Abstract
Next-generation sequencing (NGS) technologies have become the standard for data generation in studies of population genomics, as the 1000 Genomes Project (1000G). However, these techniques are known to be problematic when applied to highly polymorphic genomic regions, such as the human leukocyte antigen (HLA) genes. Because accurate genotype calls and allele frequency estimations are crucial to population genomics analyses, it is important to assess the reliability of NGS data. Here, we evaluate the reliability of genotype calls and allele frequency estimates of the single-nucleotide polymorphisms (SNPs) reported by 1000G (phase I) at five HLA genes (HLA-A, -B, -C, -DRB1, and -DQB1). We take advantage of the availability of HLA Sanger sequencing of 930 of the 1092 1000G samples and use this as a gold standard to benchmark the 1000G data. We document that 18.6% of SNP genotype calls in HLA genes are incorrect and that allele frequencies are estimated with an error greater than ±0.1 at approximately 25% of the SNPs in HLA genes. We found a bias toward overestimation of reference allele frequency for the 1000G data, indicating mapping bias is an important cause of error in frequency estimation in this dataset. We provide a list of sites that have poor allele frequency estimates and discuss the outcomes of including those sites in different kinds of analyses. Because the HLA region is the most polymorphic in the human genome, our results provide insights into the challenges of using of NGS data at other genomic regions of high diversity.
Keywords: NGS, mapping bias, 1000 Genomes, HLA
Whole-genome resequencing data for large numbers of human individuals, as generated by the 1000 Genomes Project (www.1000genomes.org), provide unprecedented amounts of information about microevolutionary processes and demographic histories. Such inferences rely on either genotypic or allelic frequency information for each variable position, which constitute the data for downstream analyses and hypothesis testing.
The calling of single-nucleotide polymorphisms (SNPs) and genotypes and the estimation of allele frequencies from next-generation sequencing (NGS) has undergone rapid development, along with likelihood-based and Bayesian methods created to deal with challenges associated to heterogeneity in read quality and coverage (Nielsen et al. 2011). In Phase I of the 1000 Genomes Project, genotypes were called using a combination of different approaches: first, primary call sets were independently generated by different centers with different sequencing platforms, alignment, and variant calling methods; then, a consensus SNP call set was generated and made publicly available (The 1000 Genomes Project Consortium 2012).
The data generated by the 1000 Genomes Project frequently have been used to make inferences about evolutionary processes affecting our species, including the detection of targets of natural selection (Hernandez et al. 2011; Ward and Kellis 2012; Andersen et al. 2012) and understanding the genetic basis of complex phenotypes (Lappalainen et al. 2013). In addition, the detailed catalog of genetic variation it provides across multiple human populations has been used to understand the processes affecting specific genes.
Among the well-documented targets of selection is the major histocompatibility complex region of the human genome, which harbors the highly polymorphic classical human leukocyte antigen (HLA) class I and II loci. The interest in these loci stems from their strong association to various autoimmune disorders (Sollid et al. 2014), susceptibility and resistance to infection (Chapman and Hill 2012), and striking signatures of genetic variation indicating strong balancing selection (Meyer and Thomson 2001). Such types of investigations can naturally be extended to the analysis of the 1000 Genomes data, which provide a rich resource of population genetic variation within and around HLA genes.
Despite this interest, the use of NGS data for HLA loci is hampered by a major technical hurdle, which is the mapping of short sequence reads to genes that are both highly polymorphic and which constitute a multigene family. The high polymorphism may decrease the probability that short reads will be successfully mapped to the reference genome, in the event that the sequenced individual carries a variant that is highly diverged from that used in the index (Nielsen et al. 2011). In addition, the fact that many HLA genes have close paralogues increases the chance that a read will map to two or more genomic regions, leading it to be discarded from most sequencing analyses pipelines, and thus decreasing the amount of usable information for genotype calling (Treangen and Salzberg 2012).
In previous studies authors explored the applicability of NGS to genotype the HLA alleles of an individual, where an allele typically is defined as the haplotype determined by a combination of SNPs within a given HLA gene [e.g., Erlich et al. (2011); Major et al. (2013)]. To this end, Erlich et al. (2011) proposed NGS methodologies in which different steps—from sample preparation to haplotype level allele calling—were adapted to deal with the issues of high polymorphism and paralogy of HLA genes. In this way, they were able to successfully validate their methodology in a study of 270 samples that had been typed previously by sequence-specific oligonucleotide hybridization, which they treated as a gold standard dataset. The same gold standard dataset was used by Major et al. (2013), who also examined the reliability of calling HLA alleles using NGS, but using the 1000 Genomes alignment data, and showed that this publicly available dataset can be used for this purpose, after appropriate filters (e.g., coverage) are applied.
Both Erlich et al. (2011) and Major et al. (2013) were interested in using NGS data to determine HLA alleles. Information regarding HLA alleles is of biomedical relevance because HLA genotypes often are an important covariate to account for in association studies, and HLA typing is critical to hematopoietic transplantation. In this study, however, we evaluate the quality of SNP level genotype calls from the 1000 Genomes at the HLA genes.
The analysis of genotype and allele frequencies for SNPs contained within HLA genes has proven of great value in biomedical and evolutionary studies, and the 1000 Genomes dataset is a resource used recurrently in this context. Examples of the use of HLA SNP data from the 1000 Genomes Project include: (1) In genome-wide association studies (GWAS), SNPs in HLA genes often are associated with phenotypes of interest, and it is useful to understand the prevalence of these variants in additional populations; (2) GWAS studies benefit from knowledge of the haplotype structure surrounding HLA genes, which can be inferred from the dense SNP data of the 1000 Genomes for multiple populations (e.g., Hill-Burns et al. 2011); and (3) when testing for selection, many studies have found strong evidence associated to the HLA region, using the 1000 Genomes as a source of polymorphism data (e.g., Leffler et al. 2013).
All the aforementioned applications of the 1000 Genomes Project SNP data in HLA genes are dependent on the reliability of genotype calls at each SNP. However, no study to date has provided a detailed survey of the reliability of individual genotype calls and allele frequency estimates at the SNPs in HLA genes, despite their frequent usage. We address this issue, discuss likely causes for cases of incorrect genotype calls, and provide a list of reliable sites for the HLA loci in the 1000 Genomes data. As in previous studies (Erlich et al. 2011; Major et al. 2013), we used a dataset in which individuals had their HLA genes genotyped using Sanger sequencing as a gold standard to benchmark the genotypes called at the 1000 Genomes Project. However, differently from these other studies, which were interested in reconstructing the HLA haplotypes using NGS, here we have deconstructed the haplotypes determined from Sanger sequencing data into SNPs, and compared genotypes at the SNP level to the 1000 Genomes data. We took advantage of the recent availability of a dataset of Sanger sequencing based HLA genotyping of HLA-A, -B, -C, -DQB1, and -DRB1 for 930 of the samples from the 1000 Genomes Project (Gourraud et al. 2014). Our results have implications for other studies that use SNP data from the 1000 Genomes in order to estimate allele frequencies. Because HLA loci are the most polymorphic in the human genome, they most likely represent the worst case scenario for mapping bias and, consequently, allele frequency estimation error.
Methods
In this study, we compare NGS genotype calls and allele frequency estimates reported by the 1000 Genomes Project with those obtained in a study which used Sanger sequencing to genotype HLA genes. For the purpose of our analysis we assembled a dataset comprising the intersection of the 1000 Genomes and Sanger sequencing samples, resulting in 930 individuals from 12 populations. Supporting Information, Figure S1 summarizes the preprocessing of both datasets, which preceded genotype and allele frequency comparisons.
1000 Genomes dataset (1000G)
SNP genotypes were acquired from the chromosome 6 integrated Variant Call Format (VCF) file from version 3 of the 1000 Genomes Project Phase I data, which is available at ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20110521/ (The 1000 Genomes Project Consortium 2012). We selected only SNPs in exons encoding the antigen recognition sites (ARS), which are exons 2 and 3 for HLA-A, -B, and -C (Bjorkman et al. 1987) and exon 2 for HLA-DQB1 and -DRB1 (Brown et al. 1993). Sites were selected based on the most inclusive coordinates of the RefSeq database in July 22, 2014 (see File S1). Both SNP and sample selection were carried out using VCFtools v0.1.12b (Danecek et al. 2011).
HLA reference panel by Gourraud et al. (2014) (PAG2014)
Gourraud et al. (2014) typed class I HLA-A, -B and -C, and class II HLA-DRB1 and -DQB1 genes of 1266 individuals from 14 different populations in Africa, Europe, Asia, and America. The HLA sequence-based typing was performed with specific polymerase chain reaction amplification of ARS exons followed by Sanger sequencing. Data are available at the dbMHC Web site (http://www.ncbi.nlm.nih.gov/gv/mhc/xslcgi.fcgi?cmd=cellsearch; Helmberg et al. 2014).
Data from Gourraud et al. (2014) are available in the form of HLA allele names per individual. Allele naming for HLA genes follows specific rules (Marsh et al. 2010). To summarize, allele names are composed of a letter indicating the locus, followed by 2−4 numeric fields separated by colons. Each numeric field indicates specific forms of variation: the 1st field distinguishes groups of alleles by serological type, and the following fields distinguish nonsynonymous polymorphisms, synonymous polymorphisms, and noncoding differences, respectively. To obtain SNP genotypes and frequencies from the Sanger sequencing data, we converted all allele names to their associated sequences for ARS encoding exons. Sequences were acquired from the IMGT (i.e., international ImMunoGeneTics information system) database (Robinson et al. 2013), which keeps a well-curated repository of all known HLA allele sequences.
Our analysis was restricted to ARS exons because the HLA typing method used by Gourraud et al. (2014) only probed genetic variation in these specific exons. As a consequence, multiple HLA alleles are compatible with the sequencing results, because the sites that differentiate them are in other exons. This results in what we refer to as an “ambiguous allele call” for an HLA allele (e.g., the allele is identified as B*35:03, but we cannot establish whether it is B*35:03:01 or B*35:03:02, or a group of alleles is attributed to an individual, such as B*35:02/B*35:03/B*35:04). Ambiguous allele calls also may happen when sequencing has low quality at bases that differentiate two alleles. In addition, there are also genotypic ambiguities, which occur when different pairs of alleles are compatible with the sequencing results. For individuals that bear ambiguous alleles, we created a consensus sequence in which ambiguous sites were reported with both possible alleles (e.g., A/T, see Figure S1). In this way, we incorporate the uncertainty associated to the sequence-based typing into downstream analyses.
Although we cannot rule out technical errors in the Sanger sequencing that generated the PAG2014 data (Gourraud et al. 2014), we assume that this method provides the most reliable estimate of HLA alleles (and hence SNP genotypes), and will serve as a standard to estimate the reliability of genotype calls and allele frequencies for the 1000 Genomes data (De Santis et al. 2013).
Genotype comparisons
We initially quantified how well the 1000G and PAG2014 data agreed with respect to genotype calls. Genotypes at each site in each individual were compared between the 1000G data and the PAG2014 data, here considered as a gold standard. In the case of sites with ambiguity (e.g., T/A) in the PAG2014 data, if one of the two possible alleles matched an allele present in the 1000G, we considered this an allele match and PAG2014 was corrected, by attributing the allele present in the 1000G data to the ambiguous site. After correcting the ambiguous sites in PAG2014, we only considered genotypes to be a match if both alleles in 1000G were present in the PAG2014 data, at that site. Throughout this article, sites are numbered according to their position in the ARS exons coding sequences (1−546 at the class I loci and 1−270 at the class II loci).
Allele frequency comparisons
After correcting all possible ambiguities in PAG2014 (as described previously), we calculated allele frequencies for SNPs in both datasets. By comparing the frequency of the reference allele in 1000G to its value in PAG2014, we assessed the accuracy of allele frequency estimation. The reference allele was defined as the allele present in the hg19 build of the reference sequence of the human genome. RefSeq IDs of the reference sequences used for each HLA gene are reported on File S1.
We computed the error in 1000G frequency estimates per site i (FEi) as follows:
where and are the frequency of the reference allele at site i in 1000G and PAG2014, respectively. We also computed the mean absolute error in frequency estimates per gene as a mean of absolute for all sites within a gene (MAE):
where n is the number of SNPs in the gene.
Coverage in 1000G
Sequencing coverage per individual per site was calculated from the 1000 Genomes Project phase I BAM files for the low coverage experiments using the genomeCoverageBed program from BEDTools (Quinlan and Hall 2010). BAM files are available on ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/phase1/data/[sampleID]/alignment/. Only low-coverage BAM files were used to estimate coverage because genotype likelihoods for the data we analyzed (1000 Genomes Project Phase I integrated VCF files) were estimated from this source. Genotype likelihoods were estimated from high coverage exome BAM files only for a minority of sites that were exclusively discovered on the exome experiments, and were not used in the coverage analysis (See Table S1).
Testing for mapping bias
After demonstrating that there is an overestimation of reference allele frequency in the 1000G SNPs (see the section Results), we hypothesized that mapping bias was the underlying cause. To test this hypothesis, we examined whether reads carrying the alternative allele at a SNP are less likely to map to the reference genome than reads carrying the reference allele. First, for each HLA allele present in the PAG2014 dataset, we defined windows of 51 base pairs that were centered on each SNP (25 base pairs upstream and 25 base pairs downstream of the SNP, including non-polymorphic sites). The set of windows centered on a specific SNP was then separated in two groups: (i) those that carry the reference allele at the central site and (ii) those that carry the alternative allele at the central site. Next, all windows were compared with the reference genome (hg19) sequence (the same sequence that was used as an index in the 1000 Genomes Project), and the number of mismatches was counted, excluding the mismatch at the central SNP. If mapping bias was influencing allele frequency estimates, we expected that, for SNP positions with overestimation of the reference allele frequency in the 1000G, the alternative alleles would be flanked by additional alternative alleles (and thus have a greater mismatch count against the reference sequence).
Results
Genotypic mismatch frequency
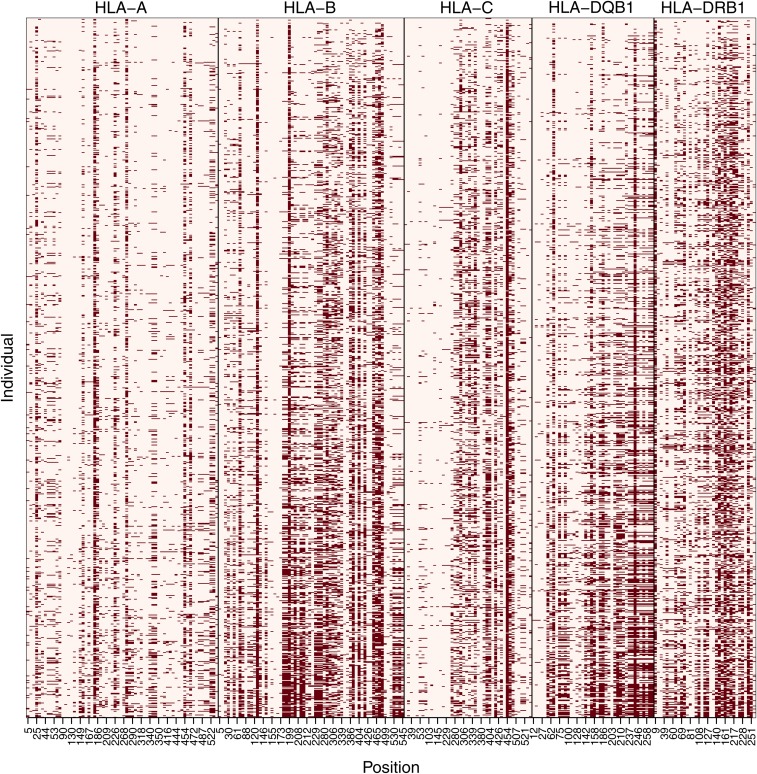
We found that, on average, 18.6% of genotypes were mismatched between 1000G and PAG2014 when individual genotypes for each site in the five classical HLA genes were compared, and exons with greater nucleotide diversity tend to have a greater proportion of genotype mismatches (Figure S2). We also observed that mismatches are specially concentrated on a few sites (Figure 1), with 18.7% of sites concentrating 50% of the mismatches over the five loci we analyzed.
Figure 1.
Genotype mismatches between the 1000G and PAG2014 datasets. Results per polymorphic site (“Position”) and per individual (930 in total). Individuals are ordered by number of mismatches (individuals with less mismatches on top). Sites are numbered according to their position in ARS exons coding sequence. Dark squares indicate mismatches between genotypes in the two datasets. ARS, antigen recognition sites; HLA, human leukocyte antigen.
Reference allele frequency accuracy
Accuracy of estimation of allele frequencies in 1000G was assessed comparing the observed frequency of the reference allele in the 1000G data with that of PAG2014, for both the global dataset (consisting of a pooled set of all individuals) and for each population separately (see Figure S3, Figure S4, Figure S5, Figure S6, and Figure S7). We chose a difference of 0.1 between the frequencies on both datasets as a threshold that determines a “large frequency difference.”
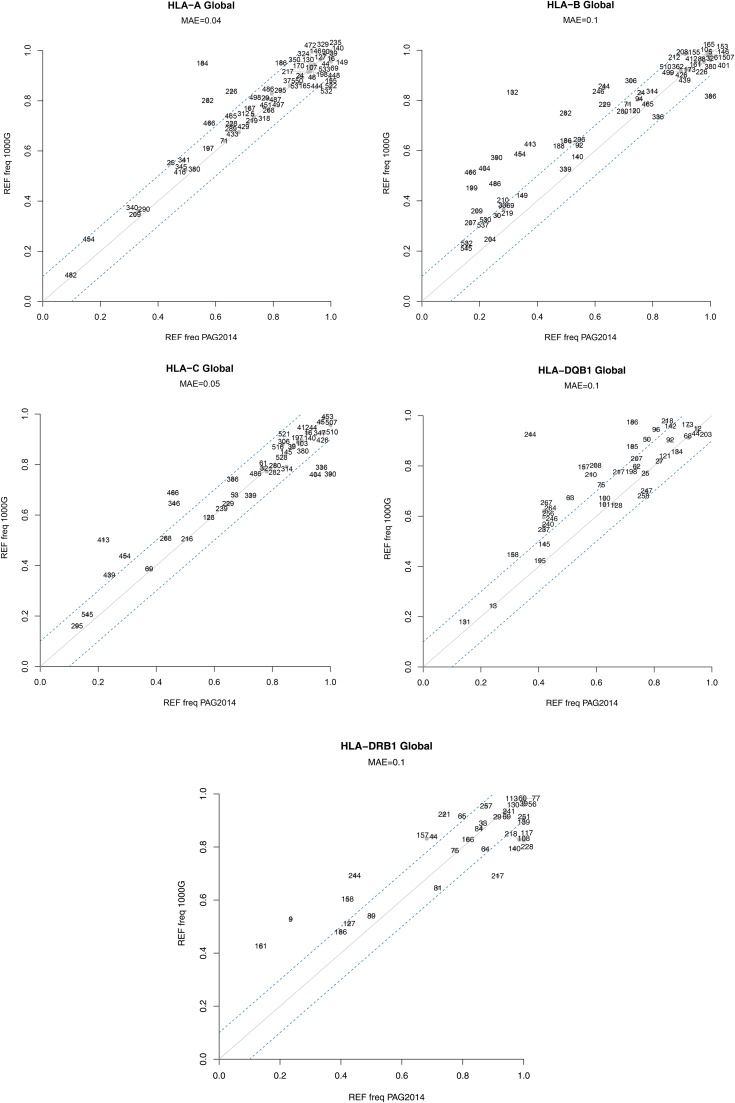
For the global dataset (Figure 2) we found that for HLA-A and -C most SNPs have similar frequency estimates for 1000G and PAG2014, with few large deviations (only 9/66 and 8/44 SNPs with absolute difference in frequencies () larger than 0.1, respectively). The HLA-DQB1 locus shows an intermediate proportion of SNPs with large deviations (10/42 SNPs with ), and HLA-B and HLA-DRB1 show the greatest proportion of sites with large frequency differences between 1000G and PAG2014 (23/64 and 15/35 sites with ). Overall, the mean absolute difference in frequency between SNPs in the 1000G and PAG2014 data are 0.08, and it is greater at the HLA genes with the greatest levels of nucleotide diversity (HLA-B, -DQB1 and -DRB1 all deviate by ).
Figure 2.
REF allele frequency per site in each HLA gene in the 1000 Genomes (1000G) and Sanger sequencing (PAG2014) datasets. Continuous line indicates the expected relationship (i.e., no difference) between 1000G and PAG2014. Dashed lines indicate a ±0.1 deviation from the expected frequency (as estimated from PAG2014 dataset). MAE (mean absolute error) defined in the section Materials and Methods. Numbers indicate site position in ARS exons sequence. REF, reference; ARS, antigen recognition sites; HLA, human leukocyte antigen.
The proportion of genotype mismatches and allele frequency deviations per site are highly correlated (Pearson correlation = 0.86, P < 10−16; Figure S8). However, some SNPs with a high proportion of genotype mismatches have well-estimated allele frequencies. One example is site 465 at HLA-B, in which 44% of genotypes are mismatched, but is only 0.007. Overall, 15 sites have more than 25% mismatched genotypes while showing (see Figure S8). This is possible when the frequency of genotype errors in which the reference allele is overrepresented is similar to the frequency of errors in which the alternative allele is overrepresented.
Allele frequency at the axiom exome genotyping array – Affymetrix:
Because genotyping arrays constitute an additional frequently used resource to genotype SNPs within HLA genes, playing an important role in GWAS studies, we also have investigated the accuracy of allele frequency estimation from this genotyping technology. We estimated allele frequencies from Axiom Exome data, and we found that those allele frequency estimates are as reliable as the ones from the 1000 Genomes NGS data, at the same SNPs (see Figure S9).
Relationship between sequencing coverage and genotypic mismatches
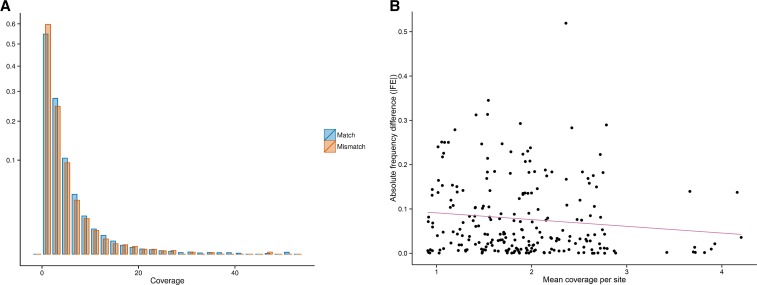
To investigate whether low sequencing coverage could explain genotype mismatches and deviations from expected allele frequencies, we compared sequencing coverage between mismatched and matched genotypes (Figure 3A) and assessed the relationship between coverage and frequency deviation (Figure 3B).
Figure 3.
(A) Distribution of coverage (x-axis) at matched and mismatched genotypes; y-axis is the square root of the relative frequency (Mann-Whitney U one-tailed test, P < 10−16); (B) Relationship between mean coverage (x-axis) and absolute frequency difference (, y-axis) between 1000G and PAG2014 (r = −0.11, P = 0.09). All polymorphic sites from HLA-A, -B, -C, -DRB1, and -DQB1 genes are included in both a and b. HLA, human leukocyte antigen.
Sites with mismatched genotypes have on average lower sequencing coverage than sites with matched genotypes (Figure 3A; Mann-Whitney U one-tailed test P < 10−16). This is the expected relationship if low sequencing coverage explains genotype mismatches between datasets. However, the difference in sequencing coverage between sites with matched and mismatched genotypes is small (mean coverage in matching genotypes is 1.95, and 1.75 in nonmatching genotypes, a difference of 6.2%) and has likely achieved very high significance only due to the large number of observations. Similarly, correlation between allele frequency deviation and sequencing coverage is weak and not significant (Figure 3B; r = −0.11, P = 0.09), although the direction of correlation is in agreement with what would be expected if lower coverage explained larger deviations in frequency estimation. We have also investigated the possible effect of the position of the SNPs relative to exon edges on the allele frequency deviations and found no correlation between those factors (Figure S10). We therefore investigated other factors that may account for errors in genotype calling.
Direction of frequency deviation
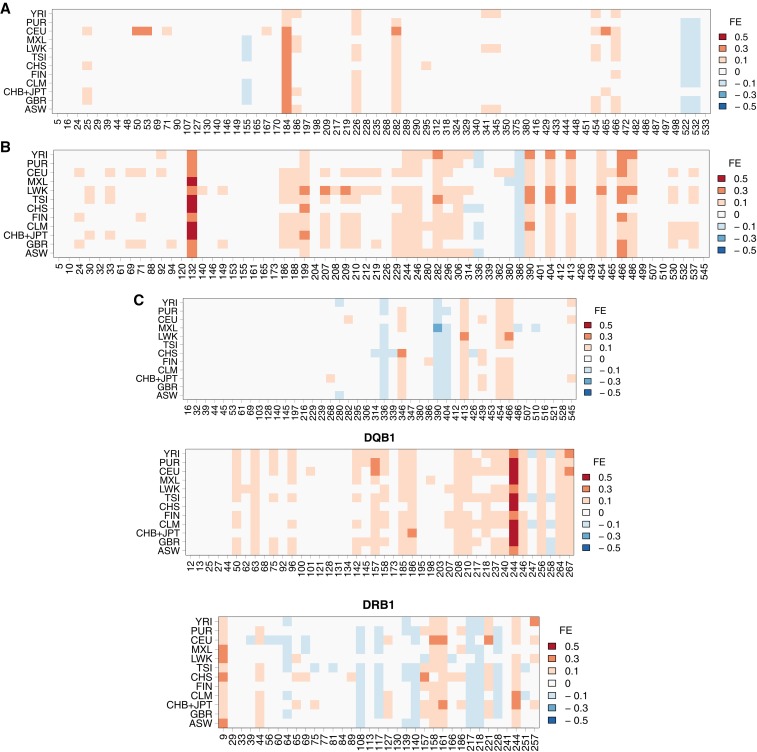
We found that most of the genotype mismatches are caused by miscalling an alternative allele as a reference allele (Table S2). Furthermore, most deviations in allele frequency estimates are in the direction of an overestimation of reference allele frequencies in the 1000 Genomes data (Figure 2). This information is summarized in Figure 4, which shows the location and magnitude of frequency deviations between the 1000G and PAG2014 data.
Figure 4.
Difference in reference allele frequency between 1000G and PAG2014, measured by (see the section Materials and Methods), at each polymorphic site, in each population. Shades of red indicate overestimation of reference allele frequency and shades of blue indicate underestimation of reference allele frequency in 1000G. Full population names are given in Table S3.
The overall shift in the direction of overestimating reference alleles is summarized in Table 1, which shows the number of SNPs with more than 0.1 frequency difference in at least two populations, for each locus. For HLA-A, -B, and -DQB1 most sites with large frequency differences between 1000G and PAG2014 are skewed in the direction of overestimating the reference allele [P = 0.057 for HLA-A and P < 10−4 for HLA-B and -DQB1, binomial test for null hypothesis of equal numbers of deviations in direction of reference (REF) or alternative (ALT)], whereas HLA-C and HLA-DRB1 show no evidence for an excess of large deviations in the direction of reference alleles.
Table 1. Number of sites with overestimation of REF or ALT allele frequency in each HLA locus ( > 0.1 in 2 or more populations).
| A | B | C | DQB1 | DRB1 | |
|---|---|---|---|---|---|
| REF | 11 | 30 | 6 | 22 | 11 |
| ALT | 3 | 2 | 3 | 2 | 11 |
Genomic coordinates of those sites are given in Table S4. HLA, human leukocyte antigen; REF, reference; ALT, alternative.
Testing for mapping bias
We hypothesized that the observed reference allele bias was caused by a lower efficiency in the mapping of reads containing the alternative allele. This is expected under the assumption that the reads carrying the alternative allele on average have more differences with respect to the reference genome (used by the 1000 Genomes Consortium as the index to align NGS reads) than reads carrying the reference allele. In this scenario, some sites would have a stronger bias than others if the alternative alleles in those sites are flanked by additional alternative alleles.
To test this hypothesis, we aligned sequences of all alleles present in PAG2014 to the HLA sequences present in the hg19 build of the reference human genome (the same sequences used for the alignment of reads in the 1000 Genomes Project) and defined windows of 51 base pairs around each SNP. We then quantified the number of differences with respect to the reference genome for windows surrounding (i) REF and (ii) ALT alleles. If REF allele mapping bias is driving errors in frequency estimation, it is expected that sites with an overestimation of REF allele frequency would present the following pattern: windows carrying the REF with fewer differences to the reference genome than sequences centered on the ALT alleles. For sites with well-estimated frequencies, on the other hand, we did not expect such a difference between REF and ALT windows.
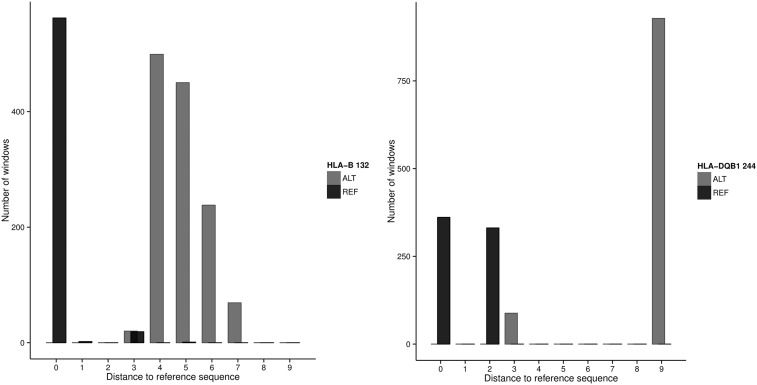
To illustrate this effect, Figure 5 shows the results for the two most extreme cases of frequency deviation shown in Figure 4: site 244 of HLA-DQB1 and site 132 of HLA-B (0.56 and 0.52 absolute increase in REF allele frequency in the 1000 Genomes data with respect to PAG2014). In both cases, ALT windows bear more differences to the reference sequence than REF windows.
Figure 5.
Number of differences to the reference genome at 1860 51-bp windows centered at sites HLA-B 132 and HLA-DQB1 244 with reference (REF) or alternative (ALT) allele at those sites. Windows were defined from all HLA alleles present in the 930 samples from the PAG2014 dataset. HLA, human leukocyte antigen.
These results support the hypothesis that these sites with poorly estimated allele frequencies have their ALT alleles residing in haplotypes with substantially more differences with respect to the reference genome than haplotypes centered on the REF allele, thus accounting for the observed bias.
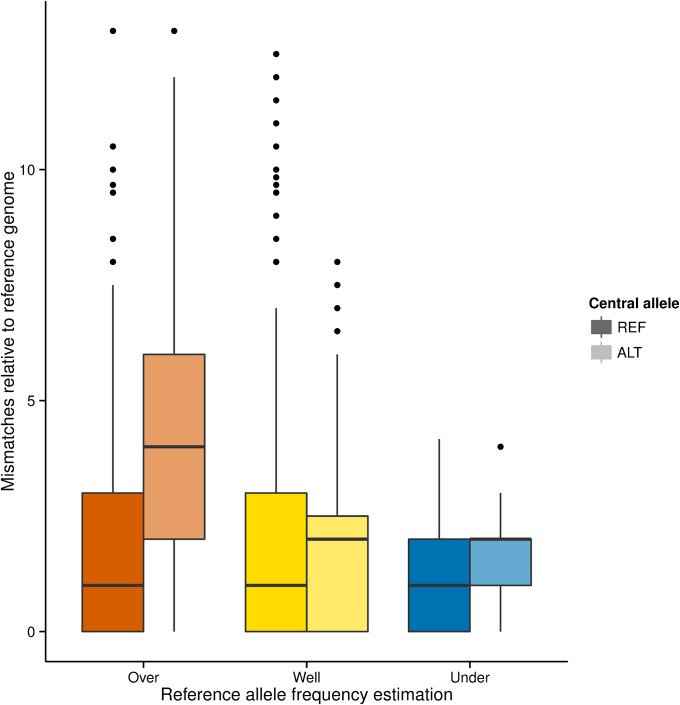
To gain a broader perspective of this issue, we classified SNPs from the HLA loci with REF allele bias (HLA-A, -B, and -DQB1) into three categories: (i) sites at which the REF allele frequency was overestimated, i.e., (“overestimated”); (ii) sites where the REF allele frequency was underestimated, i.e., (“underestimated”); and (iii) sites at which allele frequencies were well estimated (, here referred to as “well estimated”). We compared these three categories of sites with respect to the number of differences relative to the reference genome in REF and ALT windows (Figure 6). We found that the overestimated group has significant excess of differences at alternative allele bearing haplotypes. In this group of SNPs, ALT windows have on average 4.4 other differences relative to the reference genome, whereas those centered on the REF allele have 1.9 differences (excess of differences on windows centered on the ALT allele was tested with a one tailed Mann-Whitney U test; P < 10−16). Sites with well estimated or underestimated REF allele frequency, on the other hand, do not show a similar excess of differences in the haplotypes bearing the ALT allele, although the difference between REF and ALT windows is statistically significant because of the large sample size (well estimated: ALT mean = 1.7; REF mean = 1.8; one tailed Mann-Whitney U test P < 10−16; underestimated: ALT mean = 1.9 ; REF mean = 1.2; one tailed Mann-Whitney U, P < 10−16).
Figure 6.
Number of differences to the reference genome at 51-bp windows centered at each SNP in the HLA-A, -B, and -DQB1 genes. Windows around each SNP were defined from the set of 1860 alleles present in the 930 samples from the PAG2014 dataset. Next, the set of windows was divided in three groups: those centered on SNPs with overestimated, well estimated and underestimated reference allele frequencies (red, yellow and blue boxplots, respectively). Then, each group was divided in two: windows in which the central site contains the reference allele (REF, dark boxplots) and windows centered on an alternative allele (ALT, light colored boxplots). Upper and lower hinges correspond to the 25th and 75th percentiles, horizontal lines represent the median, whiskers are 1.5 times the interquartile range, and outliers are represented by dots. HLA, human leukocyte antigen; SNP, single-nucleotide polymorphism.
Impact of biases in frequency estimation to population genetic statistics
Our analysis was able to identify a subset of SNPs in the HLA genes for which genotype calls and allele frequency estimates from the 1000G showed a high error rate with respect to the PAG2014 dataset. To evaluate the impact of the errors introduced by including these sites in population genetic analyses, we compared the distribution of sample heterozygosity between the sites with low and high error rates. Heterozygosity is defined as for biallelic loci, as is the case for the 1000 Genomes Phase I SNPs, because tri- or quad-allelic SNPs were not reported on Phase I.
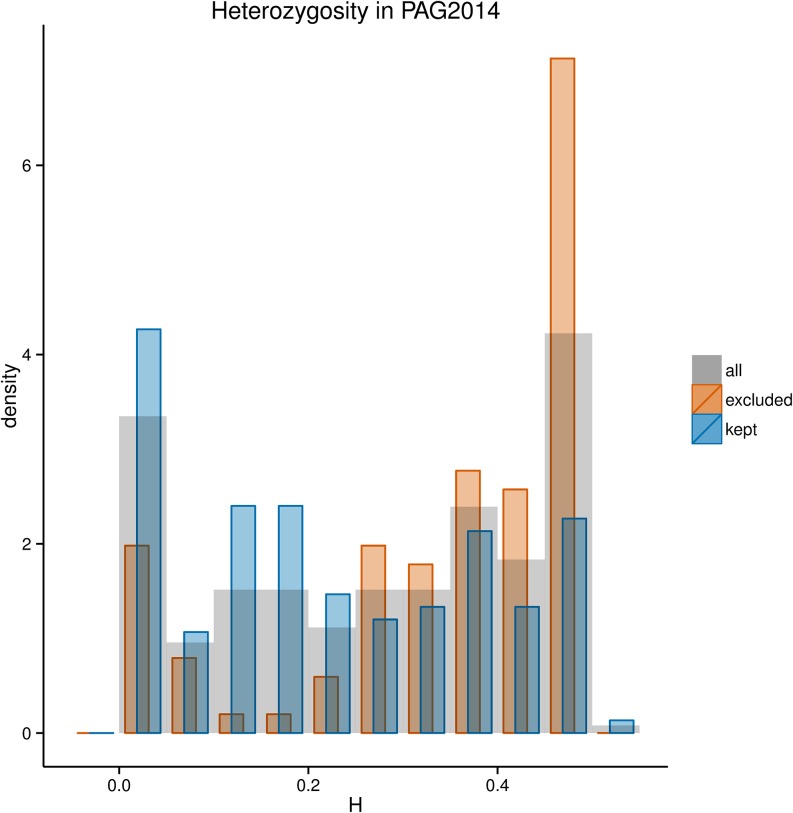
The removal of sites with poor frequency estimates ( in at least two populations) results in a marked change in the distribution of H, with a significant drop in the frequency of sites with large H and a shift in the distribution toward lower values (Figure 7). Note that the H values in Figure 7 are estimated from the PAG2014 data, implying that the high values of H among “excluded” sites are not due to the deviations in allele frequencies generated by NGS errors, but are the true heterozygosities at those sites. These results therefore document that because sites with high heterozygosity tend to have greater deviations from the “true” frequency (i.e., based on the PAG2014 dataset), the removal of poorly estimated sites results in a reduction in H values.
Figure 7.
Heterozygosity of SNPs at HLA genes estimated from the PAG2014 dataset. Orange bars show distribution of heterozygosity at sites with a high error rate in frequency estimation ( in two or more populations). Blue bars show the distribution of heterozygosity after exclusion of SNPs with high error rate. SNP, single-nucleotide polymorphism; HLA, human leukocyte antigen.
The effect of heterozygosity on allele frequency estimation bias
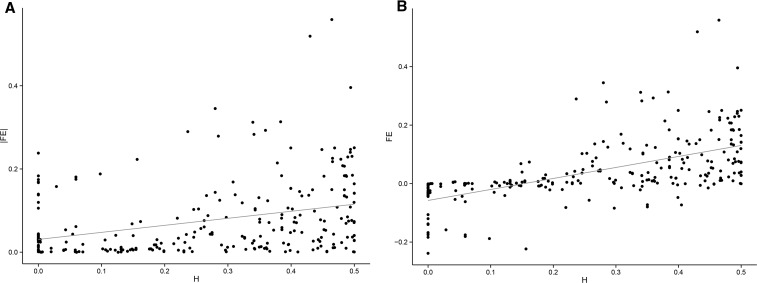
We found an overall positive correlation between SNP heterozygosity and the magnitude of error in allele frequency estimates (Figure 8A; Pearson’s correlation = 0.32; P = 1.938 × 10−7). This result provides further evidence that sites with greater heterozygosity tend to have poorer estimates for allele frequencies in the 1000G. Also, heterozygosity is even more strongly correlated to the deviation in frequency, considering the direction of the deviation (Figure 8B; Pearson’s correlation = 0.59; P < 10−16). Together, these results show that HLA SNPs with greater heterozygosities not only have more errors in frequency estimation but also a stronger bias toward overestimation of REF allele frequency.
Figure 8.
Relationship between SNP heterozygosity (H) and (A) absolute value of deviation (; Pearson’s correlation = 0.32; P = 1.938 × 10−7) or (B) magnitude and direction of deviation (FE; Pearson’s correlation = 0.59; P < 10−16). SNP, single-nucleotide polymorphism.
Discussion
The 1000 Genomes Project data were generated by various sequencing centers, which relied on different sequencing platforms, read lengths, aligners and variant and genotype calling algorithms (The 1000 Genomes Project Consortium 2012), creating challenges to an overall assessment of data reliability. In this study, we specifically examine the performance of NGS-based genotype calls and allele frequency estimates for the highly polymorphic and intensely studied classical HLA genes. We took advantage of the possibility of comparing downstream genotype calls from the 1000 Genomes and HLA typing based on Sanger sequencing for the same set of samples to assess data quality and test hypothesis about possible biases.
We show that the 1000 Genomes SNPs called in the HLA genes have many differences at the genotype level, when compared to results obtained using Sanger sequencing. However, considerably high genotype mismatching is possible with only modest deviations in allele frequencies, and we conclude that for the 1000 Genomes data allele frequency estimates for SNPs at HLA genes are considerably more reliable than the individual genotype calls.
Low coverage did not explain the errors in genotypes and allele frequencies in the 1000 Genomes dataset. Instead, we found evidence that read mapping bias was responsible for those errors. Mapping bias is well known for NGS, and highly polymorphic regions such as HLA genes are especially susceptible to its effects (Nielsen et al. 2011), particularly when a single reference genome is used as an index for the alignment of NGS reads. In this situation, many true variants fail to be identified because they are present in haplotypes that differ from the genome used as index, and thus reads generated from these regions are not aligned and are lost. Together, these results suggest that increasing coverage would not improve allele frequency estimates at those sites if a single reference sequence is still used as index. By mapping to multiple genomes [e.g., using strategies similar to Boegel et al. (2012) or Dilthey et al. (2014)], it would be possible to improve genotype calling and allele frequency estimates.
In our study, HLA-A, -B, and DQB1 show evidence of REF allele mapping bias. The HLA-DRB1 locus, on the other hand, did not present REF allele frequency overestimation, a finding that can be explained by the existence of multiple copies of this gene (both pseudogenes and functional copies), which may result in biases/errors that make REF allele bias comparatively less visible (Degner et al. 2009). The HLA-C locus also shows a weaker REF allele bias, a pattern that may be explained by its lower degree of polymorphism which leads to a decrease in the number of mismatches of reads with respect to the reference genome, thus decreasing the mapping bias.
We provide a list of unreliable SNPs within the HLA genes, defined by us as those with an absolute difference in frequency larger than 0.1 () in two or more populations (Table S4). We show that these unreliable SNPs on average have greater heterozygosities in our gold standard dataset. As a consequence, although filtering out those unreliable sites improves the overall accuracy in allele frequency estimation, it leads to an underestimation of the mean heterozygosity of SNPs in HLA genes, a bias that should be taken into account in downstream analyses. Analyses that require genotype calls at the individual level, including haplotype-based analyses, should be performed with caution when using the data from the 1000 Genomes at HLA genes.
Our results have implications to studies that use SNP data from the 1000 Genomes in other genomic regions with high variability, such as KIR and olfactory receptors. Because HLA loci are the most polymorphic in the human genome, they represent a worst case scenario for mapping bias and subsequent allele frequency estimation errors. We found a significant correlation between SNP heterozygosity and the absolute difference in frequency between 1000 Genomes data and our gold standard. This suggests that in genome-wide studies, SNPs with high heterozygosities, and contained within regions with additional SNPs, have an increased chance of presenting poor frequency estimates.
Acknowledgments
This research was financially supported by grants from São Paulo Research Foundation (FAPESP) and The Brazilian National Council for Scientific and Technological Development (CNPq). D.Y.C.B. was funded by FAPESP scholarships #2012/22796-9 and #2013/12162-5; V.R.C.A. has a FAPESP grant #2014/12123-2, B.D.B. was funded by #2011/12500-2 (FAPESP) and #152676/2011-2 (CNPq); K.N. has a FAPESP grant #2012/09950-9; and D.M. has a FAPESP research grant #12/18010-0 and a CNPq productivity grant #308167/2012-0.
Footnotes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.114.015784/-/DC1
Data available in public repositories: https://github.com/deboraycb/reliability_hla_1000g
Communicating editor: C. R. Marshall
Literature Cited
- Andersen K. G., Shylakhter I., Tabrizi S., Grossman S. R., Happi C. T., et al. , 2012. Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., et al. , 1987. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329: 506–512. [DOI] [PubMed] [Google Scholar]
- Boegel S., Löwer M., Schäfer M., Bukur T., de Graaf J., et al. , 2012. HLA typing from RNA-Seq sequence reads. Genome Med. 4: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., et al. , 1993. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364: 33–39. [DOI] [PubMed] [Google Scholar]
- Chapman S. J., Hill A. V. S., 2012. Human genetic susceptibility to infectious disease. Nat. Rev. Genet. 13: 175–188. [DOI] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G. R., Albers C. a., Banks E., et al. , 2011. The variant call format and VCFtools. Bioinformatics 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis D., Dinauer D., Duke J., Erlich H. A., Holcomb C. L., et al. , 2013. 16(th) IHIW: review of HLA typing by NGS. Int. J. Immunogenet. 40: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner J. F., Marioni J. C., Pai A. A., Pickrell J. K., Nkadori E., et al. , 2009. Effect of read-mapping biases on detecting allele-specific expression from RNA-sequencing data. Bioinformatics 25: 3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilthey, A., C. Cox, Z. Iqbal, M. R. Nelson, and G. McVean, 2014 Improved genome inference in the MHC using a population reference graph. bioRxiv. Available from: http://biorxiv.org/content/early/2014/07/08/006973. Accessed March 20, 2015. [DOI] [PMC free article] [PubMed]
- Erlich R. L., Jia X., Anderson S., Banks E., Gao X., et al. , 2011. Next-generation sequencing for HLA typing of class I loci. BMC Genomics 12: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourraud P.-A., Khankhanian P., Cereb N., Yang S. Y., Feolo M., et al. , 2014. HLA Diversity in the 1000 Genomes Dataset. PLoS One 9: e97282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmberg W., Feolo M., Dunivin R., Hoffman D., 2014. dbMHC.
- Hernandez R. D., Kelley J. L., Elyashiv E., Melton S. C., Auton A., et al. , 2011. Classic selective sweeps were rare in recent human evolution. Science 331: 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns E. M., Factor S. A., Zabetian C. P., Thomson G., Payami H., 2011. Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS One 6: e27109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts A., Feolo M., Helmberg W., 2003. The major histocompatibility complex database, dbMHC. In: National Center for Biotechnology Information NIH, ed. The NCBI Handbook. Bethesda: National Center for Biotechnology Information NIH, p.1–29. [Google Scholar]
- Lappalainen T., Sammeth M., Friedländer M. R., ’t Hoen P. C., Monlong J., et al. , 2013. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler E. M., Gao Z., Pfeifer S., Ségurel L., Auton A., et al. , 2013. Multiple instances of ancient balancing selection shared between humans and chimpanzees. Science 339: 1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E., Rigo K., Hague T., Bérces A., Juhos S., 2013. HLA typing from 1000 genomes whole genome and whole exome Illumina data. PLoS One 8: e78410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. G. E., Albert E. D., Bodmer W. F., Bontrop R. E., Dupont B., et al. , 2010. Nomenclature for factors of the HLA system, 2010. Tissue Antigens 75: 291–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D., Thomson G., 2001. How selection shapes variation of the human major histocompatibility complex: a review. Ann. Hum. Genet. 65: 1–26. [DOI] [PubMed] [Google Scholar]
- Nielsen R., Paul J. S., Albrechtsen A., Song Y. S., 2011. Genotype and SNP calling from next-generation sequencing data. Nat. Rev. Genet. 12: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R., Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Halliwell J. A., McWilliam H., Lopez R., Parham P., et al. , 2013. The IMGT/HLA database. Nucleic Acids Res. 41: D1222–D1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Pos W., Wucherpfennig K. W., 2014. Molecular mechanisms for contribution of MHC molecules to autoimmune diseases. Curr. Opin. Immunol. 31C: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium , 2012. An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen T. J., Salzberg S. L., 2012. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat. Rev. Genet. 13: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. D., Kellis M., 2012. Evidence of abundant purifying selection in humans for recently acquired regulatory functions. Science 337: 1675–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]