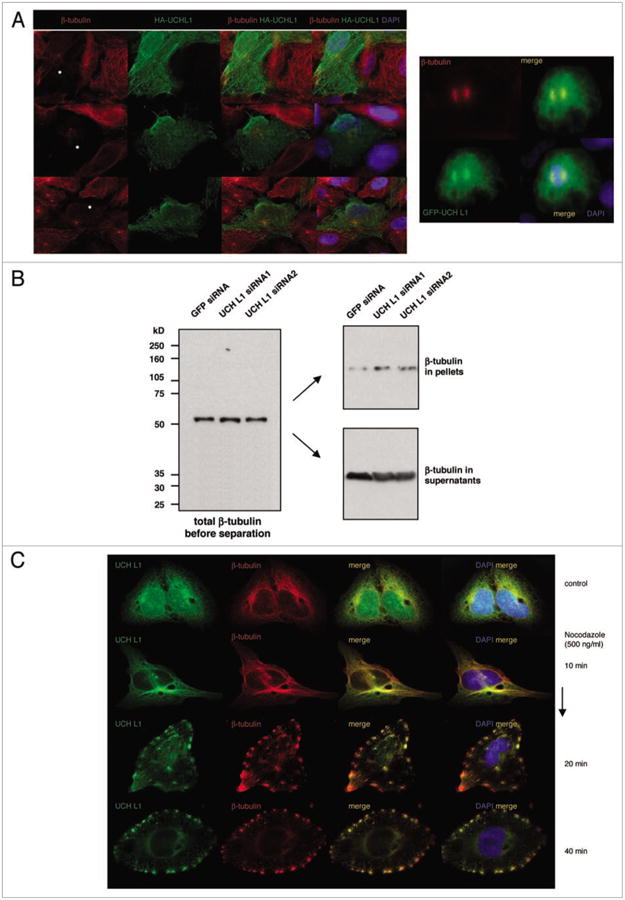
overexpression of UCH L1 inhibits microtubule formation in vivo. (A) GM00637F (SV40-transformed fibroblasts) cells were transfected with HA-UCH L1 (left) and GFp-UCH L1 (right) expression vectors. 48 hours later cells were fixed in 4% pFA and co-immunostained for HA-tag and β-tubulin (left) or β-tubulin alone (right) antibodies. DApI staining was used to visualize nuclei. (B) Microtubules from KR4 cells stably expressing control and UCH L1 siRNAs were separated by centrifugation at 25,000 g for 30 mins at room temperature. Fractions of supernatants and pellets were resolved in pAGe, and western blotting was performed with β-tubulin antibody. total tubulin in cell lysates before centrifugation served as a positive control. (C) GM00637F cells were treated with 500 ng/ml nocodazole. After indicated time cells were fixed and co-stained for UCH L1 and β-tubulin.
To investigate whether inhibition of UCH L1 expression interrupts microtubule assembly, we utilized previously described KR4 cell lines stably expressing control and UCH L1 siRNAs.34 We separated microtubules from soluble fraction of tubulin (monomers and dimers) by centrifugation and analyzed the levels of tubulin in each fraction western blotting with β-tubulin antibody (Fig. 4B). Since inhibition of UCH L1 expression slightly reduces TUBB gene expression,34 we normalized the blot for total amount of tubulin in each sample before sedimentation assay. The results in Figure 4B demonstrate an increase in tubulin levels in the pellets from UCH L1 siRNA-expressing cells, which correlates with a slight reduction of free tubulin in the supernatants. Although these data support the hypothesis that inhibition of UCH L1 expression reduces microtubule formation in the cells, it is important to remember that UCH L1 is a multifunctional molecule, and the observed effect might be the result of indirect dysruption of cellular processes on different levels.
Additionally, we analyzed whether endogenous UCH L1 is still associated with depolymerizing microtubules in the cells. We treated GM00637F cells with high doses of nocodazole or DMSO (control) for different time courses (Fig. 4C). The cells were fixed and co-immunostaining was performed for UCH L1 and β-tubulin. As seen in Figure 4C, the expression of UCH L1 in control is dispersed in both nucleus and cytoplasm. As soon as 10 min after nocodazole treatment, UCH L1 is detectable only in cytoplasm where it still partnered with microtubules. Towards the end of the treatment, punctate cytoplasmic UCH L1 staining was observed with depolymerized microtubules.
Again, this result demonstrates a tight association of UCH L1 with microtubules which contradicts the results of sedimentation assays after tubulin polymerization in vitro (Fig. 3B).
UCH L1 is involved in ubiquitination of tubulin in vitro: detection of higher molecular weight UCH L1 complexes associated with purified tubulin
Since UCH L1 is a deubiquitinating enzyme, the next step was to determine whether UCH L1 deubiquitinates tubulin. This was done using in vitro tubulin ubiquitination assay with Fraction II reticulocyte lysates as a source of ubiquitin-conjugating enzymes. The reactions were performed with 800 ng/reaction of HTS-tubulin (97% pure) in the presence or absence of recombinant UCH L1 in the presence of Fraction II (Fig. 5). The results of the western blot with ubiquitin antibody demonstrate that ubiquitinated substrate(s) in the tubulin fraction was slightly detectable without adding recombinant ubiquitin (Fig. 5, Panel I, lane 1), but ubiquitination was significantly increased in the presence of activated free ubiquitin (Fig. 5, Panel I, lane 2). Surprisingly, instead of inhibiting this ubiquitination, the addition of exogenous UCH L1 further enhanced it (Fig. 5, panel I, lane 3). Moreover, when we probed the same samples with UCH L1 antibody, besides the expected 25 kDa band, we also detected an additional band at molecular weight close to 50 kDa (Fig. 5, Panel III), but lower than β-tubulin bands (Fig. 5, Panel II). Moreover, we detected this band not only in the lane with recombinant UCH L1 (Panel III, lane 3), but also in the samples to which exogenous UCH L1 was not added (Panel III, Lanes 1 and 2).
Figure 5.
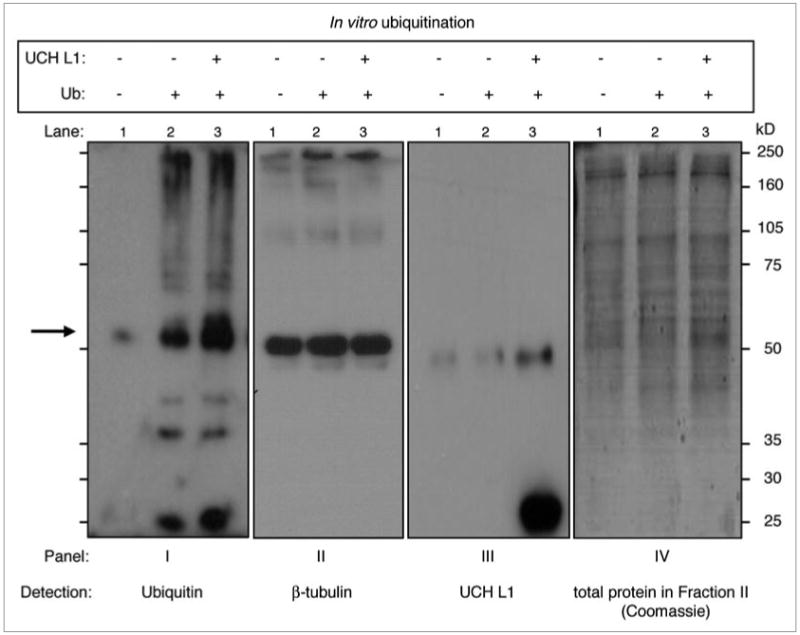
UCH L1 increases in vitro tubulin ubiquitination in the presence of reticulocyte fraction II. HtS-tubulin (97%) was incubated with Mg-Atp, rabbit reticulocyte lysates (fraction II) with or without ubiquitin at 37°C for 1 hour, after which the reaction containing ubiquitin was divided into equal parts with and without recombinant UCH L1. the reactions were incubated at 37°C for an additional 1 hour. At the end of the assay, samples were separated in 10% SDS-pAGe, and western blotting with ubiquitin, β-tubulin and UCH L1 antibodies was performed. the gel stained with Gel-Code blue Coomassie after transferring shows amount of total (Fraction II + tubulin fraction) protein used in the reactions.
Based on earlier findings that UCH L1 has ubiquitin ligase activity in its dimer form (∼50 kDa),24 and our observation that the 50 kDa band was more pronounced in the lane with recombinant UCH L1 (Fig. 5, Panel III), we decided to investigate whether this band is specific for UCH L1, and whether it belongs to reticulocyte or tubulin fractions.
To rule out the possibility of an artifact due to non-specific cross-reaction of UCH L1 antibody with proteins from fraction II, we ran two sets of the same samples from the in vitro polymerization assay described in Figure 3, along with recombinant UCH L1. After transferring to membrane, the two sets were separated: one set was incubated with UCH L1 antibody alone, the other one, with UCH L1 antibody in the presence of a blocking peptide (C-terminus peptide which was the antigen). The results of western blot in Figure 6A led to two important conclusions: (1) the 50 kDa band belongs to the tubulin fraction (since it is detectable in total lysates after tubulin polymerization in the presence of exogenous UCH L1), and (2) this band is specific for UCH L1 (since the presence of peptide completely blocked the detection of both 25 and 50 kDa bands).
Figure 6.
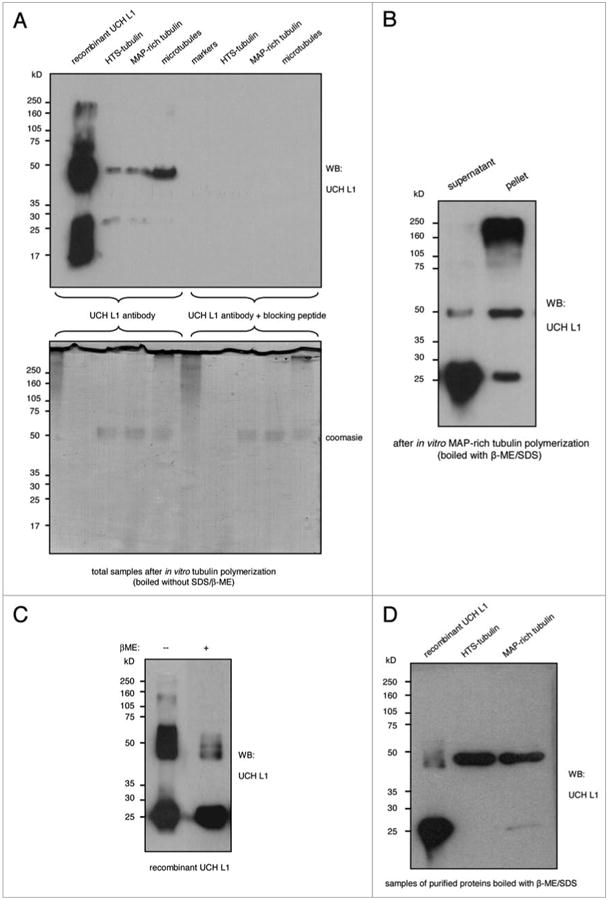
Detection of different UCH L1 complexes associated with purified tubulin fractions. (A) Samples of HtS- and MAp-rich tubulin after in vitro polymerization in the presence of recombinant UCH L1, and pre-made microtubules incubated with recombinant UCH L1 in polymerization buffer, were boiled without β-mercaptoethanol and run in duplicates on SDS-pAGe. the gel stained with Gelcode blue for loading control, and the membrane was cut into two parts: one was incubated with UCH L1 antibody, and the other with UCH L1 antibody in the presence of blocking peptide. (B) Sample from HtS-tubulin polymerization assay in the presence of recombinant UCH L1 was subjected to sedimentation assay. pellet and supernatant fractions were boiled in β-mercaptoethanol/SDS bufer. After SDS-pAGe, the blot was probed with UCH L1 antibody. (C) Sample of recombinant UCH L1 was divided in two parts and boiled in loading bufer with or without β-mercaptoethanol. 50 μg of the protein fractions were run on SDS-pAGe and probed with UCH L1 antibody. (D) Samples of recombinant UCH L1 (from Biomol), HtS- and MAp-tubulin (from Cytoskelton) were boiled in loading bufer with β-Me and SDS, run in SDS-pAGe and probed with UCH L1 antibody.
We repeated the reaction of tubulin polymerization in vitro in the presence of recombinant UCH L1, followed by the sedimentation assay (Fig. 6B). Analysis of the samples under reducing conditions, done by boiling them with β-ME and SDS and western blotting for UCH L1. Figure 6B reveals a 25 kDa UCH L1 band in both soluble and insoluble fractions of tubulin. However the intensity of the 50 kDa UCH L1 was greater in the insoluble tubulin fraction even under reducing conditions, providing further assurance that 50 kDa UCH L1 interacts with microtubules. Since a large amount of proteins was loaded on the gel (about 50 μg), we also saw much higher molecular weight bands of UCH L1 in the assembled microtubules, which lead us to ask whether recombinant UCH L1 alone was capable of forming these complexes or if UCH L1 was forming these complexes only in presence of microtubules. To answer this, we compared recombinant UCH L1 under non-reducing and reducing conditions. Western blot for UCH L1 showed that under non-reducing conditions, the percentage of 25 kDa band versus 50 kDa UCH L1 band was about equal, whereas under reducing conditions, the intensity of 50 kDa UCH L1 band decreased by almost 80% and the 25 kDa band increased (Fig. 6C). These results indicate that the 50 kDa UCH L1 band is a complex of UCH L1 joined by disulphide bonds. Additionally, we also detected the 50 kDa UCH L1 band in commercially purified tubulin fractions (purified and MAP-rich) (Fig. 6D) indicating that this form of UCH L1 has a high binding affinity for tubulin. We also detected 50 kDa UCH L1 formation during tubulin polymerization in vitro using different antibody (Invitrogen, Cat.# 480012).
Detection of UCH L1 50 kDa form in vivo: possible association with mitosis
To investigate whether UCH L1 forms 50 kDa complexes in vivo as well, we performed co-sedimentation assay in the presence or absence of paxitaxel with cell lysates from lymphoid (KR4) and epithelial (293) cells (both cell lines express relatively high levels of UCH L1). Upon separation into supernatants and pellets, the samples were analyzed by western blotting for UCH L1 and β-tubulin. As seen from Figure 7A, the 50 kDa UCH L1 band was detected in the assembled microtubules, whereas 25 kDa UCH L1 was detected in the supernatants, indicating the association of 50 kDa UCH L1 with microtubules. To determine whether overexpressed UCH L1 forms complexes similar to the endogenous protein we transiently expressed HA-tagged UCH L1 in 293 cells, probed the total lysates with HA-tag antibody and observed higher molecular weight complexes of overexpressed HA-UCH L1 including ∼50 kDa band (Fig. 7B).
Figure 7.
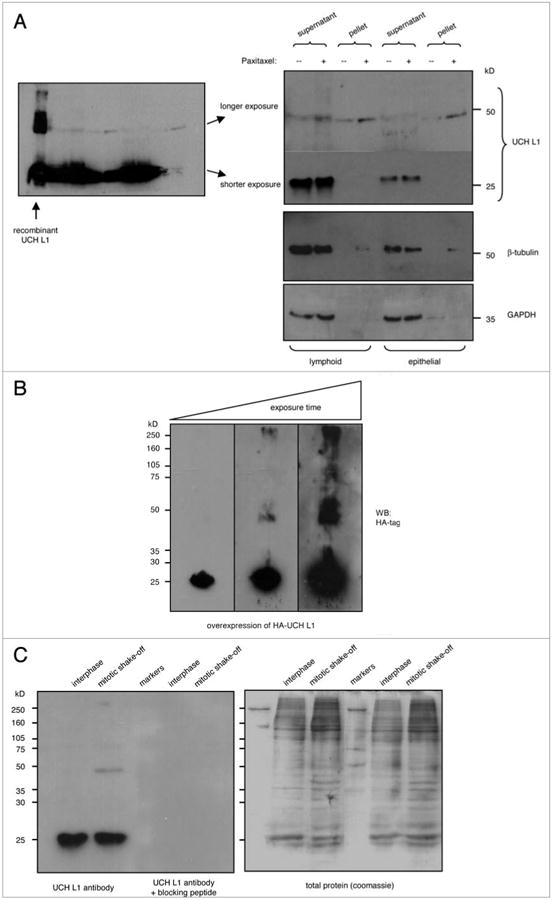
Detection of high molecular weight UCH L1 complexes in vivo. (A) total cell lysates from KR4 and 293 cells were incubated in presence or absence of taxol (4 mg/ml) in microtubule stabilization bufer (0.1 M pIpeS, 1 mM eGtA, 1 mM MgSo4, 2 M glycerol, pH 8.0, 1× protease inhibitor cocktail) for 30 mins on ice. the reaction was then separated into supernatant (soluble) and pellet (insoluble) tubulin fractions by centrifugation at 25,000 g for 30 mins at 4°C. the microtubule pellet was resuspended in MtS bufer. Samples were analyzed by SDS-pAGe followed by western blot with UCH L1 antibody. (B) 293 cells were transfected with HA-UCH L1, and after 48 hours total lysates were analyzed with SDS-pAGe, followed by western blotting for UCH L1. (C) Mitotic cells were collected by shaking-of from 293 cell monolayers. total lysates from interphase and mitotic cells were separated on SDS-pAGe in duplicate, and after transfer the membrane was cut in two parts: one was probed with UCH L1 antibody, and the other with UCH L1 antibody in the presence of blocking peptide.
Finally, to determine if the 50 kDa band of UCH L1 can be detected in total cell lysates, we performed western blot analysis of 293 cells comparing interphase and mitotic shake-off cell lysates. As demonstrated in Figure 7C, the specific 50 kDa UCH L1 band was detected in mitotic shake-off samples, indicating that endogenous UCH L1 50 kDa can be detected in vivo, and that formation of this band might be associated with G2/M phase of the cycle. For additional validation of the specificity, we analyzed the blot with the blocking peptide as described in Figure 6A.
UCH L1 enhances ubiquitination that occurs during in vitro tubulin polymerization
Since UCH L1 is an enzyme of the ubiquitin system,20 we suggested that UCH L1-dependent reduction of microtubule formation is mediated by ubiquitination. To test this hypothesis, we performed a tubulin polymerization assay in the presence or absence of free ubiquitin, recombinant UCH L1, or the combination of both (Fig. 8). The results monitored by OD demonstrate that the ability of 97% pure tubulin to polymerize was slightly reduced in the presence of UCH L1 or ubiquitin alone, and that combination of both enhanced this inhibitory effect (Fig. 8A, top). The OD results were confirmed by sedimentation assay followed by western blot for β-tubulin under reducing conditions (Fig. 8A and bottom). Additionally, we separated the total samples (without centrifugation) after tubulin polymerization assays in SDS-PAGE and probed with indicated antibodies (Fig. 8B). Western blot with ubiquitin antibody (Fig. 8B) clearly shows a certain level of ubiquitination of the component(s) of tubulin fraction in the presence of ubiquitin alone (Fig. 8B, lane 2), and further increase in the presence of exogenous UCH L1 (Fig. 8B, lane 4). The results of this experiment indicate that: (a) ubiquitination occurs during tubulin polymerization, and that ubiquitination has an inhibitory effect on microtubule formation (at least under these conditions); (b) 50 kDa UCH L1 in purified tubulin fraction is involved in this process, since the addition of recombinant UCH L1 (probably its complexes) increased ubiquitination, observed with ubiquitin alone. A possible explanation as to why the addition of UCH L1 alone had very little effect on tubulin polymerization and none on ubiquitination might be the lack of ubiquitin in the 97% pure tubulin fraction.
Figure 8.
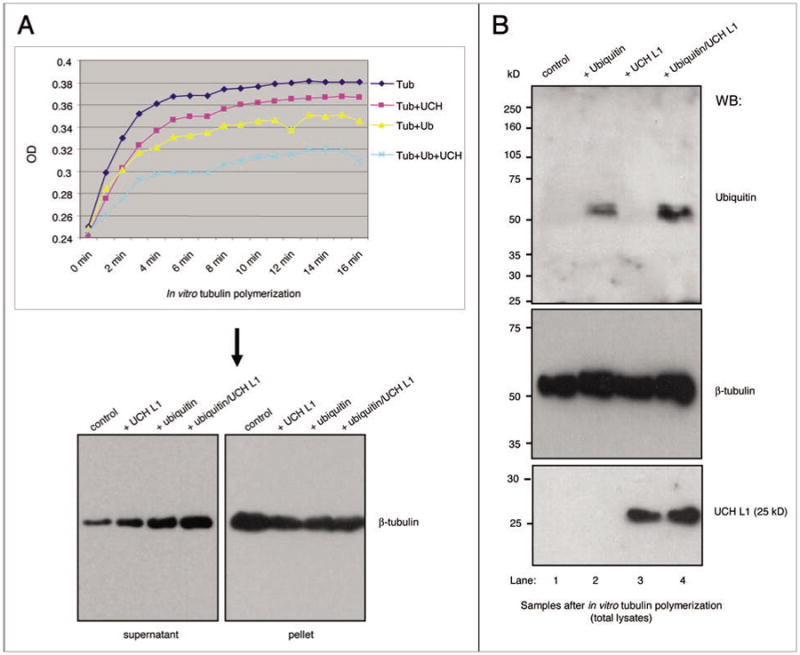
Recombinant UCH L1 increases ubiquitin-dependent inhibition of microtubule formation in vitro. (A) HtS-tubulin was allowed to assemble in polymerization buffer. the assembly of HtS-tubulin was measured every minute and monitored over the indicated period of time by light scattering at 340 nm with a temperature controlled spectrophotometer. Where indicated, the assay was performed in presence of 1 μg/reaction of recombinant UCH L1 and 500 ng/reaction of free ubiquitin (both from Biomol). Following the polymerization assay, the samples were centrifuged at 25,000 g for 30 mins at room temperature to pellet microtubules and all associated proteins. the samples were separated in SDS-pAGe and probed with β-tubulin antibody. (B) total samples after HtS-tubulin in vitro polymerization assay as in (A) (but without sedimentation) were separated in SDS-PAGE, transferred to membrane and probed with indicated antibodies.
Discussion
The results of our experiments taken together lead to three major conclusions:
UCH L1 is a part of microtubule network in different transformed cell lines;
UCH L1 is tightly associated with mitotic spindle through all stages of G2/M phase of the cell cycle;
UCH L1 complexes are directly or indirectly involved in negative regulation of microtubule dynamics through ubiquitination of microtubule components.
The association of UCH L1 with microtubules is more prominent in lymphoid and fibroblastic than in epithelial cells (Fig. 1A), and the level of endogenous UCH L1 in some cells is extremely low (for example in 3T3 and HeLa cells). Nevertheless, from the moment of centriole duplication, and through all stages of M phase, UCH L1 is always present and co-localized with microtubules in the mitotic spindle (Figs. 1B and 2). Moreover, we observed de novo expression of UCH L1 and co-localization with the mitotic spindle of mitogen-stimulated normal human lymphocytes and fibroblasts cells as well (Shackelford J, unpublished data). Since in the recent study on deubiquitinating enzymes association of several UCHs with mitotic spindle was reported,42 it is likely that members of the UCH family have a general role associated with microtubule function during the M phase of the cell cycle with the possibility to compensate each other functions.
It is important to mention the differences observed in microtubule network formation with endogenous and overexpressed UCH L1 (Fig. 4A and B). It seems that the levels of UCH L1 are critical for effects on microtubule organization (which in turn should affect intracellular functions as well). Overexpressed UCH L1 is localized in mitotic spindle in cells transfected with low concentrations of UCH L1 expression vector (Fig. 4A), the microtubule array in transfected cells was visually less “organized”. These observations might explain inhibitory effects of UCH L1 over-expression in some transformed cells.43,44 At the same time, we did not detect any significant differences in expression patterns or association with microtubules between wild type and deubiquitinase-dead point mutants of UCH L1, C90S and H161D,21 in both interphase and mitotic cells (unpublished observation). Nevertheless, it does not mean that the deubiquitinating activity of UCH L1 is irrelevant to its function during mitosis; additional studies are necessary to clarify this issue.
Although the inhibition of microtubule formation in the presence of UCH L1 (Fig. 3A and B) was somewhat surprising, the turning point of the study was the result of in vitro tubulin ubiquitination assay. The increased ubiquitination and the presence of 50 kDa UCH L1 (at first considered to be a non-specific band) (Fig. 5) in combination with a previous study on UCH L1's two opposite enzymatic activities,24 led us to the hypothesis that perhaps UCH L1 dimerization occurs in association with microtubules, and then the enzyme acts as a ubiquitin ligase.
The first question was whether this 50 kDa band is specific for UCH L1; western blot analysis in the presence of blocking peptide proves that both, in vitro (Fig. 6A) and in vivo (Fig. 7C) the observed 50 kDa bands contain at least the C-terminus of UCH L1 (the region the antibody was raised against).
We detected 50 kDa UCH L1 in all fractions of commercially purified brain tubulin (Fig. 6A and D). Studies on the association of Parkin ubiquitin ligase with microtubules demonstrate strong binding between tubulin and Parkin: high concentrations of NaCl or urea could not remove Parkin from microtubules.14,45 It seems that the association between 50 kDa UCH L1 and tubulin might be a similar case. There is another interesting detail about the 50 kDa UCH L1: in different fractions of purified tubulin (less or more MAPs) we detect approximately the same amount of 50 kDa UCH L1 (Fig. 6D). The amount would be different if the 50 kDa UCH L1 was bonded only to MAPs. At the same time, the crude fraction of tubulin contains large amounts of UCH L1 with molecular weight 25 kDa. We suggest that each cycle of tubulin polymerization (but not depolymerization) induces the formation of 50 kDa UCH L1 as well as its association with tubulin (Bheda A, manuscript in preparation). Considering that commercially available tubulin goes through several cycles of polymerization during purification, it might explain the equal amounts of 50 kDa UCH L1 detected in each fraction. This hypothesis still does not eliminate MAPs as participants in the process. It is also worth mentioning that the 50 kDa is not the only UCH L1-containing complex: UCH L1 exogenously added to the reaction of polymerization, shows not only ∼50 kDa, but also much higher molecular weight complexes associated only with microtubules (Fig. 6B).
In any case, the question of what this 50 kDa UCH L1 band consists remains unanswered. The fact that recombinant UCH L1 forms a dimer (Fig. 6A and C) is not a proof that the tubulin-associated 50 kDa band is a UCH L1 homodimer as well. Dimerization of recombinant proteins (and even a covalent dimerization) might occur during purification. The treatment with β-mercaptoethanol visibly affected only recombinant UCH L1 dimer (Fig. 6D), indicating that binding in the tubulin-associated band might be different from (or not only) a disulfide bond. We plan to study whether mutations in UCH L1 cysteines will affect its dimerization. In the meantime, mass spectrometric analysis of the 50 kDa band in tubulin fractions seems the obvious choice to identify the potential partner of UCH L1.
The detection of 50 kDa UCH L1 in vivo (Fig. 7) is important evidence of its biological significance, and the accumulation of 50 kDa UCH L1 in insoluble cellular protein fractions in the presence of paxitaxel provides a link between microtubules and dimer formation (Fig. 7A), which might explain the presence of the 50 k Da band of UCH L1 in mitotic cells (Fig. 7C). Additionally, overexpressed UCH L1 forms higher molecular weight bands as well (Fig. 7B). Interestingly, mutations in C90 and H161 that abolish UCH L1 deubiquitinating activity21 did not affect the ability of the overexpressed protein to form higher molecular weight complexes (data not shown). We plan to study whether these mutants are still able to affect microtubule formation. Also, another UCH L1 mutant—D30A(K)—which lacks not only deubiquitinating activity, but also the ability to bind ubiquitin,25 will be helpful in analyzing UCH L1 functions in the context of microtubule dynamics.
So far, ubiquitination has been implicated in μ/β-tubulin life mostly as a mark for proteasomal degradation,14 although ubiquitination of γ-tubulin has been shown to be important for the regulation of tubulin nucleation and centrosome function during mitosis.6,7,9,10,46 For the first time to our knowledge, we demonstrate that the process of ubiquitination occurs during the reaction of tubulin polymerization which correlates with decreased microtubule formation (Fig. 8). Incorporation of ubiquitin into a substrate in the presence of free ubiquitin alone (Fig. 8B, lane 2) indicates that purified fractions of tubulin already have components to complete the reaction of ubiquitination. The tubulin fraction we used is purified from brain tissue and contains 97% α/β-tubulin and 3% MAPs. We suggest that among those MAPs are ubiquitin conjugases/ligases (such as UCH L1 50 kDa, Parkin and probably, others), which are involved in regulation of microtubule assembly through ubiquitination.
At the same time, our results raise many questions. Are α/β-tubulin direct targets of the ubiquitination we detected? We did not observe differences (such as a shift) in β-tubulin bands in reaction of ubiquitination (Fig. 4), but it might be explained by a small portion of ubiquitinated tubulin and low affinity of the antibody to the modified form. It also might be γ-tubulin (which is present in this fraction). The involvement of UCH L1 in γ-tubulin ubiquitination could explain the association of endogenous UCH L1 with centrioles from the moment of their duplication (Fig. 1B). It could also explain the nature of UCH L1-dependent inhibition of microtubule formation in vitro through the disruption of the nucleation step. We also can not eliminate the possibility that tubulins are not targets for the ubiquitination we observed. Low molecular weight MAPs (such as tau47,48) might be substrates for UCH L1-mediated increase in ubiquitination during tubulin polymerization. What type of ubiquitination is it? We suggest that this is mono-ubiquitination of a relatively small portion of the substrate, but the fact that we did not detect additional bands does not mean they do not exist. In vitro ubiquitination assays with different ubiquitin mutants may provide clearer answers.
The addition of exogenous UCH L1 increased the ubiquitination during tubulin polymerization in vitro (Fig. 8). This result indicates that UCH L1 (presumably its dimer) is involved in the process, but does not prove that this is a direct effect. The ubiquitinating activity of UCH L1 dimer is ATP-independent,24 and the presence of GTP in the reaction of tubulin polymerization does not eliminate the possibility that other E2/E3 enzymes in tubulin fractions use GTP as a substitute. In vitro ubiquitination assays with recombinant α/β/γ-tubulin and UCH L1 in the absence of ATP will be necessary to support the hypothesis that it is UCH L1 dimer-dependent ubiquitination.
Nevertheless, we believe that our findings provide enough evidence to claim that UCH L1 is a tubulin-associated protein which is involved in regulation of microtubule dynamics through ubiquitination. All the recent information on UCH L1 suggests that this ubiquitin-editing enzyme is a multi-functional protein that is likely to contribute to more diverse cellular processes than were previously thought, and needs further serious investigation.
Materials and Methods
Cell culture
U20S osteosarcoma, NIH 3T3 mouse fibroblasts, human fetal fibroblasts, and 293 human embryonic kidney cell lines were cultured in DM EM (Sigma) supplemented with 10% FBS (Sigma) and penicillin-streptomycin. GM00637F, SV40-transformed cell lines were cultured in RPMI (Sigma) supplemented with 15% newborn serum (Sigma) and penicillin-streptomycin. The EBV-positive lymphoblastoid cell line KR4 and Burkitt's lymphoma cells were cultured in RPMI 1640 medium plus 10% heat-inactivated FBS and 100 units/ml penicillin-streptomycin. All cell lines were maintained at 37°C in 5% with CO2. For nocodazole experiments, a time course treatment (up to 40 mins) with 500 ng/ml nocodazole was performed on GM00637F cells in complete media. At the end of the treatment, cells were fixed and processed for immunofluorescence staining.
Transient transfections of UCH L1 expression vector
GM00637F and U20S cells were grown on poly-L-Lysine (Sigma)-treated coverslips in 6-well dishes until 40% confluent. The cells were transiently transfected with HA-UCH L1 expression vector or GFP-UCH L1 expression vector using Fugene HD (Roche) and 48 h post transfections, cells were processed for immunofluorescence staining.
Immunofluorescence studies
The cells were grown on poly-L-Lysine (Sigma)-coated coverslips in 6-well dishes until 75% confluent. The cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min, permeablized with 0.5% triton, washed three times with PBS and blocked with 5% normal donkey serum. Cells were then incubated with rabbit anti-UCH L1 antibody (Invitrogen, 1:50), mouse anti-P-tubulin antibody (Molecular probes, 1:50), rabbit anti-HA (Santa Cruz, 1:500) in 1:5 dilution of blocking solution for 1 h at 37°C and then washed three times with PBS. Next, cells were incubated with Alexafuor-594-conjugated goat anti-rabbit and Alexafuor-488-conjugated goat anti-mouse antibodies (Vector Laboratories, 1:500) for 1 h at 37°C, washed four times with PBS and mounted. Fluorescent images were created using Openlab software (Improvision Inc., MA, USA).
FACS analysis
To sort cells in different phases, asynchronously growing cells were labeled with 0.01 mM Hoechst 33342 (Sigma) at 37°C for 45 min and sorted with a DAKO Cytomation fluorescence-activated cell sorting (FACS) Mofo sorter. Cells within the G0/G1, or G2/M phases were collected in sterile PBS at 4°C.
RNA extraction and reverse transcriptase PCR
NIH 3T3 cells were treated with 100 ng/ml Nocodazole for 4 h. Mitotic U20S cells were collected after “shake off” over a period of 2 days. Total RNA from control and treated was extracted with the use of Agilent's Total RNA isolation mini kit (Agilent Technologies). 500 ng of total RNA were used to perform RT-PCR reactions with Qiagen's one step RT-PCR kit (Qiagen) as per manufacturer's instructions at an annealing temperature of 55°C. The samples were analyzed on 1% agarose gel. Primers used:
UCH L1: 5′-GGA TGG CCA CCT CTA TGA AC-3′, 5′-AGA CCT TGG CAG CGT CCT-3′, GAPDH: 5′-AGG TGA AGG TCG GAG TCA ACG-3′, 5′-AGG GGT CAT TGA TGG CAA CA-3′.
Western blotting
Total cell lysates or immunocomplexes were resolved on 12% SDS-PAGE, transferred to PVDF membrane (GE Healthcare), blocked in 5% milk-Tris-buffered saline solution, and incubated at 4°C overnight with UCH L1 (1:7,500, Invitrogen), α/β-Tubulin (1:1,000, Cell signaling), β-Tubulin (1:5,000, Santa Cruz), anti-ubiquitin (1:500, Sigma) and GAPDH (1:5,000, Sigma) antibodies followed with horseradish peroxidase-conjugated secondary antibody. Proteins were detected with the Super Signal West Pico Chemiluminescence Detection Kit (Pierce Biotechnology, Rockford, IL, USA) and exposed to Kodak XAR-5 film. Gels were stained with Gel-Code blue coomassie stain.
Blocking peptide against UCH L1 antibody
The blocking peptide against UCH L1 antibody was obtained from Invitrogen. The blocking peptide used was the synthetic antigen used as an immunogen to produce antibodies. All the samples to be tested for blocking peptide were run on one gel. After the transfer the blot was cut into half vertically. One part of the blot was incubated with UCH L1 antibody (Invitrogen) and the second part was incubated with UCH L1 antibody + blocking peptide against the antibody, followed with horse-radish peroxidase-conjugated secondary antibody. Proteins were detected with the Super Signal West Pico Chemiluminescence Detection Kit (Pierce Biotechnology, Rockford, IL, USA) and exposed to Kodak XAR-5 film. In order to eliminate any differences both the blots were processed at the same time.
In vitro ubiquitination assay
For the in vitro ubiquitination assay, each reaction mixture contained 50 mM Tris (pH 7.5), 2 mM Mg-ATP, 2 mM dithiothreitol, 20 μM His-ubiquitin/reaction (Biomol), 25 μM MG132 (Sigma), 10% (vol/vol) rabbit reticulocyte lysate (Boston Biochem), 1× protease inhibitors and 2.5 g of purified Tubulin protein. The reaction mixtures were incubated at 37°C for 1 hour. For deubiquitination assays, after the initial incubation, the reaction containing Ub was treated with 1 ug of recombinant UCH L1 (Biomol), followed by incubation at 37°C for additional 1 hour. The samples were then analyzed by western blotting for tubulin, ubiquitin and UCH L1.
In vitro tubulin polymerization and microtubule binding assays
Three mg/ml purified bovine brain tubulin (99% tubulin) or two mg/ml bovine brain microtubule associated protein (MAP)-rich tubulin (70% tubulin and 30% MAPs) or four mg/ml of purified porcine brain HTS-tubulin (3% MAPs and 97% tubulin) (Cytoskeleton Inc.,) was assembled in tubulin polymerization buffer (TP buffer). TP buffer was made with general tubulin buffer (80 nM Pipes, pH 6.9, 2 mM MgCl2, 0.5 mM EGTA and 2.5 mM of GTP) and tubulin cushion buffer (containing 15% glycerol + general tubulin buffer). The half area 96-well plate was pre-warmed to 37°C for 30 min. The assembly of tubulin was measured every 1 min and monitored over a period of 30 mins by light scattering at 340 nm using temperature controlled spectrophotometer. The assay was performed in presence or absence of 1 μg/reaction of recombinant UCH L1 and where indicated 500 ng/reaction of free ubiquitin was added.
Following the polymerization assay, the samples were centrifuged at 25,000 g for 30 mins at RT to pellet microtubules and all associated proteins. The pellet was washed twice with room temperature TP buffer and resuspended in TP buffer. The samples were then analyzed by PAGE under reducing or native (with or without SDS respectively) conditions.
In vivo tubulin polymerization assay
In order to determine if UCH L1 bound to microtubules in vivo, cell lysates from KR4 LCLs and 293 cells were incubated in presence or absence of taxol (4 mg/ml) in microtubule stabilization buffer (0.1 M PIPES, 1 mM EGTA, 1 mM MgSO4, 2 M glycerol, pH 8.0, 1× protease inhibitor cocktail) for 30 mins on ice. The reaction was then separated into supernatant (soluble) and pellet (insoluble) tubulin fractions by centrifugation at 25,000 g for 30 mins at 4°C. The microtubule pellet was resuspended in MTS buffer. The samples were analyzed by SDS-PAGE.
References
- 1.Fukushima N, Furuta D, Hidaka Y, Moriyama R, Tsujiuchi T. Post-translational modifications of tubulin in the nervous system. J Neurochem. 2009;109:683–93. doi: 10.1111/j.1471-4159.2009.06013.x. [DOI] [PubMed] [Google Scholar]
- 2.Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–6. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beghin A, Galmarini CM, Dumontet C. Tubulin folding pathways: implication in the regulation of microtubule dynamics. Curr Cancer Drug Targets. 2007;7:697–703. doi: 10.2174/156800907783220426. [DOI] [PubMed] [Google Scholar]
- 4.Verhey KJ, Gaertig J. Cell Cycle. Vol. 6. Georgetown, Tex: 2007. The tubulin code; pp. 2152–60. [DOI] [PubMed] [Google Scholar]
- 5.Parvin JD, Sankaran S. The BRCA1 E3 ubiquitin ligase controls centrosome dynamics. Cell Cycle. 2006;5:1946–50. doi: 10.4161/cc.5.17.3208. [DOI] [PubMed] [Google Scholar]
- 6.Parvin JD. The BRCA1-dependent ubiquitin ligase, gamma-tubulin and centrosomes. Environ Mol Mutagen. 2009;50:649–53. doi: 10.1002/em.20475. [DOI] [PubMed] [Google Scholar]
- 7.Sankaran S, Crone DE, Palazzo RE, Parvin JD. BRCA1 regulates gamma-tubulin binding to centrosomes. Cancer Biol Ther. 2007;6:1853–7. doi: 10.4161/cbt.6.12.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Molecular and Cellular Biology. 2005;25:8656–68. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP, et al. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Molecular and Cellular Biology. 2004;24:8457–66. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuschieri L, Nguyen T, Vogel J. Control at the cell center: the role of spindle poles in cytoskeletal organization and cell cycle regulation. Cell Cycle. 2007;6:2788–94. doi: 10.4161/cc.6.22.4941. [DOI] [PubMed] [Google Scholar]
- 11.Alonso AC, Li B, Grundke-Iqbal I, Iqbal K. Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2008;5:375–84. doi: 10.2174/156720508785132307. [DOI] [PubMed] [Google Scholar]
- 12.Pevalova M, Filipcik P, Novak M, Avila J, Iqbal K. Post-translational modifications of tau protein. Bratisl Lek Listy. 2006;107:346–53. [PubMed] [Google Scholar]
- 13.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm. 2005;112:813–38. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–24. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol. 2009;186:601–13. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson WE, Ramalho-Santos J, Sutovsky P. Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod. 2003;69:254–60. doi: 10.1095/biolreprod.102.010975. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal K, Grundke-Iqbal I. Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer's disease. Molecular Neurobiology. 1991;5:399–410. doi: 10.1007/BF02935561. [DOI] [PubMed] [Google Scholar]
- 18.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News & Perspectives. 2007;20:365–70. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 19.Ventii KH, Wilkinson KD. Protein partners of deubiquitinating enzymes. Biochem J. 2008;414:161–75. doi: 10.1042/BJ20080798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem Int. 2007;51:105–11. doi: 10.1016/j.neuint.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry. 1996;35:6735–44. doi: 10.1021/bi960099f. [DOI] [PubMed] [Google Scholar]
- 22.Betarbet R, Sherer TB, Greenamyre JT. Ubiquitin-proteasome system and Parkinsons diseases. Exp Neurol. 2005;191:17–27. doi: 10.1016/j.expneurol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Day IN, Hinks LJ, Thompson RJ. The structure of the human gene encoding protein gene product 9.5 (PGP9.5), a neuron-specific ubiquitin C-terminal hydrolase. The Biochemical Journal. 1990;268:521–4. doi: 10.1042/bj2680521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell. 2002;111:209–18. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 25.Osaka H, Wang YL, Takada K, Takizawa S, Setsuie R, Li H, et al. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Human Molecular Genetics. 2003;12:1945–58. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- 26.Tezel E, Hibi K, Nagasaka T, Nakao A. PGP9.5 as a prognostic factor in pancreatic cancer. Clin Cancer Res. 2000;6:4764–7. [PubMed] [Google Scholar]
- 27.Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5 as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol. 1999;155:711–5. doi: 10.1016/S0002-9440(10)65169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeffler-Ragg J, Skvortsov S, Sarg B, Skvortsova I, Witsch-Baumgartner M, Mueller D, et al. Gefitinib-responsive EGFR-positive colorectal cancers have different proteome profiles from non-responsive cell lines. Eur J Cancer. 2005;41:2338–46. doi: 10.1016/j.ejca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Yang YC, Li X, Chen W. Characterization of genes associated with different phenotypes of human bladder cancer cells. Acta Biochim Biophys Sin (Shanghai) 2006;38:602–10. doi: 10.1111/j.1745-7270.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi Y, Nakayama S, Torikoshi Y, Tanaka S, Ishihara H, Taguchi T, et al. High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Science. 2006;97:523–9. doi: 10.1111/j.1349-7006.2006.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ovaa H, Kessler BM, Rolen U, Galardy PJ, Ploegh HL, Masucci MG. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc Natl Acad Sci USA. 2004;101:2253–8. doi: 10.1073/pnas.0308411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuki T, Yata K, Takata-Tomokuni A, Hyodoh F, Miura Y, Sakaguchi H, et al. Expression of protein gene product 9.5 (PGP9.5)/ubiquitin-C-terminal hydrolase 1 (UCHL-1) in human myeloma cells. Br J Haematol. 2004;127:292–8. doi: 10.1111/j.1365-2141.2004.05205.x. [DOI] [PubMed] [Google Scholar]
- 33.Rolen U, Freda E, Xie J, Pfirmann T, Frisan T, Masucci MG. The Ubiquitin C-terminal Hydrolase UCH-L1 regulates B-cell proliferation and integrin activation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PloS One. 2009;4:6764. doi: 10.1371/journal.pone.0006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Kim YM, Lim S, Nam YK, Jeong J, Kim HJ, Lee KJ. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene. 2008;28:117–27. doi: 10.1038/onc.2008.364. [DOI] [PubMed] [Google Scholar]
- 36.Long EM, Long MA, Tsirigotis M, Gray DA. Stimulation of the murine Uchl1 gene promoter by the B-Myb transcription factor. Lung Cancer. 2003;42:9–21. doi: 10.1016/s0169-5002(03)00279-4. [DOI] [PubMed] [Google Scholar]
- 37.Bheda A, Yue W, Gullapalli A, Whitehurst C, Liu R, Pagano JS, Shackelford J. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/TCF signaling. PloS One. 2009;4:5955. doi: 10.1371/journal.pone.0005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8:1688–97. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 39.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–75. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Tian Q, Zhang Q, Zhou X, Liu S, Wang JZ. Hyperphosphorylation of microtubule-associated tau protein plays dual role in neurodegeneration and neuroprotection. Pathophysiology. 2009;16:311–6. doi: 10.1016/j.pathophys.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Kabuta T, Setsuie R, Mitsui T, Kinugawa A, Sakurai M, Aoki S, et al. Aberrant molecular properties shared by familial Parkinson's disease-associated mutant UCH-L1 and carbonyl-modified UCH-L1. Hum Mol Genet. 2008;17:1482–96. doi: 10.1093/hmg/ddn037. [DOI] [PubMed] [Google Scholar]
- 42.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichikawa T, Li J, Dong X, Potts JD, Tang DQ, Li DS, Cui T. Ubiquitin carboxyl terminal hydrolase L1 negatively regulates TNFalpha-mediated vascular smooth muscle cell proliferation via suppressing ERK activation. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, et al. Overexpression of ubiquitin carboxy terminal hydrolase-L1 induces apoptosis in breast cancer cells. Int J Oncol. 2008;33:1037–45. doi: 10.3892/ijo_00000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Jiang Q, Zhao J, Ren Y, Sutton MD, Feng J. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J Biol Chem. 2005;280:17154–62. doi: 10.1074/jbc.M500843200. [DOI] [PubMed] [Google Scholar]
- 46.Raynaud-Messina B, Merdes A. Gamma-tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Rossi G, Dalpra L, Crosti F, Lissoni S, Sciacca FL, Catania M, et al. A new function of microtubule-associated protein tau: involvement in chromosome stability. Cell Cycle. 2008;7:1788–94. doi: 10.4161/cc.7.12.6012. [DOI] [PubMed] [Google Scholar]
- 48.Connolly JA, Kalnins VI, Cleveland DW, Kirschner MW. Immunoflourescent staining of cytoplasmic and spindle microtubules in mouse fibroblasts with anti-body to tau protein. Proc Natl Acad Sci USA. 1977;74:2437–40. doi: 10.1073/pnas.74.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]


