Abstract
Fungi from the genus Candida are common members of the human microbiota; however, they are also important opportunistic pathogens in immunocompromised hosts. Several morphological transitions have been linked to the ability of these fungi to occupy the different ecological niches in the human body. The transcription factor Efg1 from the APSES family plays a central role in the transcription circuits underlying several of these morphological changes. In Candida albicans, for example, Efg1 is a central regulator of filamentation, biofilm formation, and white-opaque switching, processes associated with survival in the human host. Orthologs of Efg1 are present throughout the Candida clade but, surprisingly, the genome sequence of Candida tropicalis failed to uncover a gene coding for Efg1. One possibility was that the paralog of Efg1, Efh1, had assumed the function of Efg1 in C. tropicalis. However, we show that this gene has only a minor role in the morphological transitions mentioned above. Instead, we report here that C. tropicalis does have an ortholog of the EFG1 gene found in other Candida species. The gene is located in a different genomic position than EFG1 in C. albicans, in a region that contains a gap in the current genome assembly of C. tropicalis. We show that the newly identified C. tropicalis EFG1 gene regulates filamentation, biofilm formation, and white-opaque switching. Our results highlight the conserved role of Efg1 in controlling morphogenesis in Candida species and remind us that published genome sequences are drafts that require continuous curation and careful scrutiny.
Keywords: Candida morphogenesis, biofilm, filamentation, phenotypic switch, transcriptional regulation
Several species of the Candida genus belong to a monophyletic clade of ascomycetous fungi that translate the CTG codon as serine instead of leucine (Butler et al. 2009). Members of this CTG clade include species that are commensals of the human microbiota with no known environmental reservoirs outside of animals. However, these commensals can also cause mucosal disease in healthy individuals as well as systemic infections in immunocompromised hosts (Calderone 2002). Members of the CTG clade, therefore, are important fungal human pathogens, both in terms of their prevalence and their mortality rate.
Although they are most commonly encountered in the yeast form (unicellular spherical cells), most CTG species are able to undergo a variety of changes in cell and colony morphology. The best studied of these is the ability to switch between the yeast and hyphal (filamentous) forms, a transition that is closely linked to pathogenesis and biofilm formation (Sudbery 2011). Biofilms are communities of cells associated with a biologic or inert surface. In the case of Candida species, these communities are typically composed of several layers of yeast cells and hyphae that are embedded within an extracellular matrix (Finkel and Mitchell 2011; Nobile et al. 2012). Other morphological transitions include the phenomenon of heritable phenotypic switching. In C. albicans, this includes the white-opaque switch, in which cells transition between two different cell types that generate either shiny and dome-shaped colonies (white) or dull and flat colonies (opaque) (Slutsky et al. 1987; Lohse and Johnson 2009). White and opaque cells are morphologically distinct, have different metabolic preferences, and differ in their ability to mate (Slutsky et al. 1987; Lan et al. 2002; Miller and Johnson 2002). Several lines of evidence suggest that morphological transitions allow C. albicans to adapt to different ecological niches in the human host (Kvaal et al. 1997; Lohse and Johnson 2009; Pande et al. 2013; Tao et al. 2014). For example, filamentous cells are more effective at invading epithelia, whereas biofilms often form on indwelling medical devices and confer tolerance to antifungal drugs (Cutler 1991; Donlan 2001; Donlan and Costerton 2002; Kojic and Darouiche 2004; Sudbery 2011). White and opaque cells are also known to differ in their ability to colonize different anatomical locations and are distinct in terms of their virulence (Kvaal et al. 1997; Lachke et al. 2003; Lohse and Johnson 2009). Therefore, elucidating the molecular mechanisms underlying phenotypic changes will help determine how these fungi are able to colonize and infect multiple niches in the human body.
At the molecular level, the morphological changes described above have been extensively investigated in C. albicans. Filamentation, biofilm formation, and white-opaque switching are regulated by specific sets of transcription factors that control the expression of large numbers of target genes (Kadosh and Johnson 2005; Nobile et al. 2012; Hernday et al. 2013). Interestingly, the transcription factor Efg1 is unique in being common to all three transcription circuits. This transcription factor was originally identified as a regulator of hyphae formation and a member of the fungal-specific APSES family of DNA binding proteins (Stoldt et al. 1997). In C. albicans it acts as an activator or repressor of hyphae formation depending on the environmental conditions (Lo et al. 1997; Stoldt et al. 1997; Tebarth et al. 2003). Efg1 is also one of the six core transcription factors that control biofilm formation in C. albicans. Its deletion has the strongest effect on biofilm formation in vitro among the six core biofilm transcription factors (Nobile et al. 2012). In white-opaque switching, Efg1 is a critical transcription factor for formation of the white phenotypic state (Sonneborn et al. 1999; Srikantha et al. 2000; Zordan et al. 2007). Efg1 binds to the regulatory sequences of the five other transcription factors of the circuit and represses the expression of the master regulator of the opaque state, Wor1 (Zordan et al. 2007; Hernday et al. 2013). In addition to its role in these phenotypes and possibly as a consequence, Efg1 plays an important role in mediating colonization of the gastrointestinal tract (Pierce and Kumamoto 2012; Pande et al. 2013; Hirakawa et al. 2014). Together, these studies establish the central role of Efg1 in regulating multiple facets of C. albicans biology, including both commensalism and pathogenicity.
Relatively little is known about the role of EFG1 orthologs in other CTG species. In Candida parapsilosis, Efg1 is involved in biofilm formation and also regulates a colony morphology switch, although the morphologies appear distinct from C. albicans white and opaque states (Connolly et al. 2013). Interestingly, the role of Efg1 as a regulator of morphological change appears to be conserved even in species outside the CTG clade. For example, the ortholog of Efg1 in the model yeast Saccharomyces cerevisiae, Sok2, controls pseudohyphal growth (Pan and Heitman 2000), whereas in the filamentous ascomycetes Neurospora crassa and Aspergillus nidulans, the corresponding orthologs regulate ascospore maturation and conidiosphore development, respectively (Aramayo et al. 1996; Dutton et al. 1997).
Given the central role that EFG1 plays in adaptation of C. albicans to the human host and in morphological transitions across multiple fungal species, it was surprising that the genome sequence of C. tropicalis appeared to be missing an ortholog of this gene (Butler et al. 2009; Maguire et al. 2013). C. tropicalis is a CTG species related to C. albicans [approximately 55 million years since their last common ancestor (Mishra et al. 2007)] and is a common commensal and pathogen of humans (Butler et al. 2009). C. tropicalis is also known to undergo the yeast-to-hyphae switch, to form biofilms, and to exhibit white-opaque switching (Porman et al. 2011; Xie et al. 2012; Porman et al. 2013). Recent experiments have revealed that the C. tropicalis white-opaque switch involves a third stable state, termed the "intermediate" state, and therefore exhibits tristable rather than bistable switching (A. M. Porman, M. Anderson, N. Wang, E. Mancera, and R. J. Bennett, unpublished data). Among all CTG species whose genomes have been sequenced, C. tropicalis is the only one apparently missing an ortholog of EFG1 (Maguire et al. 2013).
One possibility to explain the absence of EFG1 in C. tropicalis is that its functional role has been assumed by the APSES paralog EFH1, but we show here that EFH1 plays only a minor role, if at all, in the morphological transitions mentioned above. Instead, we report here that the genome of C. tropicalis does contain an ortholog of EFG1, but it was not incorporated in the final assembly of the genome sequence. We first uncovered the gene by PCR amplification from genomic DNA using primers designed on EFG1 orthologs from related CTG species. We subsequently determined the sequence and genomic location of C. tropicalis EFG1. The C. tropicalis gene lies in a different genomic location than that of its ortholog in C. albicans and yet clearly represents a true EFG1 ortholog. Deletion of both alleles of the gene revealed that it is critical for filamentation, biofilm formation, and white-opaque switching in C. tropicalis, similar to its role in C. albicans. Overall, our results stress the conserved role of EFG1 in regulating physical transitions in fungi and provide a cautionary note for the analysis of published genome sequences.
Materials and Methods
Strain/plasmid construction
Transformations were performed as previously described for C. tropicalis (Porman et al. 2011). Nutritional gene deletions were constructed using the SAT1 flipper strategy (Reuss et al. 2004) in two different C. tropicalis genetic backgrounds: MYA3404 (the strain used by the genome sequencing project) and AM2005/0093. Plasmids to delete HIS1, LEU2, and ARG4 were made as described (Noble and Johnson 2005). For the deletion of EFH1 and EFG1, the 5′ and 3′ ∼300-bp regions flanking the ORFs were PCR-amplified. HIS1, LEU2, and ARG4 auxotrophic markers were PCR-amplified from plasmids pSN52, pSN40, and pSN69, respectively. Fusion PCR was then performed to fuse 5′ and 3′ flanks to nutritional markers. This PCR product was transformed into auxotrophic strains lacking HIS1 and LEU2 or HIS1 and ARG4 (Supporting Information, Table S2) and the process repeated to delete the second copy of the ORF. When using strains that were only leu minus, the first allele was deleted using the LEU2 marker from plasmid pEM002 and the second allele using the HYG marker from plasmid pYM70 (Basso et al. 2010). PCR was used to confirm correct genomic integration of the markers and homozygous deletion of the genes. Two independent isolates were generated for each deleted gene in each genetic background (Table S2).
Cloning EFG1 from C. tropicalis
The amplification of the first fragment of EFG1 was performed by PCR using degenerate primers designed against the portion of the EFG1 sequence that is most conserved from C. albicans to C. parapsilosis (Table S1, primers EMO348 and EMO352). C. tropicalis genomic DNA from the EFH1 deletion mutant was used as template and annealing temperatures of between 40° and 50° were used. Based on the sequence of the amplified fragment and conserved regions further out on the C. albicans gene, more primer pairs were designed to further extend the amplified region by PCR (Table S1).
For the final cloning of the gene, two flanking ∼300-bp segments in the center of the EFG1 sequence identified above were PCR-amplified from genomic DNA to be used as homology for integration. These two regions were PCR-fused to the edges of a segment containing the SAT1 marker, the CamR marker, and an E. coli origin of replication that was PCR-amplified from plasmid pSFS2A (Reuss et al. 2004). The resulting cassette (5′ EFG1 homology—drug markers and origin of replication—3′ EFG1 homology) was transformed into C. tropicalis cells as previously described. Transformants with the correct insertion at the center of the endogenous EFG1 sequence were selected on YEPD plates containing 400 μg/ml of nourseothricin and verified by colony PCR. Genomic DNA from the transformants was extracted, digested with XhoI and NheI separately, circularized by ligation, and electroporated into E. coli XL1-Blue Electroporation-Competent Cells (Agilent Technologies) following the manufacturer’s protocol. Clones containing the EFG1 gene with the SAT1- CamR cassette were selected on LB plates containing 34 μg/ml chloramphenicol. Sequencing of the clones was performed by primer walking.
Characterization of hyphae formation
Similarly to what has been previously described (Lackey et al. 2013), cells from a YEPD, 30°, overnight culture, were washed twice with water and used to inoculate SD medium supplemented with 0.75% glucose and 50% fetal bovine serum at an OD600 of 1.5. This culture was grown with shaking at 37°. Samples were taken at the time of inoculation (0-hr time point) and at 2, 4, and 22 hr after inoculation and were visualized by differential interference contrast (DIC) microscopy.
Characterization of biofilm formation
Biofilm formation was assayed by confocal scanning laser microscopy and by determining biomass dry weight as previously described (Nobile et al. 2012). Briefly, overnight YEPD cultures grown at 30° were used to inoculate silicon squares pretreated with adult bovine serum at an OD600 of 0.5. Cells were left to adhere to the silicon square in Spider medium without mannitol and with 1% glucose at 37° and shaking at 200 rpm for 90 min. The squares were then washed with PBS and transferred to fresh Spider 1% glucose medium to be incubated for 48 hr at 37° and shaking at 200 rpm. Biofilms formed on the squares were stained for 1 hr with 50 mg/ml of concanavalin A Alexa Fluor 594 conjugate and visualized on a confocal microscope using a 40×/0.80W Nikon objective. For the determination of biomass dry weights, biofilms were grown in the same way, but directly on the bottom of six-well polystyrene plates. After 48 hr of growth at 37°, the medium was aspirated, biofilms were scraped from the bottom of each well, and they were dried on top of preweighed 0.8-μm nitrocellulose filter papers. Five replicate biofilms grown in separate wells were used for each strain.
Phenotypic switching assays on C. tropicalis mutant strains
Strains were grown overnight at 30° in Spider medium. Cells were diluted in water and plated onto Spider medium at a concentration of ∼100 colonies per plate. Plates were incubated for 7–10 days at 30° and colonies were examined for sectors. Cellular morphology was assessed by DIC microscopy of cells from the resulting colonies. Starting cells for each strain were in the corresponding default state: white, intermediate, and opaque for the wild-type, efg1Δ/EFG1, and efg1Δ/efg1Δ strains, respectively. For the heterozygous and homozygous mutants, we were not able to obtain stable populations in the white state.
Results
The role of EFH1 in C. tropicalis morphogenesis
Given the apparent absence of an ortholog of EFG1 in C. tropicalis, it could be hypothesized that the role of EFG1 had been assumed by its APSES paralog, EFH1. To test this, we generated deletion mutants of this gene in C. tropicalis and evaluated filamentation, biofilm, and white-opaque switching phenotypes. Filamentation was assayed by growth at 37° in SD medium supplemented with 0.75% glucose and 50% fetal bovine serum. This medium has been previously reported to be optimal for the induction of C. tropicalis filamentation (Lackey et al. 2013). Hyphae formation was monitored at 2, 4, and 22 hr of growth in filamentation-inducing medium. No filamentation defect was observed in the efh1Δ/efh1Δ mutant compared to the wild-type strain at any of the examined time points. Biofilms were generated by growth of C. tropicalis cells on silicon or polystyrene surfaces for 48 hr (see Materials and Methods). Similar to what has been observed in C. albicans (Nobile et al. 2012), biofilms formed by C. tropicalis efh1Δ/efh1Δ deletion mutants were indistinguishable from those formed by the wild-type strain when grown on a silicon surface and visualized by confocal microscopy. When grown on polystyrene plates, efh1Δ/efh1Δ biofilms did appear different from the wild-type in terms of their structure but showed similar overall thickness and biomass (10.87 mg ± 0.5 in efh1Δ/efh1Δ vs. 11.62 mg ± 0.4 in WT). The deletion of EFH1 in C. tropicalis also had no significant effect on the frequency with which this species switches from the white to the opaque state (A. M. Porman, M. Anderson, N. Wang, E. Mancera, and R. J. Bennett, unpublished data). This is in agreement with previous reports showing that the expression levels of EFH1 in C. tropicalis are similar between white and opaque cells (Porman et al. 2011). Taken together, these results indicate that the role of EFG1 in C. tropicalis has not simply been assumed by its paralog EFH1.
Identification of an ortholog of EFG1 in C. tropicalis
To test whether C. tropicalis has an EFG1 gene that was missed by sequencing analysis, we performed PCR on C. tropicalis genomic DNA using degenerate primers designed to anneal to conserved regions of EFG1 orthologs from the CTG clade (Table S1). We used low annealing temperatures to allow for mismatches between primers and template, and we used genomic DNA from the efh1Δ/efh1Δ mutant to avoid PCR amplification of the paralog gene. This approach allowed us to PCR amplify a ∼260-bp sequence that was sequenced and shown to be more similar to C. albicans EFG1 than to C. tropicalis EFH1. Additional rounds of PCR using primers that anneal to the amplified region or to the C. albicans EFG1 gene allowed us to extend the sequenced region to 900 bp. This 900-bp region did not match any sequences in the assembled C. tropicalis genomic sequence that would allow placement of the gene in the genome.
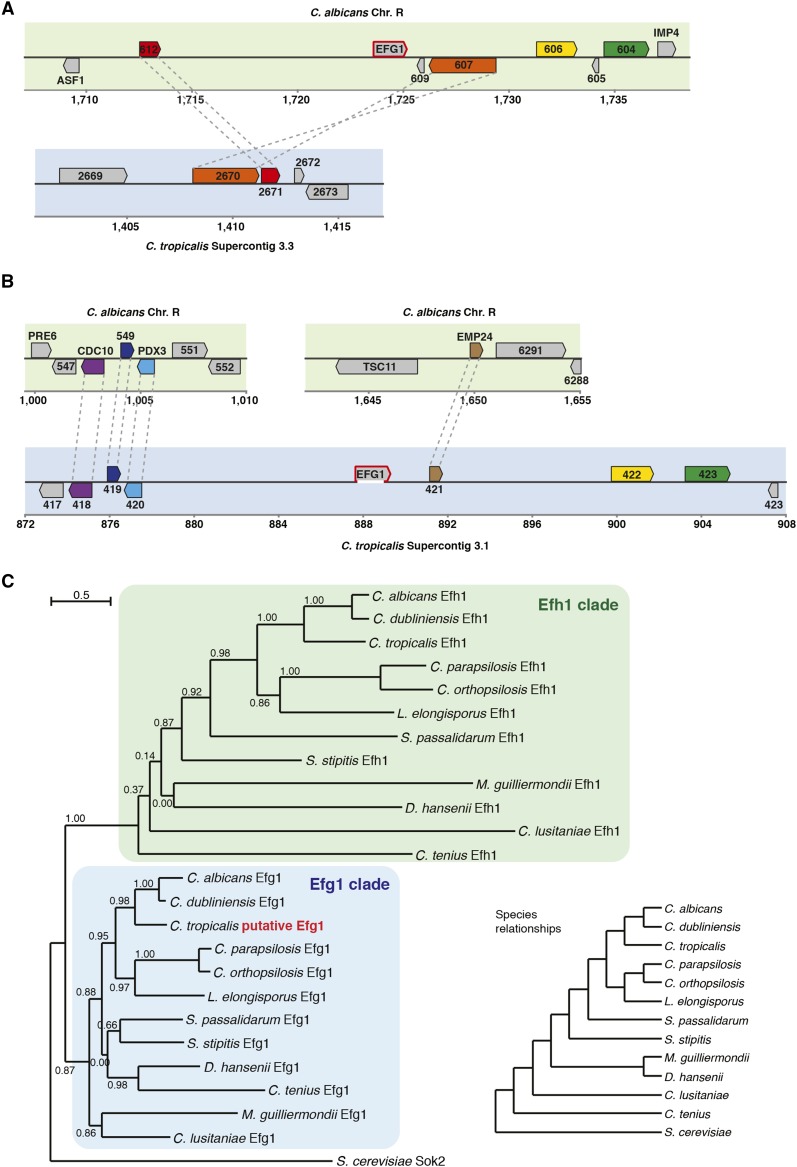
To identify the complete sequence of the C. tropicalis EFG1 gene and to map its location in the genome, we used a targeting plasmid to integrate into the endogenous EFG1 locus. The plasmid contained the CamR drug marker selectable in Escherichia coli and the SAT1 marker selectable in Candida species. From this plasmid we generated a PCR cassette that contained the two selectable markers and an E. coli origin of replication, together with flanking arms with homology to the sequenced region of C. tropicalis EFG1. This cassette was transformed into C. tropicalis cells, where it inserted at the endogenous EFG1 gene by homologous recombination. Genomic DNA from these transformants was fragmented, circularized by ligation, and transformed into E. coli. Clones containing the putative C. tropicalis EFG1 gene were selected and sequencing of these plasmids revealed an ORF of 1701 bp, found to map to Supercontig 3.1 of the C. tropicalis genome sequence. Notably, the ORF spans a gap in the Supercontig beginning at 887,643 bp and ending at 888,943 bp. The ORF starts 13 bp upstream of the gap and ends 342 bp downstream of it (see Figure 1). Based on the neighboring genes, the genomic location of EFG1 in C. tropicalis is different from that of EFG1 in C. albicans (Figure 1). This is highlighted by the fact that the C. tropicalis orthologs of the genes neighboring EFG1 in C. albicans are located on a different Supercontig (Figure 1).
Figure 1.
Genomic and phylogenetic position of EFG1 in C. tropicalis. (A) Schematic depiction of the genomic location of EFG1 in C. albicans and the corresponding genomic location in C. tropicalis. (B) As (A), but for C. tropicalis EFG1 and the corresponding location in C. albicans. In (A and B) the upper panel depicts C. albicans chromosomes (green background) and the lower panel depicts C. tropicalis chromosomes (blue background). Coordinates are in kb. One-to-one orthologs defined by CGOB are shown using the same color and are connected by dashed gray lines if in the same panel. The prefixes “orf19.” and “CTRG_” for C. albicans and C. tropicalis ORF names, respectively, were omitted. The white segment at the bottom of the C. tropicalis EFG1 gene depicts the location of the sequence gap. (C) Gene phylogeny of the EFG1 and EFH1 genes in the CTG clade and S. cerevisiae. Ortholog sequences were obtained from CGOB (Maguire et al. 2013), aligned using MUSCLE, and a phylogenetic tree was generated using PhyML in Seaview (Gouy et al. 2010). Branch support values are SH-like approximate likelihood ratios and the branch-length scale bar represents substitutions per site. Generating the tree by bootstrapping (100 trees) gave the same tree topology. The species relationships depicted in the cladogram (lower right) were obtained from (Maguire et al. 2013).
Sequence analysis of the EFG1 ortholog in C. tropicalis
Translation of the C. tropicalis EFG1 ORF results in a polypeptide of 567 amino acids that has 54% sequence identity and 60% sequence similarity to the C. albicans Efg1 protein. The two Efg1 proteins also represent the best reciprocal BLAST hit among the genes of the C. tropicalis and C. albicans genomes. As can be observed in Figure 1, the identified gene is at the precise position in the phylogenetic tree where it would be expected given the species evolutionary relationships. It groups with the Efg1 orthologs in the CTG clade, closest to C. albicans and Candida dubliniensis, and in a distinct part of the tree from the EFH1 orthologs. C. tropicalis Efg1 has a single conserved domain between amino acids 190 and 296 that matches the helix-turn-helix DNA binding domain characteristic of the APSES transcription factors (Stoldt et al. 1997). The domain in the C. tropicalis gene is almost identical to the APSES domain of C. albicans Efg1, differing only by two amino acids (sequence identity of 98%). Thus, by several criteria, the C. tropicalis EFG1 is an ortholog of the family of EFG1 genes from related CTG species.
We were able to reconstruct the two alleles of the C. tropicalis EFG1 gene by comparing differences between sequenced clones (Sequence accession numbers KP314278 and KP314279). The two alleles differ by seven nucleotides, five of which are nonsynonymous. None of the allelic differences lie within the APSES DNA binding domain of the protein. These are the two alleles present in the C. tropicalis strain MYA3404, which was the strain used for the genome sequencing project (Butler et al. 2009). Although we also used a strain of another genetic background to confirm our functional results (described below), all of the findings presented here are for the MYA3404 strain background.
The upstream intergenic region of EFG1 in C. tropicalis is strikingly long (10,110 bp compared with an average intergenic region in the C. tropicalis genome of 902 bp (Butler et al. 2009) and, in this sense, resembles the control region of C. albicans EFG1 (9,831 bp or 10,077 bp if the tRNA in the intergenic region is excluded). In C. albicans it is believed that the extended control region helps explain the complex regulation of EFG1 expression (Lachke et al. 2003; Tebarth et al. 2003; Nobile et al. 2012; Hernday et al. 2013).
C. tropicalis EFG1 is involved in filamentation, biofilm formation, and white-opaque switching
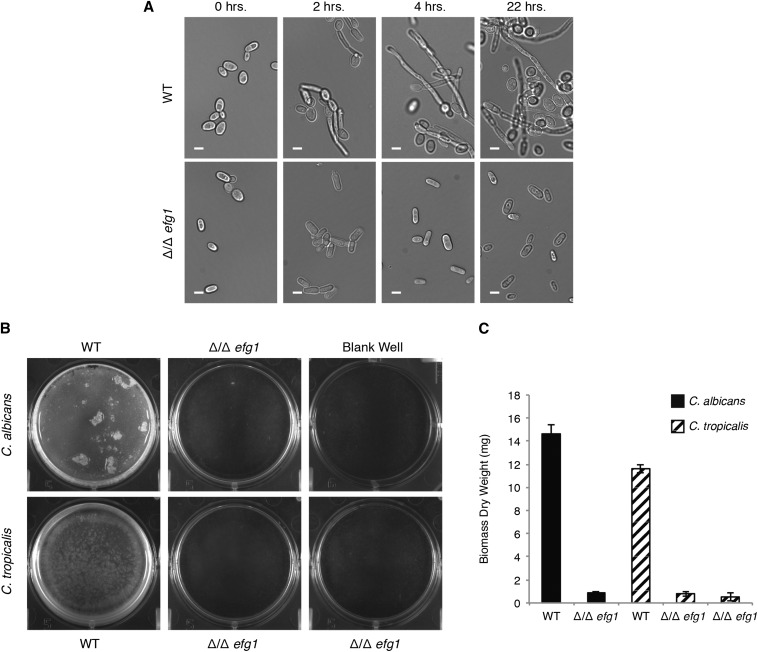
We generated deletion mutants of C. tropicalis EFG1 to characterize the role of the gene in morphogenesis (Table S2). Two independent isolates of the deletion mutant were tested to ensure that the phenotypes observed were not due to off-target effects. The deletion mutants were assayed for filamentation as described above for the EFH1 deletion mutants. As can be seen in Figure 2, the deletion of EFG1 in C. tropicalis had a strong effect on hyphae formation, with cells remaining in the yeast form even after 22 hr in the filamentation-inducing conditions. In contrast, cells from the wild-type strain showed hyphal projections even at the 2-hr time point. This phenotype is in agreement with the observed phenotype in C. albicans when EFG1 is deleted (Lo et al. 1997).
Figure 2.
EFG1 is involved in filamentation and biofilm formation in C. tropicalis. (A) Formation of filamentous cells by wild-type and EFG1 deletion strains of C. tropicalis in the MYA3404 genetic background. Representative DIC microscopy images are shown for each time point. Scale bars are 5 μm. (B) Images of representative biofilms for EFG1 deletion mutants in C. albicans and C. trpicalis (two independent isolates) grown in wells of six-well polystyrene plates. (C) Biomass dry weights of the same strains shown in (B), grown in the same conditions. Error bars represent the SD of five replicates.
To assess whether EFG1 is involved in biofilm formation in C. tropicalis, we determined the ability of the deletion mutant to form biofilms on two different in vitro surfaces, silicon and polystyrene, both pretreated with bovine serum. Biofilm accumulation was determined by confocal microscopy and dry weight measurements. After testing a variety of media, we performed assays in Spider medium with mannitol replaced by 1% glucose, because this medium was optimal for C. tropicalis biofilm formation. The ability to form biofilms by C. tropicalis was severely impaired in the efg1Δ/efg1Δ mutant compared to the wild-type strain (Figure 2). Using confocal microscopy, biofilms formed by the efg1Δ/efg1Δ mutant were composed only of layers of yeast cells with very few filamentous cells. Also, the few filamentous cells that were present resembled pseudohyphae rather than true hyphae. In contrast, wild-type strains formed biofilms that are multilayered with a thick layer of hyphal cells on top of basal layers of yeast cells. The biofilm deficiency in the efg1Δ/efg1Δ mutant was also evident in the overall biomass of the biofilm (Figure 2). As shown in Figure 2, the biofilm defect observed in the C. tropicalis deletion mutant resembles the C. albicans phenotype when the orthologous gene is deleted.
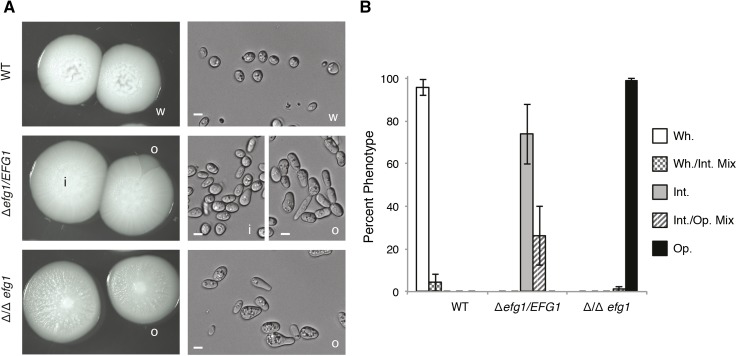
To test whether EFG1 plays a role in C. tropicalis white-opaque switching, we plated single colonies on Spider medium and grew them at 30° for 7 days. Phenotypic switching in C. tropicalis involves transitions between white, opaque, and a third phenotypic state that is intermediate to both the white and opaque forms (A. M. Porman, M. Anderson, N. Wang, E. Mancera, and R.J. Bennett, unpublished data). As shown in Figure 3, deletion of EFG1 had a major effect on the phenotypic states in C. tropicalis. The white state was stable in the wild-type background, but heterozygous efg1Δ/EFG1 and homozygous efg1Δ/efg1Δ strains, respectively, formed the "intermediate" or the opaque state as the default state. In terms of switching frequencies, white isolates from wild-type strains showed a low percentage of switching events, with only 4% of colonies showing sectoring to the "intermediate" phenotypic state. In contrast, isolates from heterozygous or homozygous strains showed only switching between the intermediate and opaque states (Figure 3B). These experiments establish that the role of EFG1 in C. tropicalis is similar to that of its ortholog in C. albicans, where it acts to promote stable formation of the white state (Srikantha et al. 2000; Zordan et al. 2007). We also generated efg1Δ/efg1Δ strains in a C. tropicalis genetic background different from the background used for the genome sequencing project and observed qualitatively the same phenotypes in biofilm formation and white-opaque switching (data not shown).
Figure 3.
EFG1 is essential to maintain the white state in C. tropicalis. (A) Photos of representative colony and cellular morphologies of the default states of the wild-type, heterozygous efg1Δ/EFG1, and homozygous efg1Δ/efg1Δ strains in C. tropicalis MYA3404 genetic background. Scale bars are 5 μm. w, white; i, intermediate; and o, opaque cell types are indicated. (B) Phenotype frequencies of the same strains starting from a population of white, intermediate, or opaque cells. Cells in these states were used because these were the default states for wild-type, efg1Δ/EFG1, and efg1Δ/efg1Δ cells, respectively. Error bars are the SD of five independent experiments.
In summary, the phenotypes of the C. tropicalis EFG1 deletion mutants establish that this gene plays a key role in filamentation, biofilm formation, and white-opaque switching. These findings are consistent with a conserved role for EFG1 in regulating morphological transitions in both C. tropicalis and C. albicans.
Discussion
The absence of an ortholog of EFG1 in the assembled genome of C. tropicalis was surprising given the conservation of this gene among other CTG species and its importance for regulating several key morphological transitions in C. albicans. Here, we report that C. tropicalis does indeed contain a true EFG1 ortholog. The C. tropicalis gene is located on the homologous chromosome to the C. albicans EFG1 gene (chromosome R), but it occupies a different position on this chromosome (Figure 1). Despite its different genomic location, the gene sequence reveals a shared evolutionary history with EFG1 genes from other species. It is difficult to recreate the exact genomic rearrangements that led to repositioning of EFG1, but the fact that the C. albicans and C. tropicalis EFG1 orthologs are on the same chromosome is consistent with the idea that most genomic rearrangements in the CTG clade are intrachromosomal (Butler et al. 2009).
We demonstrate, through gene knockouts, that C. tropicalis EFG1 regulates filamentation, biofilm formation, and white-opaque switching, indicating functional conservation with its ortholog in C. albicans. In contrast, analysis of the EFG1 paralog, EFH1, revealed a relatively minor role, if any, in each of these processes in both C. tropicalis (this work) and C. albicans (Doedt et al. 2004). EFG1 also regulates biofilm formation and a phenotypic switch in C. parapsilosis, although the latter appears distinct from that of the white-opaque switch (Connolly et al. 2013; Holland et al. 2014). It is therefore evident that this master regulator of morphological transitions has a conserved function between several of the most pathogenic Candida species. The molecular mechanisms underlying filamentation, biofilm formation, and white-opaque switching have been extensively studied in C. albicans, but little is known about these mechanisms in C. tropicalis. Identification of the ancient transcription factor EFG1 in C. tropicalis is a key step forward in understanding the strategies used by this species to adapt to the human host.
To gain insight into why the C. tropicalis EFG1 gene was not present in the published version of the genomic sequence (Butler et al. 2009), we examined the assembly process used for C. tropicalis. Initially, the genomic sequence was assembled using Arachne with standard parameters (Jaffe et al. 2003; Butler et al. 2009). However, it was found that many of the genes produced by this assembly were interrupted with stop codons caused by collapsing both haplotypes into a consensus haploid sequence. To address this problem, a new assembly was generated targeting highly polymorphic regions of the genome for iterative reassembly to minimize disagreement in the consensus and phase through a single haplotype for a given region (Butler et al. 2009). This strategy solved the problem of stop codon interruptions but reduced the total size of the assembly (8 kb smaller) and created new breaks in three scaffolds. Thus, assembling the C. tropicalis genome was a compromise between completeness, contiguity, and accuracy, made particularly challenging by the high number of allelic polymorphisms in the genome. In retrospect, we found that EFG1 was present in the initial assembly (where many genes were interrupted by stop codons), but was lost in the subsequent assembly that was published. Overall, our results stress the fact (often overlooked by investigators, including us) that published genome sequences are drafts in various stages of completeness. As was the case with the C. albicans genome sequence, further curation by the scientific community is critical to complement the computational assembly of genome sequences. This is an especially important and timely reminder in light of the exponential generation of new genomic sequences.
Acknowledgments
We thank Matthew B. Lohse and Katarzyna Oktaba for critical comments on the manuscript. This work was supported by a Human Frontier Science Program grant (to E.M.) and National Institutes of Health grants AI083311 (to A.D.J.), AI081704 (to R.J.B.) and F31DE022703 (to A.M.P.). This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200900018C.
Footnotes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.017566/-/DC1
Communicating editor: B. J. Andrews
Literature Cited
- Aramayo R., Peleg Y., Addison R., Metzenberg R., 1996. Asm-1+, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics 144: 991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso L. R., Jr, Bartiss A., Mao Y., Gast C. E., Coelho P. S., et al. , 2010. Transformation of Candida albicans with a synthetic hygromycin B resistance gene. Yeast 27: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G., Rasmussen M. D., Lin M. F., Santos M. A., Sakthikumar S., et al. , 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R., 2002. Candida and candidiasis, ASM Press, Washington, D.C. [Google Scholar]
- Connolly L. A., Riccombeni A., Grozer Z., Holland L. M., Lynch D. B., et al. , 2013. The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol. Microbiol. 90: 36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45: 187–218. [DOI] [PubMed] [Google Scholar]
- Doedt T., Krishnamurthy S., Bockmuhl D. P., Tebarth B., Stempel C., et al. , 2004. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol. Biol. Cell 15: 3167–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R. M., 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33: 1387–1392. [DOI] [PubMed] [Google Scholar]
- Donlan R. M., Costerton J. W., 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15: 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton J. R., Johns S., Miller B. L., 1997. StuAp is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J. 16: 5710–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel J. S., Mitchell A. P., 2011. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 9: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O., 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27: 221–224. [DOI] [PubMed] [Google Scholar]
- Hernday A. D., Lohse M. B., Fordyce P. M., Nobile C. J., DeRisi J. L., et al. , 2013. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol. Microbiol. 90: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa M. P., Martinez D. A., Sakthikumar S., Anderson M., Berlin A., et al. , 2014. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res.25: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. M., Schroder M. S., Turner S. A., Taff H., Andes D., et al. , 2014. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 10: e1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D. B., Butler J., Gnerre S., Mauceli E., Lindblad-Toh K., et al. , 2003. Whole-genome sequence assembly for mammalian genomes: Arachne 2. Genome Res. 13: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh D., Johnson A. D., 2005. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol. Biol. Cell 16: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic E. M., Darouiche R. O., 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal C. A., Srikantha T., Soll D. R., 1997. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect. Immun. 65: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke S. A., Srikantha T., Soll D. R., 2003. The regulation of EFG1 in white-opaque switching in Candida albicans involves overlapping promoters. Mol. Microbiol. 48: 523–536. [DOI] [PubMed] [Google Scholar]
- Lackey E., Vipulanandan G., Childers D. S., Kadosh D., 2013. Comparative evolution of morphological regulatory functions in Candida species. Eukaryot. Cell 12: 1356–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan C. Y., Newport G., Murillo L. A., Jones T., Scherer S., et al. , 2002. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA 99: 14907–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H. J., Kohler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., et al. , 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949. [DOI] [PubMed] [Google Scholar]
- Lohse M. B., Johnson A. D., 2009. White-opaque switching in Candida albicans. Curr. Opin. Microbiol. 12: 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire S. L., Oheigeartaigh S. S., Byrne K. P., Schroder M. S., O’Gaora P., et al. , 2013. Comparative Genome Analysis and Gene Finding in Candida Species Using CGOB. Mol. Biol. Evol. 30: 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. G., Johnson A. D., 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110: 293–302. [DOI] [PubMed] [Google Scholar]
- Mishra P. K., Baum M., Carbon J., 2007. Centromere size and position in Candida albicans are evolutionarily conserved independent of DNA sequence heterogeneity. Mol. Genet. Genomics 278: 455–465. [DOI] [PubMed] [Google Scholar]
- Nobile C. J., Fox E. P., Nett J. E., Sorrells T. R., Mitrovich Q. M., et al. , 2012. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. M., Johnson A. D., 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Heitman J., 2000. Sok2 regulates yeast pseudohyphal differentiation via a transcription factor cascade that regulates cell-cell adhesion. Mol. Cell. Biol. 20: 8364–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande K., Chen C., Noble S. M., 2013. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet.45: 1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. V., Kumamoto C. A., 2012. Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. MBio 3: e00117–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porman A. M., Alby K., Hirakawa M. P., Bennett R. J., 2011. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc. Natl. Acad. Sci. USA 108: 21158–21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porman A. M., Hirakawa M. P., Jones S. K., Wang N., Bennett R. J., 2013. MTL-independent phenotypic switching in Candida tropicalis and a dual role for Wor1 in regulating switching and filamentation. PLoS Genet. 9: e1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss O., Vik A., Kolter R., Morschhauser J., 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341: 119–127. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., et al. , 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn A., Tebarth B., Ernst J. F., 1999. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect. Immun. 67: 4655–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha T., Tsai L. K., Daniels K., Soll D. R., 2000. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J. Bacteriol. 182: 1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoldt V. R., Sonneborn A., Leuker C. E., Ernst J. F., 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16: 1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E., 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9: 737–748. [DOI] [PubMed] [Google Scholar]
- Tao L., Du H., Guan G., Dai Y., Nobile C. J., et al. , 2014. Discovery of a “white-gray-opaque” tristable phenotypic switching system in Candida albicans: roles of non-genetic diversity in host adaptation. PLoS Biol. 12: e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebarth B., Doedt T., Krishnamurthy S., Weide M., Monterola F., et al. , 2003. Adaptation of the Efg1p morphogenetic pathway in Candida albicans by negative autoregulation and PKA-dependent repression of the EFG1 gene. J. Mol. Biol. 329: 949–962. [DOI] [PubMed] [Google Scholar]
- Xie J., Du H., Guan G., Tong Y., Kourkoumpetis T. K., et al. , 2012. N-acetylglucosamine induces white-to-opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot. Cell 11: 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan R. E., Miller M. G., Galgoczy D. J., Tuch B. B., Johnson A. D., 2007. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 5: e256. [DOI] [PMC free article] [PubMed] [Google Scholar]