Abstract
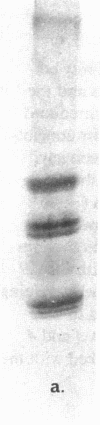
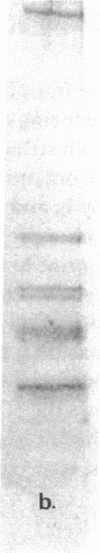
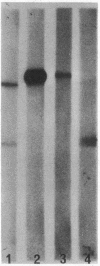
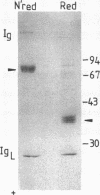
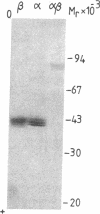
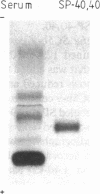
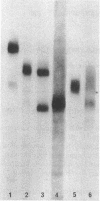
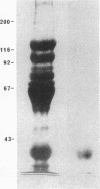
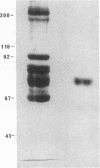
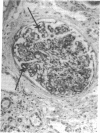
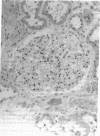
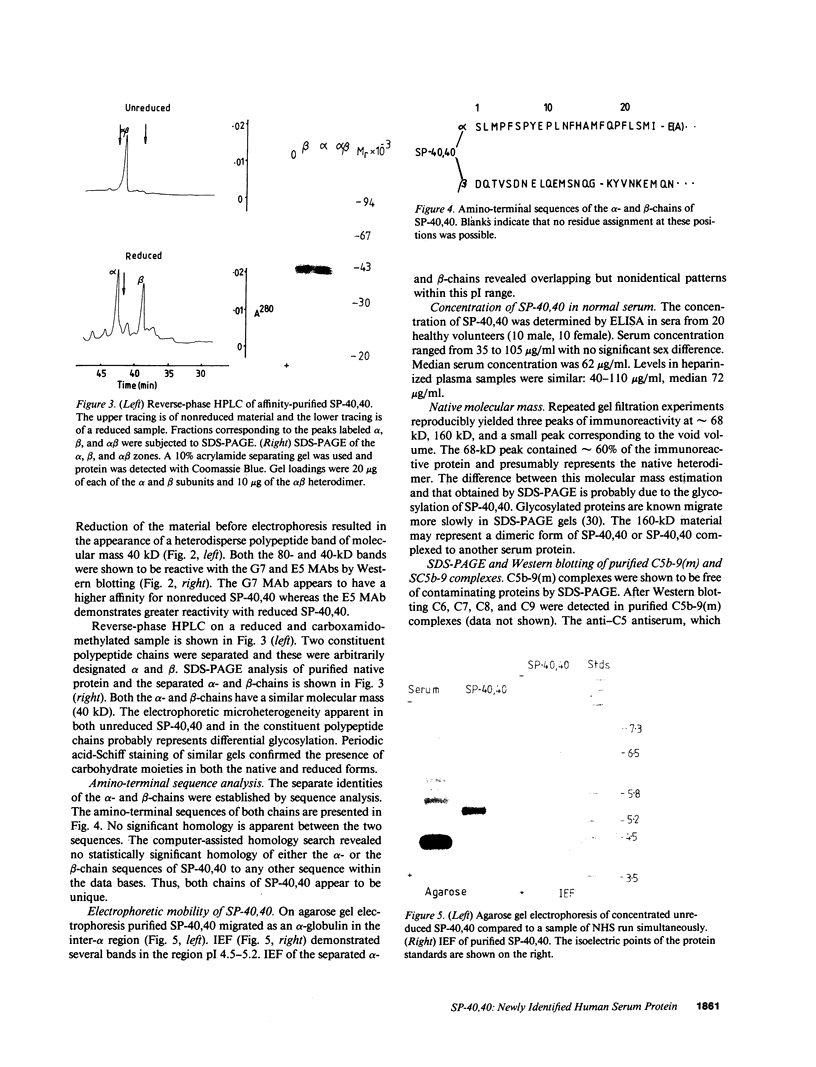
We report herein the isolation and initial characterization of a novel protein, termed SP-40,40, which is present at moderate levels (35-105 micrograms/ml) in normal human serum. SP-40,40 is deposited in the renal glomeruli of patients with glomerulonephritis but is not found in normal glomeruli. The protein is a heterodimeric structure of relative molecular mass 80 kD, both chains of which are of a similar size (40 kD). The amino-terminal sequences of both chains are unrelated to one another and possess no significant homology to any known protein sequence. The tissue distribution of SP-40,40 closely resembles that of the terminal complement components and its physicochemical properties are similar to, but distinct from, those of the S protein of complement. We have identified SP-40,40 in the SC5b-9 complex of complement and have demonstrated incorporation of labeled SP-40,40 into this complex. These data suggest that SP-40,40 is an additional component of SC5b-9.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Roth M. Fluid-phase SC5b-8 complex of human complement: generation and isolation from serum. J Immunol. 1981 Aug;127(2):576–580. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Hydrophilic-amphiphilic transition of the terminal SC5b-8 complement complex through tryptic modification: biochemical and ultrastructural studies. Mol Immunol. 1982 Sep;19(9):1167–1177. doi: 10.1016/0161-5890(82)90327-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Chambers G. W., Walker I. D., Bateman J. F. Deletion of 24 amino acids from the pro-alpha 1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1986 Apr 25;261(12):5496–5503. [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R. J., Podack E., Dalmasso A. P., Jennette J. C. Localization of S protein and its relationship to the membrane attack complex of complement in renal tissue. Am J Pathol. 1987 Apr;127(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Hancock W. W., Becker G. J., Atkins R. C. A comparison of fixatives and immunohistochemical technics for use with monoclonal antibodies to cell surface antigens. Am J Clin Pathol. 1982 Dec;78(6):825–831. doi: 10.1093/ajcp/78.6.825. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Kraft N., Clarke F., Atkins R. C. Production of monoclonal antibodies to fibronectin, type IV collagen and other antigens of the human glomerulus. Pathology. 1984 Apr;16(2):197–206. doi: 10.3109/00313028409059105. [DOI] [PubMed] [Google Scholar]
- Jenne D., Hugo F., Bhakdi S. Monoclonal antibodies to human plasma protein X alias complement S-protein. Biosci Rep. 1985 Apr;5(4):343–352. doi: 10.1007/BF01116907. [DOI] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J. 1985 Dec 1;4(12):3153–3157. doi: 10.1002/j.1460-2075.1985.tb04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lems-Van Kan P., Verspaget H. W., Peña A. S. ELISA assay for quantitative measurement of human immunoglobulins IgA, IgG, and IgM in nanograms. J Immunol Methods. 1983 Feb 25;57(1-3):51–57. doi: 10.1016/0022-1759(83)90064-9. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew T. H., Mathews D. C., Hobbs J. B., Kincaid-Smith P. Glomerular lesions after renal transplantation. Am J Med. 1975 Aug;59(2):177–190. doi: 10.1016/0002-9343(75)90352-6. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: reaction with C7, C8, C9. J Immunol. 1978 Aug;121(2):484–490. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauterberg E. W., Lieberknecht H. M., Wingen A. M., Ritz E. Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int. 1987 Mar;31(3):820–829. doi: 10.1038/ki.1987.72. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Ware C. F., Wetsel R. A., Kolb W. P. Physicochemical characterization of fluid phase (SC5b-9) and membrane derived (MC5b-9) attack complexes of human complement purified by immunoadsorbent affinity chromatography or selective detergent extraction. Mol Immunol. 1981 Jun;18(6):521–531. doi: 10.1016/0161-5890(81)90130-9. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane. Preparation and composition. Biochemistry. 1970 Sep 15;9(19):3837–3846. doi: 10.1021/bi00821a025. [DOI] [PubMed] [Google Scholar]