Abstract
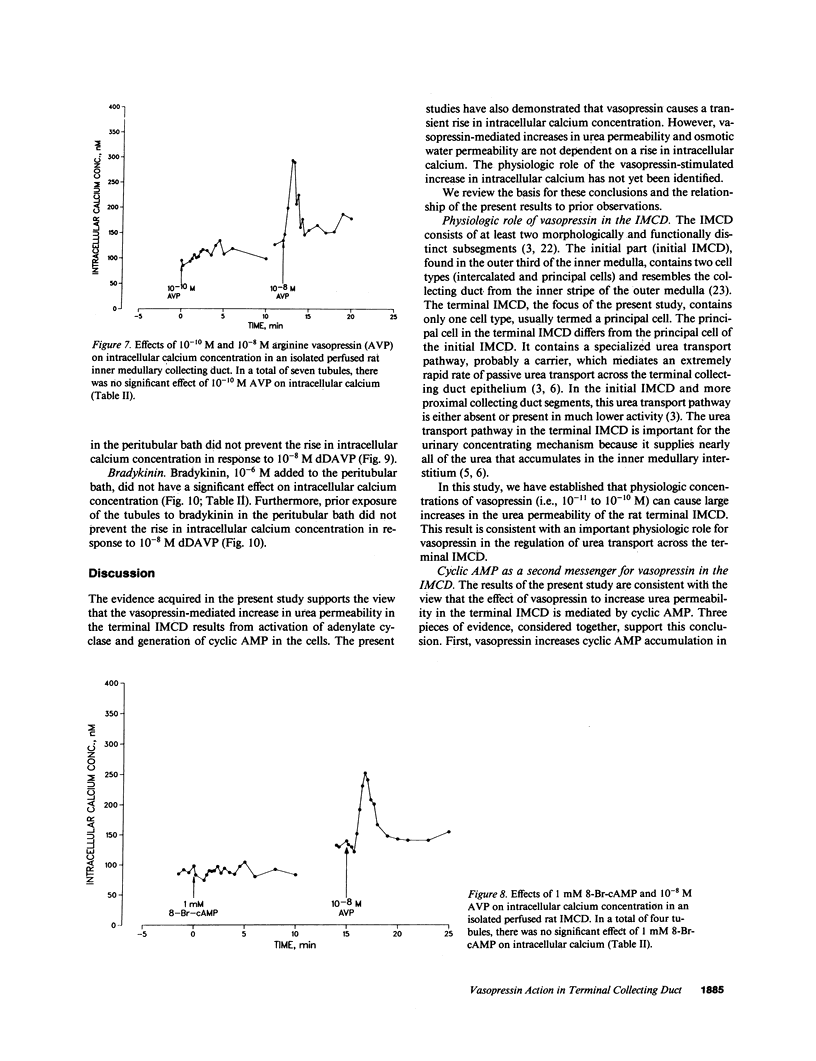
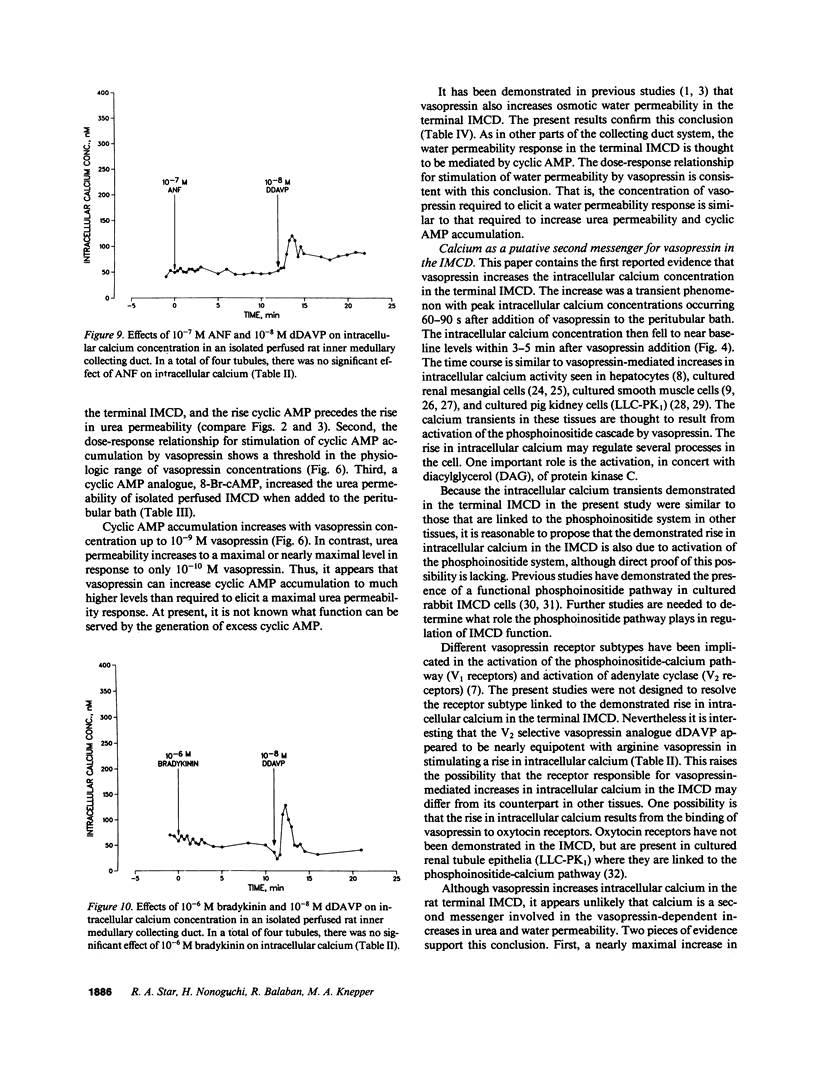
Vasopressin increases both the urea permeability and osmotic water permeability in the terminal part of the renal inner medullary collecting duct (terminal IMCD). To identify the second messengers that mediate these responses, we measured urea permeability, osmotic water permeability, intracellular calcium concentration, and cyclic AMP accumulation in isolated terminal IMCDs. After addition of vasopressin, a transient rise in intracellular calcium occurred that was coincident with increases in cyclic AMP accumulation and urea permeability. Half-maximal increases in urea permeability and osmotic water permeability occurred with 0.01 nM vasopressin. The threshold concentration for a measurable increase in cyclic AMP accumulation was approximately 0.01 nM, while measurable increases in intracellular calcium required much higher vasopressin concentrations (greater than 0.1 nM). Exogenous cyclic AMP (1 mM 8-Br-cAMP) mimicked the effect of vasopressin on urea permeability but did not produce a measurable change in intracellular calcium concentration. Conclusions: (a) Cyclic AMP is the second messenger that mediates the urea permeability response to vasopressin in the rat terminal IMCD. (b) Vasopressin increases the intracellular calcium concentration in the rat terminal IMCD, but the physiological role of this response is not yet known.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Skorecki K. L., Kreisberg J. I., Cheung J. Y. Vasopressin increases cytosolic free calcium concentration in glomerular mesangial cells. Am J Physiol. 1986 Jul;251(1 Pt 2):F94–102. doi: 10.1152/ajprenal.1986.251.1.F94. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Vasopressin increases cytosolic free calcium in LLC-PK1 cells through a V1-receptor. Am J Physiol. 1987 Aug;253(2 Pt 2):F328–F332. doi: 10.1152/ajprenal.1987.253.2.F328. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Cytosolic free calcium levels in monolayers of cultured rat aortic smooth muscle cells. Effects of angiotensin II and vasopressin. J Biol Chem. 1985 Jul 5;260(13):7836–7842. [PubMed] [Google Scholar]
- Clapp W. L., Madsen K. M., Verlander J. W., Tisher C. C. Intercalated cells of the rat inner medullary collecting duct. Kidney Int. 1987 May;31(5):1080–1087. doi: 10.1038/ki.1987.111. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of calcium and phosphoinositides in the actions of certain hormones and neurotransmitters. J Clin Invest. 1985 Jun;75(6):1753–1757. doi: 10.1172/JCI111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hassid A., Pidikiti N., Gamero D. Effects of vasoactive peptides on cytosolic calcium in cultured mesangial cells. Am J Physiol. 1986 Dec;251(6 Pt 2):F1018–F1028. doi: 10.1152/ajprenal.1986.251.6.F1018. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Good D. W., Burg M. B. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985 Dec;249(6 Pt 2):F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Roch-Ramel F. Pathways of urea transport in the mammalian kidney. Kidney Int. 1987 Feb;31(2):629–633. doi: 10.1038/ki.1987.44. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Balaban R. S. Fluorescence emission spectroscopy of 1,4-dihydroxyphthalonitrile. A method for determining intracellular pH in cultured cells. Biophys J. 1985 Sep;48(3):499–508. doi: 10.1016/S0006-3495(85)83805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz I., Star R., Balaban R. S., Garvin J. L., Knepper M. A. Spontaneous luminal disequilibrium pH in S3 proximal tubules. Role in ammonia and bicarbonate transport. J Clin Invest. 1986 Oct;78(4):989–996. doi: 10.1172/JCI112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Nonoguchi H., Knepper M. A., Manganiello V. C. Effects of atrial natriuretic factor on cyclic guanosine monophosphate and cyclic adenosine monophosphate accumulation in microdissected nephron segments from rats. J Clin Invest. 1987 Feb;79(2):500–507. doi: 10.1172/JCI112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kudo L. H. Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct. Kidney Int. 1982 Nov;22(5):485–491. doi: 10.1038/ki.1982.201. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Knepper M. A. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest. 1987 Jan;79(1):138–147. doi: 10.1172/JCI112774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. M., Nonoguchi H., Knepper M. A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987 Nov;253(5 Pt 2):F823–F832. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Hruska K. A., Morrison A. R. Bradykinin stimulates increased intracellular calcium in papillary collecting tubules of the rabbit. Biochem Biophys Res Commun. 1986 Jan 14;134(1):299–304. doi: 10.1016/0006-291x(86)90562-0. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Morrison A. R. Bradykinin-induced changes in phosphatidyl inositol turnover in cultured rabbit papillary collecting tubule cells. J Clin Invest. 1985 Sep;76(3):978–984. doi: 10.1172/JCI112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M. J., Weinberg J. M. Vasopressin-induced increases of cytosolic calcium in LLC-PK1 cells. Am J Physiol. 1986 Dec;251(6 Pt 2):F1090–F1095. doi: 10.1152/ajprenal.1986.251.6.F1090. [DOI] [PubMed] [Google Scholar]
- Troyer D. A., Schwertz D. W., Kreisberg J. I., Venkatachalam M. A. Inositol phospholipid metabolism in the kidney. Annu Rev Physiol. 1986;48:51–71. doi: 10.1146/annurev.ph.48.030186.000411. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]



