Significance
A recombinant soluble version of the transmembrane death ligand Apo2L/TRAIL has shown compelling preclinical results as a potential cancer therapeutic, but studies in cancer patients have demonstrated little efficacy. Supported membrane display of Apo2L/TRAIL, to mimic the endogenous ligand more faithfully, markedly augments receptor clustering and apoptosis stimulation in cancer cells. Covalent attachment of Apo2L/TRAIL to the surface of liposomes offers a therapeutically tractable approach to membrane display that substantially increases tumor exposure, caspase activation, and antitumor potency. These findings open new avenues for clinical investigation of Apo2L/TRAIL as a cancer therapeutic and may apply to other members of the TNF superfamily, such as FasL and CD70, which are expressed on immune-cell surfaces and are important candidates for cancer immunotherapy.
Keywords: cell death, membrane display, caspase, apoptosis, death receptor
Abstract
TNF superfamily death ligands are expressed on the surface of immune cells and can trigger apoptosis in susceptible cancer cells by engaging cognate death receptors. A recombinant soluble protein comprising the ectodomain of Apo2 ligand/TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) has shown remarkable preclinical anticancer activity but lacked broad efficacy in patients, possibly owing to insufficient exposure or potency. We observed that antibody cross-linking substantially enhanced cytotoxicity of soluble Apo2L/TRAIL against diverse cancer cell lines. Presentation of the ligand on glass-supported lipid bilayers enhanced its ability to drive receptor microclustering and apoptotic signaling. Furthermore, covalent surface attachment of Apo2L/TRAIL onto liposomes—synthetic lipid-bilayer nanospheres—similarly augmented activity. In vivo, liposome-displayed Apo2L/TRAIL achieved markedly better exposure and antitumor activity. Thus, covalent synthetic-membrane attachment of a cell-surface ligand enhances efficacy, increasing therapeutic potential. These findings have translational implications for liposomal approaches as well as for Apo2L/TRAIL and other clinically relevant TNF ligands.
Apoptosis is a mode of regulated cell death that is critical for the elimination of unwanted cells in metazoans (1). Extensive efforts have been aimed at developing therapeutic strategies to reactivate apoptosis in cancer cells (2–4). Two distinct signaling pathways converge on “executioner” caspases -3 and -7 to trigger apoptosis: the intrinsic pathway, controlled by Bcl-2 (B-cell lymphoma 2) family members (5), and the extrinsic pathway, controlled by extracellular death ligands (6, 7). Certain immune-effector cells express surface death ligands such as TNFα, FasL, and Apo2 ligand/TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) to engage cognate death receptors on susceptible target cells. Ligation promotes death receptor microclustering, which drives recruitment and activation of caspase-8 to initiate apoptosis (7–9). Although significant clinical progress has been made with small-molecule Bcl-2 antagonists to reactivate the intrinsic pathway in tumors (2), efforts to engage the extrinsic pathway with protein-based proapoptotic receptor agonists (PARAs) have been disappointing to date (10–14). A leading strategy is to target death receptor 4 (DR4) and/or DR5, which are overexpressed on various tumors. A recombinant soluble version of the cognate ligand, human Apo2L/TRAIL (dulanermin), and several agonistic anti-DR4 or anti-DR5 antibodies have been studied in preclinical models and in patients (10–16). Despite remarkable preclinical efficacy, only rare clinical responses have been observed. Potential reasons for the lack of clinical activity include insufficient tumor exposure (16, 17) and/or weak engagement of the extrinsic pathway (10, 13). Such PARAs have been relatively well tolerated in clinical trials, suggesting there might be a therapeutic window for increasing tumor exposure and/or potency of these agents (18). Because recombinant soluble Apo2L/TRAIL more faithfully resembles the endogenous ligand, we used it as a starting point for augmenting efficacy.
Results
Cross-Linking of Apo2L/TRAIL Enhances Activity Against a Wide Range of Cancer Cell Lines.
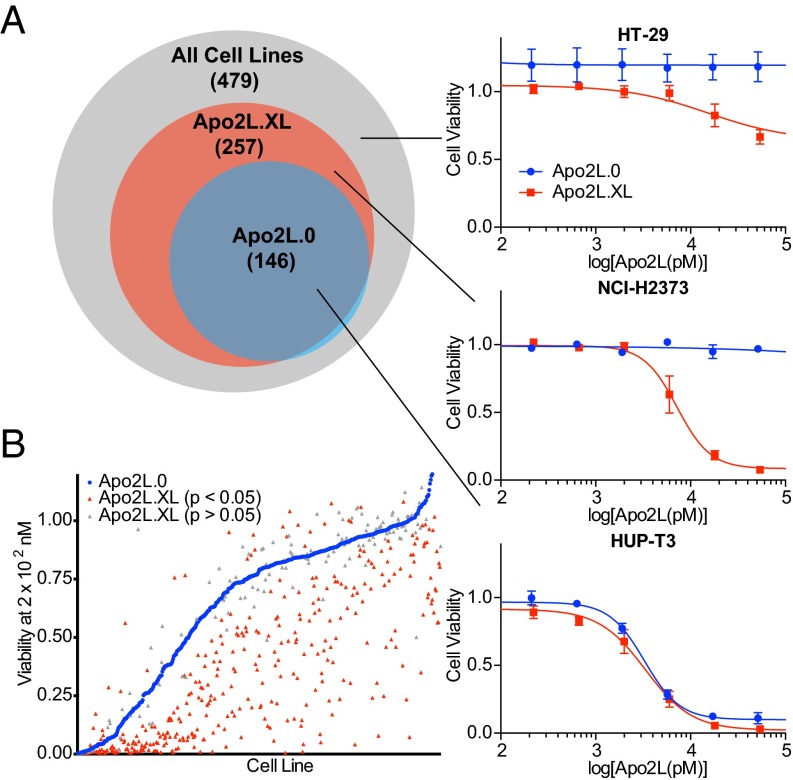
Apo2L/TRAIL cross-linking can increase proapoptotic activity (19, 20); however, it remains unclear how broadly this might apply to different cancer types. We therefore compared activity of soluble Apo2L/TRAIL (herein Apo2L.0) with that of a FLAG-epitope-tagged, antibody-cross-linked version (herein Apo2L.XL) across a panel of 479 cell lines from a wide range of human malignancies (Dataset S1). Whereas Apo2L.0 reduced viability by at least 50% in 146 (30%), Apo2L.XL did so in 257 (54%) of the cell lines (Fig. 1A). Furthermore, Apo2L.XL induced significantly more extensive death than did Apo2L.0 in 380 (79%) of the cell lines (Fig. 1B). A number of cell lines seemed unexpectedly to be more sensitive to Apo2L.0 vs. Apo2L.XL. However, further analysis of the six most divergent of these cell lines individually showed that they were either similarly sensitive to both ligand variants or more sensitive to Apo2L.XL (Fig. S1), indicating that the apparently greater sensitivity to Apo2L.0 likely reflects experimental variation in the high-throughput analysis of a large cell line panel. In agreement with previous findings, the sensitivity to Apo2L.XL, either in absolute terms or relative to that of Apo2L.0, was not more strongly correlated with the expression levels of DR4 or DR5 than sensitivity to Apo2L.0 (SI Materials and Methods). Thus, whereas responsiveness to Apo2L/TRAIL requires at least some expression of DR4 or DR5, variation in receptor levels does not strongly predict changes in sensitivity. Nevertheless, these results suggest that increasing potency might improve the efficacy of Apo2L/TRAIL against a wide spectrum of cancers.
Fig. 1.
Cross-linking of Apo2L/TRAIL augments activity against a wide range of cancer cell lines. Human cancer cell lines were treated with concentrations ranging from 0 to 5 × 102 nM of either Apo2L.0 or cross-linked Apo2L/TRAIL (Apo2L.XL). Cell viability was measured after 72 h of treatment. (A) Of the 479 cell lines tested, 146 showed at least a 50% decrease in cell viability in response to Apo2L.0 (blue circle), and 257 showed the same in response to Apo2L.XL (red circle). Representative cell lines of different populations are highlighted in detail, with fitted dose–response curves. Error bars denote SEM; n = 3 per cell line for Apo2L.0 treatments and n = 4 per cell line for Apo2L.XL treatments. (B) Cell viability in response to 2 × 102 nM of Apo2L.0 (blue points) or Apo2L.XL (red or gray points). Red points denote values for Apo2L.XL that vary from corresponding Apo2L.0 values by a statistically significant degree (P < 0.05), and gray points denote values for which P > 0.05.
Membrane Display of Apo2L/TRAIL Augments Proapoptotic Activity.
A number of approaches to increase oligomerization of Apo2L/TRAIL have been reported (21, 22). However, a key caveat with these is the addition of significant exogenous peptide sequences likely to cause immunogenicity in humans. We reasoned instead that it might be possible to increase potency by displaying recombinant soluble human Apo2L/TRAIL on lipid bilayers to mimic the native cell-surface protein. To create a minimally modified variant that could be covalently attached to other moieties, we leveraged X-ray crystallographic data (23) and mutagenesis results (24). Based on these analyses, we selected a specific variant of Apo2L.0 (amino acids 114–281) with Cys in place of Lys-179 (Apo2LK179C). For supported membrane display, we attached a biotin moiety to the sulfhydryl of Cys-179 via maleimide chemistry. Next, we deposited supported membranes (25–27) containing 0.1 mol % biotinylated lipids onto glass. We used Alexa Fluor 647-labeled streptavidin to couple biotinylated phospholipids and biotinylated Apo2L/TRAIL and used the fluorescent streptavidin to track the membrane-displayed ligand (Fig. 2A). NB-7 neuroblastoma cells express DR5 but lack endogenous DR4 and caspase-8 and resist Apo2L/TRAIL; however, their transduction with GFP-tagged caspase-8 confers marked sensitivity to Apo2L/TRAIL (28). Engagement of NB-7GFP-C8 cells with membrane-displayed Apo2L/TRAIL (herein Apo2L.M) (Fig. 2B, Top) induced lateral ligand transport across the supported membrane, leading to Apo2L.M accumulation at the center of the intermembrane interface (Fig. 2B, Middle, Fig. S2A, and Movie S1). Lateral ligand transport was followed by recruitment of caspase-8 from the cytosol to Apo2L.M clusters (Fig. 2B, Bottom and Movie S1) and subsequent cell-membrane blebbing, consistent with apoptosis. After blebbing had begun, one fraction of caspase-8 was localized at the membrane interface, and a second fraction appeared in cytosolic pockets above the intermembrane junction (Fig. 2B, Bottom and Movie S1). Upon engagement of parental NB-7 cells with Apo2L.M, the same lateral transport of Apo2L.M was observed (Fig. S2A). Fixation and staining of these cells with an anti–DR5-specific antibody revealed colocalization between DR5 and Apo2L.M (Fig. 2C), confirming receptor ligation. However, the parental cells did not display membrane blebbing, verifying resistance to Apo2L.M in the absence of caspase-8. The pan-caspase inhibitor zVAD-fmk did not prevent lateral transport of Apo2L.M-DR5 microclusters or caspase-8 recruitment, but did block cell blebbing and prolonged Apo2L.M lateral transport (Fig. 2D and Fig. S2A). Without Apo2L.M, neither caspase-8 recruitment nor blebbing occurred (Fig. S2B), confirming ligand-dependent DR5 activation. Thus, Apo2L.M induces DR5 microclustering independent of caspase-8 or downstream signaling but requires caspase-8 activity for apoptosis induction. This finding is distinct from earlier data implicating both caspase-8 and Fas-associated protein with death domain (FADD) in clustering of the death receptor Fas in response to its cognate ligand, FasL (8). To further examine the importance of caspase-8 for Apo2L/TRAIL-induced death receptor clustering, we treated mutant Jurkat cell lines deficient in either caspase-8 or FADD expression relative to parental Jurkat cells (Fig. S2C) with 1 μg/mL Apo2L/TRAIL (biotinylated and labeled as above with fluorescent streptavidin). Regardless of caspase-8 or FADD expression, Apo2L/TRAIL clusters were readily visible on the cell surface immediately after addition, and these clusters grew in size over 45 min, although nuclear fragmentation indicative of apoptosis was only observed in parental Jurkat cells expressing both caspase-8 and FADD (Fig. S2D). Size-exclusion chromatography supported these findings, in that Apo2L/TRAIL stimulation induced a shift of DR5 to higher-molecular-weight fractions in all Jurkat clones, although caspase-8 was only recruited to these fractions in the parental cells, which express both FADD and caspase-8 (Fig. S2E). These data suggest a differential involvement of FADD and caspase-8 in ligand-induced clustering of DR4 and DR5 compared with Fas.
Fig. 2.
Membrane display of Apo2L/TRAIL augments proapoptotic activity. (A) Scheme illustrating generation of membrane-displayed Apo2L/TRAIL (Apo2L.M). Biotinylated Apo2L/TRAIL is linked to a supported membrane via fluorescent streptavidin. Death receptor-expressing cells are then reacted with this surface and their response is observed via live cell fluorescence microscopy. DISC, death-inducing signaling complex. (B) NB-7GFP-C8 cells were engaged with Apo2L.M. Cells were identified using bright field microscopy; the corresponding fields were imaged in the Apo2L.M (Alexa Fluor 647) and caspase-8 (GFP) channels, and the three fields were overlaid to examine colocalization between Apo2L.M and caspase-8. (C) Parental NB-7 cells were engaged with Apo2L.M then fixed and immunostained for DR5. Bright field, Apo2L.M (Alexa Fluor 647), and DR5 (Alexa Fluor 488) channels were imaged. (D) NB-7GFP-C8 cells were pretreated with the pan-caspase inhibitor zVAD-fmk and the bright-field, Apo2L.M, and caspase-8 channels were imaged. (E–G) HT-29 cells were treated with the noted reagents and analyzed for caspase-8 activity after 4 h (E), caspase-3/7 activity after 6 h (F), or cell viability after 24 h (G). Error bars denote SEM, n = 3 per treatment.
To examine the potency of membrane-displayed ligand, we used HT-29 human colorectal carcinoma cells, which express DR4, DR5, and caspase-8, yet resist Apo2L.0-induced apoptosis (Fig. 1A, top inset). As expected, Apo2L.0 did not stimulate significant caspase activation or cell death. In contrast, Apo2L.M induced fourfold higher caspase-8 activity (Fig. 2E), 10-fold higher caspase-3/7 activity (Fig. 2F), and an 85% decrease in cell viability (Fig. 2G). The integrin-binding cyclic Arg-Gly-Asp (RGD) peptide, used as a control to allow cell attachment to supported membranes (29), had no proapoptotic activity. Apo2L.M similarly induced caspase activation and death in KPP pancreatic adenocarcinoma cells, whereas Apo2L.0 did not (Fig. S3 A–C) when used at similar levels based on detection by immunoblot (Fig. S3D). Thus, membrane display substantially augments the proapoptotic potency of Apo2L/TRAIL.
Liposome Attachment of Apo2L/TRAIL Yields Functional Ligand That Can Induce Apoptosis in Tumors.
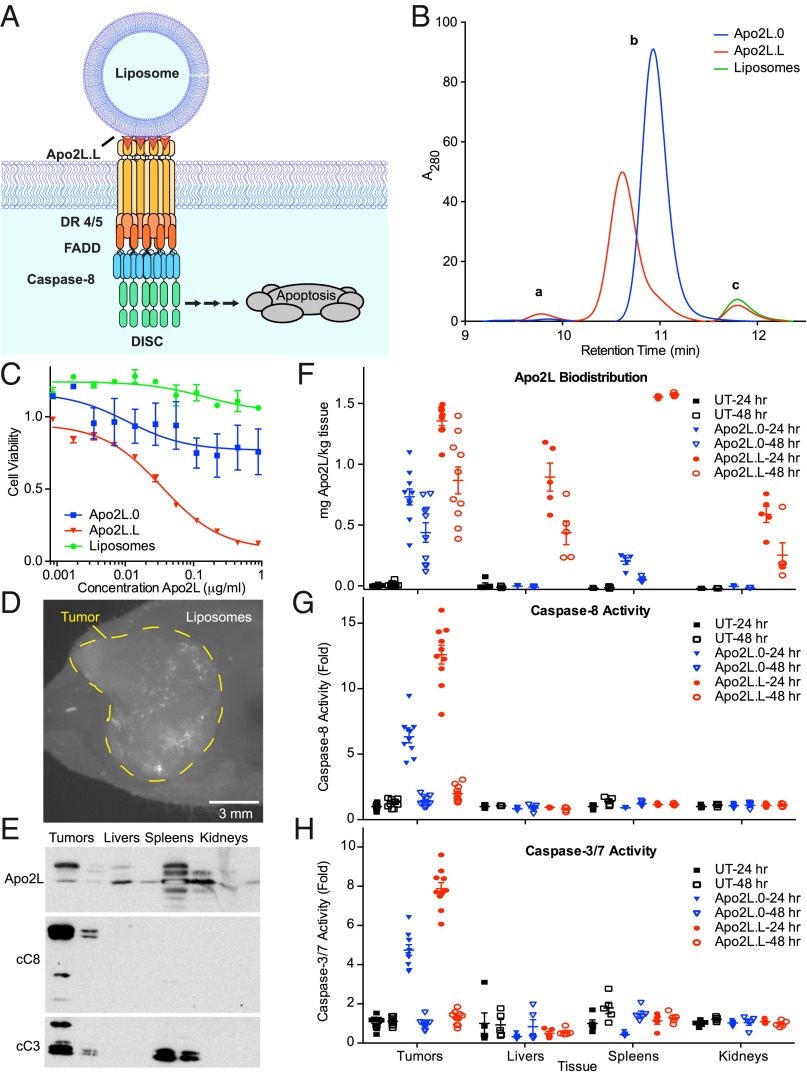
We reasoned that display of Apo2L/TRAIL on liposomal surfaces (Fig. 3A) might provide a more translatable approach. Polyhistidine-tagged Apo2L/TRAIL can be linked to liposomes via noncovalent nickel coordination (30). However, polyhistidine modification of Apo2L/TRAIL causes uncontrolled aggregation and hepatotoxicity (19). To circumvent aggregation and maximize stability, we covalently attached Apo2LK179C directly to liposomes. We prepared liposomes containing ∼10 mol % of PEG-modified lipids, because such “stealth” liposomes possess improved serum stability (31, 32). Moreover, to enable covalent attachment via Cys-179, we derivatized 50% of the PEGylated lipids with maleimide. Finally, to facilitate in vivo visualization of liposome-displayed Apo2L/TRAIL (herein Apo2L.L), we included 0.1 mol % fluorescent lipids. Dynamic light scattering indicated that the liposomes ranged in diameter from ∼100–140 nm. Immunoblot analysis of Apo2L.L showed an approximate 3-kDa shift in molecular weight (MW) (Fig. S4A); this corresponds to the mass of DSPE-mPEG(2000)-maleimide, consistent with lipidation of Apo2L/TRAIL. SDS-HPLC analysis revealed three peaks (Fig. 3B and Fig. S4B). By comparison with Apo2L.0 and bare liposomes, as well as to DTT-reduced samples (SI Materials and Methods), we assigned these as follows: (i) lipidated dimer, (ii) lipidated monomer, and (iii) free fluorescent lipids. Furthermore, by titrating Apo2L.0 and comparing the integrated area under the curves of the i and ii peaks, we were able to quantify Apo2L/TRAIL protein and assess the degree of lipidation. Based on this analysis, ∼70% of the starting protein was retained after lipidation and purification, and all detected peaks corresponded to the mass of lipidated Apo2L/TRAIL (Fig. S4B). These results confirmed efficient attachment of Apo2LK179C to liposomes. Whereas bare liposomes and Apo2L.0 had minimal activity against HT-29 cells, Apo2L.L induced a strong, dose-dependent decrease in cell viability (Fig. 3C). Thus, liposomal display of Apo2L/TRAIL markedly increases potency, recapitulating presentation on glass-supported membranes.
Fig. 3.
Liposome attachment of Apo2L/TRAIL yields functional, membrane-displayed ligand that can induce apoptosis in tumors. (A) Scheme illustrating generation of liposome-displayed Apo2L/TRAIL (Apo2L.L). Liposomes are decorated with Apo2L/TRAIL via covalent cysteine–maleimide bonds. (B) SDS-HPLC chromatograms of Apo2L.0 (blue), Apo2L.L (red), and bare liposomes (green). (C) HT-29 cells were treated with various concentrations of bare liposomes (green), Apo2L.0 (blue), or Apo2L.L (red) for 24 h and viability was measured. Error bars denote SEM, n = 3 per treatment. (D) Within COLO205 tumors implanted s.c. into nude mice, fluorescent liposomes were visible 6 h after injection via tail vein i.v. (E) Tumors, livers, spleens, and kidneys were collected from nude mice bearing s.c. COLO205 xenografts, homogenized, and assessed for Apo2L/TRAIL, cleaved caspase-8, and cleaved caspase-3, by immunoblot. (F–H) Nude mice bearing COLO205 xenografts (n = 10 per group) were treated with a single 25 mg/kg dose of Apo2L.0 or Apo2L.L via i.p. for 24 or 48 h then killed. Tumors from all animals and livers, spleens, and kidneys from five animals per group were collected and homogenized. Tissues were assessed for Apo2L/TRAIL using ELISA (F), caspase-8 activity (G), and caspase-3/7 activity (H). Error bars denote SEM.
To test tumor exposure, we first used the COLO205 xenograft model, previously shown to be sensitive to Apo2L.0. We hypothesized that efficient delivery of liposome-displayed ligand would generate at least as potent a proapoptotic response as would the soluble ligand, thus allowing us to gauge both tumor exposure and agonistic activity of Apo2L.L. We implanted COLO205 human colorectal carcinoma cells s.c. into nude mice, allowed tumors to grow to ∼250 mm3, injected 20 mg/kg Apo2L.L i.v., and assessed systemic liposome distribution after either one or two doses 24 h apart. We detected fluorescence in the blood vessels of ears and legs within minutes of injection, and signal persisted in both vasculature and tumors for several hours (Fig. 3D). Six hours after the first or second injection, we collected the tumors, spleens, livers, and kidneys—tissues expected to be susceptible to Apo2L/TRAIL or to mediate liposome degradation (19, 31, 32). Immunoblot analysis revealed Apo2L/TRAIL in most of the tissues, migrating as two bands that corresponded by MW to lipidated and nonlipidated protein; additional bands, possibly representing cleaved Apo2L/TRAIL, appeared in the spleen (Fig. 3E). Importantly, we detected cleaved (activated) caspase-8 (p41/43 and p18) only in tumors. Cleaved (activated) caspase-3 (p17/19) appeared both in tumors and spleens; however, it was present also in spleens from untreated mice (Fig. S5), suggesting it was caused by natural splenocyte turnover. Thus, Apo2L.L gains significant access into tumors and normal tissues. In keeping with the relative selectivity of Apo2L/TRAIL for malignant vs. normal cells (33–35), Apo2L.L induces caspase activation in tumors, but not in ligand-exposed normal tissues.
To assess the response to Apo2L.0 vs. Apo2L.L, we treated COLO205- tumor-bearing animals (10 mice/group) with a single i.p. injection of 25 mg/kg Apo2L.0 or Apo2L.L for 24 or 48 h and compared these animals to untreated controls. All tumors were collected at the end of the study, as well as livers, spleens, and kidneys from n = 5 animals per group. To examine whether liposomal presentation improves in vivo residence time, we analyzed tissue extracts by a specific, quantitative Apo2L/TRAIL ELISA. In agreement with the immunoblot results, we detected Apo2L.L in all tissues including tumors, whereas Apo2L.0 was present only in tumors (Fig. 3F), and at lower levels than Apo2L.L, likely due to the short half-life of ∼5 min of Apo2L.0 in mouse serum (16). However, despite the high levels of Apo2L.L, observed especially in the spleen, significant caspase-8 (Fig. 3G) and caspase-3/7 (Fig. 3H) induction was only detected in the tumors of treated animals. Furthermore, Apo2L.L induced approximately twofold more tumor caspase-8 and caspase-3/7 activity than did Apo2L.0, suggesting that liposome display of Apo2L/TRAIL may improve its in vivo potency, even toward tumors that are sensitive to Apo2L.0.
Liposomal Display of Apo2L/TRAIL Enhances Its Pharmacodynamic Profile and Antitumor Efficacy.
To examine more stringently whether the display of Apo2L/TRAIL on liposome surfaces augments its proapoptotic activity, we turned to a tumor xenograft model that was previously shown to be resistant to Apo2L.0, namely HT-29 colorectal carcinoma. Consistent with the COLO205 data, we observed fluorescence in s.c. HT-29 tumors 6 h after a single i.v. injection of Apo2L.L (Fig. S6A). We detected higher amounts of Apo2L.L compared with Apo2L.0 at 24 and 48 h after treatment (Fig. 4A). Furthermore, whereas Apo2L.0 induced little caspase-8 activation, Apo2L.L induced significant and durable caspase-8 activity, with higher amounts at 48 vs. 24 h (Fig. 4B). This suggests that the kinetics of caspase activation by Apo2L.L may be different in the COLO205 model and the HT-29 model. Nevertheless, in both models liposomal display of Apo2L/TRAIL markedly improves both tumor exposure time and proapoptotic potency.
Fig. 4.
Liposomal display of Apo2L/TRAIL improves its pharmacodynamic profile and enhances antitumor efficacy. (A and B) Nude mice bearing HT-29 xenografts (n = 10 per group) were treated with a single 50 mg/kg dose of Apo2L.0 or Apo2L.L via i.p. for 24 or 48 h then killed. All tumors were collected, homogenized, and assessed for Apo2L/TRAIL using ELISA (A) and caspase-8 activity (B). Error bars denote SEM. (C) Mice (n = 9 per group) bearing HT-29 tumor xenografts were treated with either Apo2L.0 or Apo2L.L via i.p. injection every 2 d. Tumor volumes were measured twice per week and then fit to a nonlinear mixed-effect model of tumor growth (SI Materials and Methods). Overlaid fitted curves are shown on the left and individual tumor volumes and their respective fitted curves are shown on the right. Dashed lines denote mice killed before the end of study.
As expected, Apo2L.0, dosed i.p. at 50 mg/kg every 2 d had no effect on HT-29 tumor growth compared with controls (Fig. 4C and Fig. S6B); in marked contrast, equivalent doses of Apo2L.L inhibited tumor growth by 65%. Of note, three animals within the Apo2L.L group lost >20% of body weight; however, dose titration in a follow-up study indicated comparable antitumor efficacy of Apo2L.L at 25 and 50 mg/kg, without significant weight loss at the lower dose level (Fig. S6 C–F). Thus, although it may be necessary to further optimize formulation and dosing, liposomal display substantially augments the antitumor activity of Apo2L/TRAIL.
Discussion
Although antibody-based PARAs have better pharmacokinetic properties than does soluble Apo2L/TRAIL, antibodies are bivalent molecules, they bind to distinct epitopes compared with the native, homotrimeric ligand, and they often require Fc-cross-linking for activity (11–14, 36). The potency of Apo2L/TRAIL can also be augmented by cross-linking with anti-DR5 antibody (37). However, liposomal display of Apo2L/TRAIL may better mimic the membrane geometry, stoichiometry, and dynamics of the natural ligand. Furthermore, it has the capacity to engage both DR4 and DR5, whereas agonistic antibodies to date have been monospecific. The latter also concerns binding to decoy receptors, which may help fine-tune selectivity for tumor vs. normal cells (3, 4).
The therapeutic use of liposomes often involves drug encapsulation (31, 32). Recent work demonstrates that anti-Her2 antibody can be displayed on liposomes to target encapsulated drug to tumors (38). Here we sought to advance liposomal surface-display strategies in a different direction, to enhance the biologic activity of a TNF superfamily ligand. We achieved this by covalently attaching Apo2L/TRAIL onto the surface of liposomes. Compared with Apo2L.0, Apo2L.L exhibited not only markedly stronger proapoptotic activity but also more durable tumor exposure. Consistent with these improvements, Apo2L.L exerted more robust and prolonged caspase activation in tumors, leading to substantial tumor-growth inhibition compared with complete tumor resistance to Apo2L.0. Thus, liposomal display could significantly affect potential clinical efficacy of Apo2L/TRAIL against a diverse range of cancers. There may be room for further optimization of liposomal size, composition, protein density, and dosing regimen to achieve the best possible balance of efficacy and safety.
Our findings open new potential avenues for further preclinical testing and clinical translation of Apo2L/TRAIL. Moreover, considering that multiple members of the TNF superfamily are expressed as cell-surface proteins, it may be possible to apply a similar approach to additional ligands that harbor therapeutic potential, for example FasL, CD70, OX40L, CD40L, 41BBL, and GITRL, among others.
Materials and Methods
Generation of Recombinant Apo2L/TRAIL Proteins.
Apo2L.XL was generated through the addition of a FLAG epitope (DYKDDDDK) to the N terminus of recombinant Apo2L/TRAIL, and subsequent cross-linking with a mouse monoclonal antibody specific for this epitope (M2; Sigma-Aldrich). Apo2LK179C was generated via mutagenesis of Apo2L.0 as previously described (24). Both Apo2L.0 and Apo2LK179C were produced in Escherichia coli. Apo2L.0 was purified to homogeneity as previously described (33), and Apo2LK179C was purified using a series of ion exchange and hydrophobic interaction chromatographies.
Additional Information.
See SI Materials and Methods for cell line screening, liposome formulation, supported membrane deposition and imaging, proapoptotic signaling measurement, immunoblotting, fluorescence imaging of Apo2L.0- and Apo2L.L-stimulated cells, size-exclusion chromatography, SDS-HPLC of Apo2L.L, and animal studies to determine pharmacodynamic profile as well as antitumor efficacy of Apo2L.L.
All procedures were approved by and conformed to the guidelines and principles set by the Institutional Animal Care and Use Committee of Genentech and were carried out in an Association for the Assessment and Accreditation of Laboratory Animal Care-accredited facility.
Supplementary Material
Acknowledgments
We thank S. Yee and B. Blackwood for tumor implantation, tumor volume and body weight measurements, and all animal treatments, as well as general guidance concerning animal studies, K. Totpal for providing cells for animal studies, R. Pai for protein purification, W. Galush for assistance collecting dynamic light scattering data, and R. Weimer for advice and comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418962112/-/DCSupplemental.
References
- 1.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Billard C. BH3 mimetics: Status of the field and new developments. Mol Cancer Ther. 2013;12(9):1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2(6):420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 5.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 7.Wallach D, et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RM, et al. SPOTS: Signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167(4):735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat Immunol. 2009;10(4):348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 10.Soria J-C, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(33):4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 11.Yang A, Wilson NS, Ashkenazi A. Proapoptotic DR4 and DR5 signaling in cancer cells: Toward clinical translation. Curr Opin Cell Biol. 2010;22(6):837–844. doi: 10.1016/j.ceb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7(12):1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: The potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26(21):3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 14.Holland PM. Death receptor agonist therapies for cancer, which is the right TRAIL? Cytokine Growth Factor Rev. 2014;25(2):185–193. doi: 10.1016/j.cytogfr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Subbiah V, et al. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther. 2012;11(11):2541–2546. doi: 10.1158/1535-7163.MCT-12-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28(17):2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 17.Kelley SK, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: Characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299(1):31–38. [PubMed] [Google Scholar]
- 18.Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J Clin Invest. 2015;125(2):487–489. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence D, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7(4):383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 20.Wilson NS, et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell. 2012;22(1):80–90. doi: 10.1016/j.ccr.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Seifert O, et al. Tetravalent antibody-scTRAIL fusion proteins with improved properties. Mol Cancer Ther. 2014;13(1):101–111. doi: 10.1158/1535-7163.MCT-13-0396. [DOI] [PubMed] [Google Scholar]
- 22.Gieffers C, et al. APG350 induces superior clustering of TRAIL receptors and shows therapeutic antitumor efficacy independent of cross-linking via Fcγ receptors. Mol Cancer Ther. 2013;12(12):2735–2747. doi: 10.1158/1535-7163.MCT-13-0323. [DOI] [PubMed] [Google Scholar]
- 23.Hymowitz SG, et al. Triggering cell death: The crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4(4):563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 24.Kelley RF, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280(3):2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 25.Salaita K, et al. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science. 2010;327(5971):1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair PM, Salaita K, Petit RS, Groves JT. Using patterned supported lipid membranes to investigate the role of receptor organization in intercellular signaling. Nat Protoc. 2011;6(4):523–539. doi: 10.1038/nprot.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310(5751):1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalvez F, et al. TRAF2 sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol Cell. 2012;48(6):888–899. doi: 10.1016/j.molcel.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119(Pt 19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Miguel D, et al. Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol Pharm. 2013;10(3):893–904. doi: 10.1021/mp300258c. [DOI] [PubMed] [Google Scholar]
- 31.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Chang H-I, Yeh M-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkenazi A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hylander BL, et al. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3(1):22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 36.Wilson NS, et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19(1):101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Graves JD, et al. Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity. Cancer Cell. 2014;26(2):177–189. doi: 10.1016/j.ccr.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.