Significance
Bone morphogenetic proteins (BMPs) are made as inactive precursor proteins that dimerize and are cleaved to generate a bioactive ligand along with prodomain fragments that lack signaling activity. BMP ligands signal as either homodimers, or as heterodimers that display significantly higher activity in vivo. Recombinant homodimeric BMP ligands are used clinically to stimulate bone healing, but this requires supraphysiological doses due to the short half-life of the implanted protein. The current studies demonstrate that properties intrinsic to the BMP4 prodomain contribute to the formation and activity of BMP homodimers and heterodimers in vivo. Understanding how the prodomain regulates the activity of the ligand when it is made in vivo may lead to changes in the way BMP ligands are used clinically.
Keywords: prodomain, heterodimer, bone morphogenetic protein, BMP4, BMP7
Abstract
Bone morphogenetic proteins 4 and 7 (BMP4 and BMP7) are morphogens that signal as either homodimers or heterodimers to regulate embryonic development and adult homeostasis. BMP4/7 heterodimers exhibit markedly higher signaling activity than either homodimer, but the mechanism underlying the enhanced activity is unknown. BMPs are synthesized as inactive precursors that dimerize and are then cleaved to generate both the bioactive ligand and prodomain fragments, which lack signaling activity. Our study reveals a previously unknown requirement for the BMP4 prodomain in promoting heterodimer activity. We show that BMP4 and BMP7 precursor proteins preferentially or exclusively form heterodimers when coexpressed in vivo. In addition, we show that the BMP4 prodomain is both necessary and sufficient for generation of stable heterodimeric ligands with enhanced activity and can enable homodimers to signal in a context in which they normally lack activity. Our results suggest that intrinsic properties of the BMP4 prodomain contribute to the relative bioactivities of homodimers versus heterodimers in vivo. These findings have clinical implications for the use of BMPs as regenerative agents for the treatment of bone injury and disease.
Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily that were originally isolated as bone-inducing morphogens and were subsequently found to play central roles during embryogenesis and in adult homeostasis (1). BMPs are clinically important therapeutic agents that are used to reverse bone loss caused by trauma, disease, and tumor resection (2). Their use as regenerative agents is limited, however, by their short half-life and low specific activity when implanted in vivo. Understanding how BMP activity is regulated is important for the development of more effective therapeutic agents for the treatment of bone injury and disease.
BMPs bind to and activate a receptor complex consisting of type I and type II transmembrane serine/threonine kinases. Following ligand binding, activated receptors propagate their signal by phosphorylating one of the SMADs that is specific for the BMP pathway (SMAD1, -5, or -8). The phosphorylated Smads then form heterooligomers with the common Smad, Smad4, and this complex translocates into the nucleus where it binds to BMP response elements and activates transcription of target genes (1).
BMPs are classified into subfamilies based on sequence homology. They signal as either homodimers, or as heterodimers from different subfamilies. For example, class I BMPs, which consist of BMP2 and BMP4, can heterodimerize with class II BMPs, consisting of BMP5–8 (3). Heterodimers composed of distinct BMP family members show a higher specific activity than do homodimers of either subunit. Homodimers of BMP2, -4, or -7, for example, can all induce bone formation, but heterodimers of BMP2 plus BMP7, or BMP4 plus BMP7 are significantly more potent (5- to 20-fold) than any of the homodimers in osteogenic differentiation assays (4–6). BMP2/7 and BMP4/7 heterodimers also show enhanced activity in Xenopus and zebrafish embryo assays (7, 8). Coinjection of RNA encoding BMP2 or BMP4 together with BMP7 into a single embryonic blastomere leads to robust induction of BMP target genes and ventralization of whole embryos, whereas injection of twice the dose of RNA encoding any single BMP, or injection of the two RNAs into separate blastomeres, has a much smaller effect on gene induction or patterning (7, 8). The observation that the two precursors must be coexpressed within the same cell to see synergy suggests that the formation of heterodimers is required to induce this effect (as opposed to synergistic activation of receptors by expressing homodimers in adjacent cells). Consistent with this interpretation, physiological concentrations of purified recombinant BMP4/7 or BMP2/7 heterodimers can activate BMP signaling in Xenopus and zebrafish, whereas much higher concentrations of either homodimer, applied individually or together, have no effect (7, 9, 10).
Biochemical and genetic evidence suggests that endogenous BMP heterodimers play physiologically relevant roles in vivo. In zebrafish, an ectopically expressed epitope-tagged form of BMP2b pulls down what appears to be endogenous BMP7 and vice versa (10). In addition, mutations in either BMP2b or BMP7 lead to a complete loss of signaling in early zebrafish embryos (8) and this can be rescued by recombinant heterodimers, but not by either homodimer (10). Drosophila decapentaplegic (DPP) and SCREW (BMP4 and BMP7 orthologs) are both required for proper spatial localization of DPP and this is proposed to be due to preferential transport of DPP/SCREW heterodimers within the embryo (11). Furthermore, mice carrying a point mutation that prevents proteolytic activation of BMP5, or humans carrying a point mutation that prevents secretion of the BMP family member growth and differentiation factor 5 (GDF5), display skeletal abnormalities that are more severe than those observed in the respective null mutants, most likely due to dominant negative effects on related BMPs that form heterodimers with endogenous BMP5 or GDF5 (12, 13).
The mechanism by which BMP heterodimers generate higher levels of signal than homodimers is unknown, but one possibility is that heterodimers form a more stable receptor ligand complex. Consistent with this mechanism, BMP2/6 heterodimers bind to both type I and type II BMP receptors with high affinity, whereas homodimers of either subunit show high-affinity binding to either the type I or the type II receptor, but not both (14). Furthermore, BMP2/7 heterodimers induce the formation of a high-affinity receptor complex containing two different type I receptors, together with a single type II receptor in zebrafish (10).
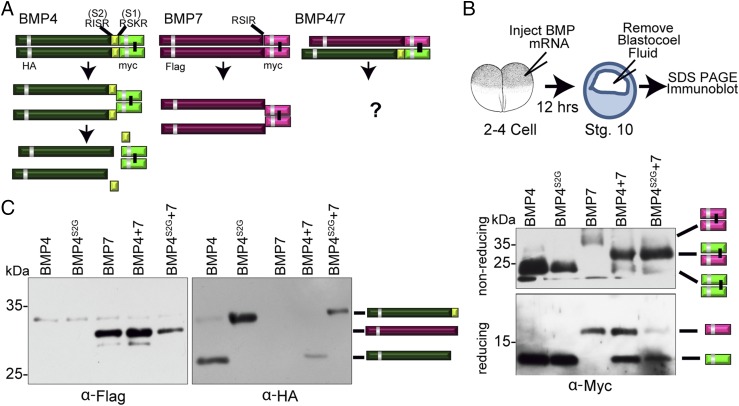
BMPs are generated as inactive precursor proteins (proBMPs) that dimerize and are then cleaved by proprotein convertases to yield prodomain fragments along with the mature active ligand (1). The question of how proprotein maturation occurs in heterodimers, as opposed to homodimers, has not been explored. This is a relevant question because class I and class II BMPs undergo different processes of proteolytic maturation. Whereas BMP2 and BMP4 precursor proteins have two highly conserved furin consensus cleavage motifs, BMP7 and other class II precursors have only a single conserved cleavage motif (7, 15–17). Among these family members, proteolytic maturation of BMP4 and BMP7 has been studied in detail (illustrated in Fig. 1A). BMP7 is cleaved at a single conserved furin site to generate a noncovalently associated prodomain/ligand complex (18, 19). By contrast, BMP4 is sequentially cleaved at two sites: initially at a site adjacent to the ligand domain (the S1 site) and subsequently at an upstream site (the S2 site) within the prodomain (Fig. 1A) (15). The BMP4 ligand forms a transient, noncovalent complex with the prodomain following cleavage at the S1 site, but subsequent cleavage at the S2 site disrupts the complex, freeing the ligand from the prodomain (20).
Fig. 1.
The S1 and S2 site of BMP4 is cleaved in BMP4/7 heterodimers. (A) Schematic illustration of cleavage patterns of BMP4 and BMP7 homodimers and position of epitope tags. (B and C) Two-cell embryos were injected with RNA encoding BMP4 (500 pg), BMP4S2G (1,000 pg), BMP7 (500 pg), or BMP4 and BMP7 (250 pg each). Blastocele fluid was extracted from the same number of embryos in each experimental group at the gastrula stage and proteins were deglycosylated and resolved by SDS PAGE. Immunoblots were probed with antibodies specific for myc (B), Flag, or HA epitopes (C) as indicated below each gel. Bands corresponding to ligand dimers, or to ligand or prodomain monomers, are indicated to the right of each gel.
The prodomain fragment that is released during proteolytic maturation lacks any known signaling activity but aids in dimerization, folding, and secretion of the active ligand (21). Moreover, prodomain swap experiments have shown that individual BMP prodomains can greatly influence the stability of cleaved mature ligands (22, 23). The prodomains of BMP7 and BMP4 play distinct roles in modulating the activity of their respective mature homodimers. The BMP7 prodomain fragment assists in solubilizing BMP7 homodimers by shielding hydrophobic residues present in the mature domain (18), and the prodomain of DPP has been suggested to play a similar role (24). The BMP7 prodomain also competes with type II receptors for binding to the BMP7 ligand, but does not confer latency because it can be easily displaced from the ligand by receptor binding (25). The BMP4 prodomain dissociates from the ligand in most tissues following cleavage at both the S1 and the S2 sites (17, 20, 26), but if only the S1 site is cleaved, the prodomain remains associated with the ligand and targets it to the lysosome for degradation (20). When only the S2 site of BMP4 is cleaved, this generates an amino-terminally extended ligand containing a small piece of the prodomain. This ligand species has not been detected in vivo, but when ectopically expressed in Xenopus embryos from a mutant precursor, the N-terminally extended ligand shows very little activity (27) or dominant negative activity (28).
In the current study we examine proteolytic maturation of BMP4/7 heterodimers and address the question of whether the higher specific activity of heterodimers is intrinsic to the ligands or whether one or both prodomains is required to reveal this enhanced activity. We find that BMP4/7 heterodimers are secreted as a complex consisting of the heterodimeric ligand noncovalently associated with the S1 and S2 cleaved BMP4 prodomain as well as the BMP7 prodomain. In addition, our results demonstrate that the BMP4 prodomain is both necessary and sufficient for the enhanced activity of heterodimers in vivo, and can enable homodimers to signal in a context in which they normally lack activity.
Results
Both Sites of BMP4 Are Cleaved in BMP4/7 Heterodimers in Xenopus Embryos.
To determine if one or both sites of BMP4 are cleaved in BMP4/7 heterodimers, we examined cleavage products generated from heterodimeric precursor proteins expressed in Xenopus embryos. Embryos were injected with RNA encoding BMP4HAmyc, BMP7Flagmyc, or both RNAs at the two-cell stage. We have previously shown that these epitope-tagged BMPs have the same activity as untagged BMPs when overexpressed (20, 27). A cleavage mutant form of BMP4 (BMP4S2G), which can only be cleaved at the S1 site (15), was included as a size control for the relative mobility of S1 only cleaved prodomain. At the early gastrula stage, fluid was aspirated from the blastocele of embryos to collect prodomain and ligand fragments that are secreted into this space (29). Proteins were deglycosylated, separated by SDS/PAGE under reducing or nonreducing conditions, and cleavage products were detected by immunoblot analysis (assay illustrated in Fig. 1B).
First, to determine the extent of heterodimer formation in embryos coexpressing BMP4 and BMP7, proteins were separated under nonreducing conditions and immunoblots were probed with antibodies specific for the myc tag in the ligand domain (Fig. 1B, Upper). A single band corresponding to mature BMP4 homodimers was detected in embryos expressing either BMP4 or BMP4S2G, and a more slowly migrating band corresponding to mature BMP7 homodimers was detected in embryos expressing BMP7 alone. In embryos coexpressing BMP4 and BMP7 precursor proteins, a single mature BMP4/7 heterodimer band of intermediate mobility was detected along with a trace amount of BMP4 homodimer. Thus, BMP4 and BMP7 preferentially form heterodimers when equivalent levels of each precursor are coexpressed in vivo. Next, to determine whether the S1 site of BMP4 is cleaved in heterodimers, proteins were separated under reducing conditions and immunoblots were probed with antibodies specific for the myc tag in the ligand domain (Fig. 1B, Lower). BMP4 monomers cleaved from heterodimeric and homodimeric precursor proteins migrate with an identical relative mobility under reducing conditions, indicating that the S1 site is cleaved in heterodimers as it is in homodimers.
To examine whether the S2 site of BMP4 is cleaved in the context of heterodimers, immunoblots were probed with antibodies specific for the HA tag in the prodomain of BMP4. The BMP4 prodomain fragment cleaved from BMP4/7 heterodimers (in embryos coexpressing BMP4 and BMP7) migrated with the same relative mobility as that cleaved from native proBMP4, and more rapidly than that cleaved from proBMP4S2G (Fig. 1C, Right), indicating that the S2 site of BMP4 is cleaved in the context of heterodimers. Collectively, these data demonstrate that BMP4 and BMP7 preferentially form heterodimers rather than either homodimer when they are coexpressed in Xenopus embryos, and that BMP4 is cleaved at both the S1 and the S2 site in heterodimers as it is in homodimers.
Cleavage of both BMP4 Sites Is Not Necessary for Heterodimer Activity.
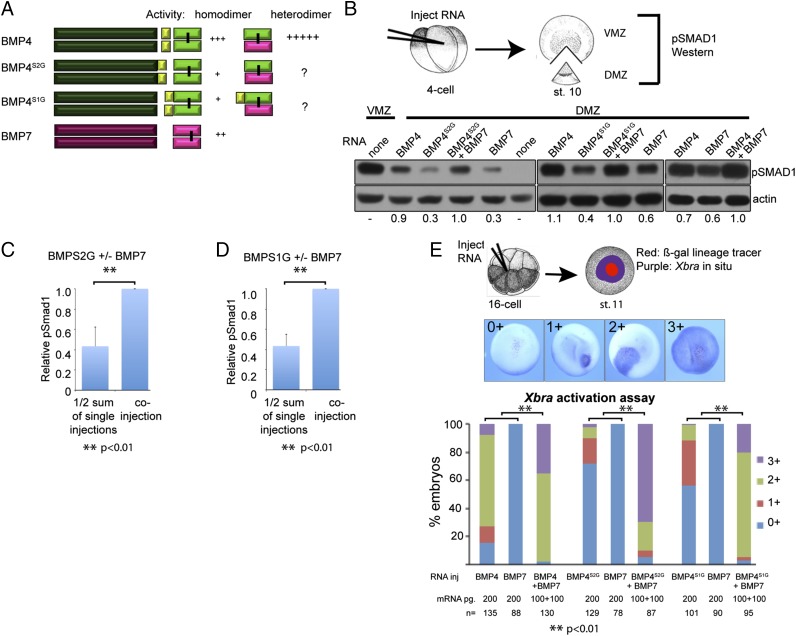
Given that proBMP4 homodimers must be cleaved at both the S1 and S2 sites to generate maximally active and stable BMP4 ligands (20, 27) (summarized in Fig. 2A), we investigated whether proBMP4, when complexed with proBMP7, also requires double cleavage for enhanced BMP4/7 heterodimer activity. To carry out this investigation, we injected RNAs encoding different combinations of BMP4 and BMP7 near the dorsal marginal zone (DMZ) of Xenopus embryos at the four-cell stage to target the presumptive dorsal mesoderm. When embryos reached the early gastrula stage, the DMZ was explanted and BMP activity was assayed by probing immunoblots of DMZ extracts for pSMAD1/5/8 (hereafter shortened to pSMAD1; assay illustrated in Fig. 2B). In each experiment, sibling embryos were cultured to the tailbud stage and scored for BMP-mediated loss of dorsal structures (Fig. S1), to verify that the relative loss of dorsal structures correlated with the relative level of pSMAD1 induced in response to each injected RNA in individual experiments. We expressed these exogenous BMPs in the DMZ because it has negligible endogenous BMP signaling (Fig. 2B, Lower Left, last lane), providing a blank slate to start from. Conversely, the ventral marginal zone (VMZ) has high endogenous BMP activity (Fig. 2B, Lower Left, first lane) and was used as a positive control.
Fig. 2.
Sequential cleavage of proBMP4 is required for maximal activity of homodimeric but not heterodimeric ligands. (A) Schematic illustration of cleavage patterns and relative bioactivity of ligands generated from wild-type and cleavage mutant BMP precursor proteins. (B) RNA encoding BMP7 or wild type or cleavage mutant forms of BMP4 were injected alone (200 pg) or in combination (100 pg of each RNA) near the dorsal midline of four-cell embryos. DMZ and VMZ explants were isolated at stage 10 and pSMAD1 levels were analyzed by immunoblot. Blots were reprobed with actin as a loading control, with the exception of the right panel, in which case duplicate samples were rerun and probed with actin. Blots shown in each of the three panels were run on different days, using samples from independent experiments, and exposed for different time periods. Thus, results can only be compared within each panel. For each panel, the relative level of pSMAD1 induced by injection of BMP4 or BMP7 alone, normalized to actin, and reported as a fraction of that induced by the two coinjected RNAs is indicated below each lane. (C and D) Quantitation of relative pSMAD1 levels in three independent experiments (mean ± SD). Normalized pSMAD1 levels in embryos injected with BMP7 or BMP4S2G alone (C), or with BMP7 or BMP4S1G alone (D) were added together, divided by two, and reported as a fraction of pSMAD1 levels induced by the two coinjected RNAs. (E) BMP RNAs were injected along with β-galactosidase RNA into one animal pole blastomere of 8- to 16-cell embryos. Embryos were stained for β-galactosidase activity (red) at stage 11 and then assayed for expression of Xbra by in situ hybridization (purple) as illustrated. The spread of Xbra signal outside of cells that express the precursor proteins (marked by red) was scored as 0+ to 3+ as illustrated. A total of 30–50 embryos were injected with each RNA per experiment and results are pooled from a minimum of three experiments.
As a control, and to validate our assay, we first asked whether wild-type BMP4/7 heterodimers induce higher levels of pSMAD1 in DMZ explants than do homodimers. Injection of RNA encoding BMP4 or BMP7 induced pSMAD1 in DMZ explants (Fig. 2B, Lower Right). To assess heterodimer function, we coinjected a half dose of BMP4 and a half dose of BMP7 into embryos, such that the total amount of RNA was equal to the single injections. The relative level of pSMAD1 induced by either BMP4 or BMP7 alone, normalized to actin and reported as a fraction of that induced by the two coinjected RNAs, is indicated below each lane. If BMP4 and BMP7 have an additive effect when coinjected, then the sum of the relative pSMAD1 levels induced by injection of each RNA alone, divided by two (to normalize for the half dose of each RNA injected alone) would be equal to the pSMAD1 level induced by coinjection of the two RNAs. As shown in Fig. 2B, last three lanes), coinjection of BMP4 and BMP7 induced a relatively higher level of pSMAD1 (1.0) than half the sum of that induced by each RNA alone [(0.7 + 0.6) ÷ 2 = 0.65]. The higher relative levels of pSMAD1 induced by heterodimers correlated with a significantly stronger inhibition of dorsal development, as indicated by a lower dorsoanterior index score (Fig. S1C).
BMP4S2G (Fig. 2B, Lower Left) or BMP4S1G (Fig. 2B, Lower Middle) alone induced lower levels of pSMAD1 than did wild-type BMP4, consistent with our previous studies showing that cleavage at both sites is required for maximal activity (27, 30). However, in three out of three experiments, coinjection of RNA encoding either BMP4 cleavage mutant together with BMP7 led to significantly enhanced activity as indicated by higher levels of pSMAD1 induction (Fig. 2 B, Lower Left and Lower Middle, C, and D), and more severe loss of dorsal structures (Fig. S1 A and B) relative to that observed when twice as much of each individual RNA was injected. Furthermore, each of the cleavage mutant forms of BMP4 preferentially formed heterodimers when coexpressed with BMP7 (Fig. 1C and Fig. S1D). These results indicate that cleavage of BMP4 at the S1 or the S2 site alone is sufficient for enhanced BMP4/7 heterodimer activity.
To further evaluate whether cleavage of BMP4 at both sites is required for full heterodimer activity, we used a second assay in which we tested whether heterodimers generated from wild type or cleavage mutant forms of BMP4 can induce ectopic Xbra expression in the animal pole of gastrula stage Xenopus embryos (assay illustrated in Fig. 2E). Xbra is normally expressed only in the mesoderm but ectopic expression can be induced in ectodermal cells by recombinant mature BMP heterodimers (7, 9). BMP RNA was coinjected into a single animal pole blastomere of 8- to 16-cell embryos along with β-galactosidase RNA (as a lineage tracer). Embryos were cultured until stage 11 when they were fixed and stained for β-galactosidase activity (red stain) followed by analysis of Xbra expression by in situ hybridization (purple stain). BMP precursor proteins are restricted to β-galactosidase–expressing cells, whereas the cleaved mature ligand can potentially signal to more distal cells to activate expression of Xbra. The extent of BMP-induced ectopic Xbra was scored in individual embryos as 0+ (no ectopic induction), 1+ (induction confined to precursor-expressing cells), 2+ (induction extending slightly beyond the precursor-expressing cells), or 3+ (complete induction throughout the animal cap) as illustrated in Fig. 2E.
BMP7 homodimers derived from injected RNA did not induce Xbra expression when they were targeted to ectodermal cells (Fig. 2E and Fig. S2A). By contrast, BMP4 homodimers derived from injected RNA induced a moderate level of Xbra expression in most embryos (mostly 2+) (Fig. 2E and Fig. S2C). Heterodimers derived from coinjection of a half dose of BMP4 and BMP7 RNA led to a significantly stronger synergistic induction of Xbra (2+ to 3+) (Fig. 2E). Homodimers generated by injection of RNA encoding either BMP4 cleavage mutant precursor induced relatively low levels of Xbra expression (mostly 0+ to 1+) (Fig. 2E). Importantly, significantly stronger and more widespread induction of Xbra was observed in embryos coinjected with half the dose of RNA encoding either of the cleavage mutant forms of BMP4 together with BMP7 (2+ to 3+; Fig. 2E). These results show that cleavage at either site of BMP4 is sufficient for enhanced heterodimer activity and signaling range.
The Prodomain of BMP7 Is Not Sufficient for Enhanced Activity of BMP4/7 Heterodimeric Ligands.
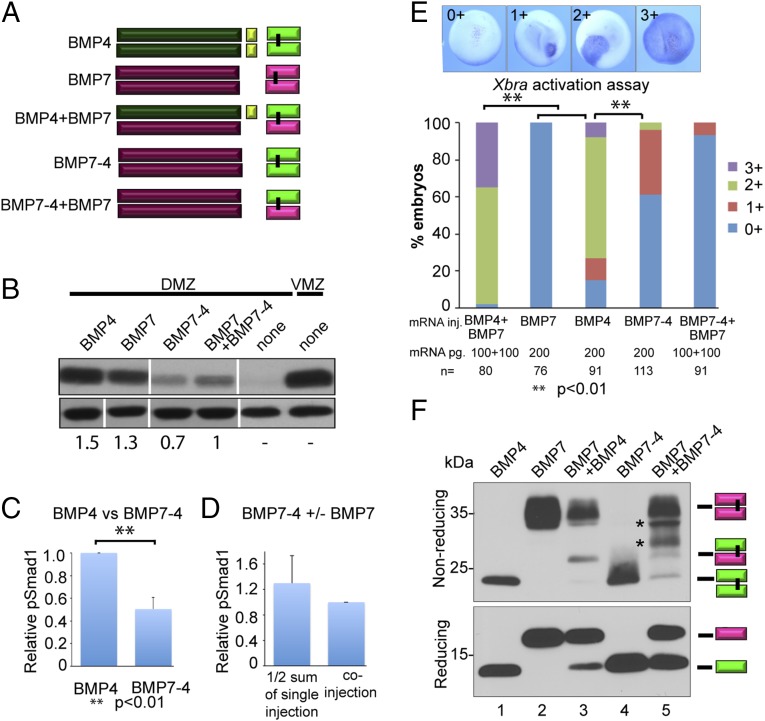
We next wanted to investigate whether the enhanced bioactivity of BMP4/7 heterodimers relative to homodimers is intrinsic to the heterodimeric ligand or whether BMP4 and/or BMP7 prodomains contribute to heterodimer activity. First, to ask whether the BMP7 prodomain is sufficient for heterodimer activity, we injected Xenopus embryos with RNA encoding a previously characterized chimeric protein consisting of the BMP7 prodomain fused to the BMP4 mature domain (BMP7–4) (27) (Fig. 3A), either alone or together with BMP7, and assayed pSMAD1 levels in explanted DMZs, inhibition of dorsal development, and Xbra induction in whole embryos as described above. Coinjection of RNA encoding BMP7–4 together with BMP7 is predicted to generate dimeric precursors containing two copies of the BMP7 prodomain, but upon cleavage, they will generate the same heterodimeric ligand (BMP4/7) that would be derived from coinjection of RNA encoding native BMP4 and BMP7 precursors (illustrated in Fig. 3A). The levels of pSMAD1 and Xbra induction in embryos expressing BMP7–4 were significantly less than those in embryos expressing wild-type BMP4 (Fig. 3 B, C, and E), and this was also reflected in the limited ability of BMP7–4 to inhibit dorsal development (Fig. S3A). These findings are consistent with our previous studies showing that the BMP7 prodomain cannot fully substitute for the BMP4 prodomain (27). Furthermore, we did not see enhanced BMP activity in embryos coexpressing BMP7–4 together with BMP7 relative to the sum of activity in embryos injected with a half dose of either RNA alone (Fig. 3 B, D, and E). Thus, the BMP7 prodomain is not sufficient for full BMP4 homodimer, or BMP4/7 heterodimer activity.
Fig. 3.
The prodomain of BMP7 is not sufficient for maximal heterodimer activity. (A) Schematic illustration of ligands generated from native and chimeric BMP precursor proteins. (B) BMP RNAs were injected individually (200 pg) or together (100 pg of each RNA) near the dorsal midline of four-cell embryos. DMZ and VMZ explants were isolated at stage 10 and pSMAD1 levels were analyzed by immunoblot. Duplicate samples were rerun and blots were probed with actin as a loading control. All lanes are from the same pSmad1 immunoblot, aligned following removal of intervening lanes (following the 2nd and 4th lanes) using Photoshop. The first four lanes of the actin immunoblot are from the same gel, with the order rearranged to match the upper samples using Photoshop. Noncontiguous lanes are marked by white bars. The last two lanes were reprobed from samples shown in Fig. 4B. The relative level of pSMAD1 induced by injection of BMP7-4 or BMP7 alone, normalized to actin and reported as a fraction of that induced by the two co-injected RNAs, is indicated below each lane. (C) Quantitation of relative pSMAD1 levels induced by BMP4 or BMP7–4 in three independent experiments (mean ± SD). (D) Normalized pSMAD1 levels in embryos injected with BMP7 or BMP7–4 alone were added together, divided by two, and reported as a fraction of pSMAD1 levels induced by the two coinjected RNAs. Data from three independent experiments (mean ± SD). (E) BMP RNAs were injected along with β-galactosidase RNA into one animal pole blastomere of 8- to 16-cell embryos. Embryos were stained for β-galactosidase activity at stage 11 and then assayed for expression of Xbra by in situ hybridization. The spread of Xbra signal outside of cells that express the precursor proteins was scored as 0+ to 3+ as illustrated. A total of 30–50 embryos were injected with each RNA per experiment and results are pooled from a minimum of three experiments. (F) Protein present in blastocele fluid from the same number of embryos in each group was deglycosylated, resolved by SDS/PAGE under reducing or nonreducing conditions as indicated, and immunoblots were probed with antibodies specific for the myc epitope in the ligand domain. Bands corresponding to each ligand dimer or monomer are indicated to the right of each gel. Asterisks indicate two bands that may correspond to partially misfolded heterodimers.
To ask whether the relatively low activity in embryos expressing BMP7–4 alone or together with BMP7 is due to the inability of the BMP7 prodomain to drive BMP4 homodimer and/or BMP4/7 heterodimer formation, we used immunoblot analysis to examine BMP ligands secreted into the blastocele of injected embryos. This assay is semiquantitative, due to the inherent variability in the amount of blastocele fluid aspirated from individual embryos, but it allows us to look for gross differences in the level of each monomeric ligand (under reducing conditions) and to compare the relative ratio of heterodimers and homodimers in a given experimental group (under nonreducing conditions). Grossly similar levels of mature BMP4 monomers (Fig. 3F, reducing) and homodimers (nonreducing) were detected in embryos expressing either BMP4 or BMP7–4 (compare lanes 1 and 4) in four independent experiments, demonstrating that the BMP7 prodomain does not impair dimerization or cleavage of the chimeric precursor nor does it destabilize the cleaved ligand. BMP4/7 heterodimers formed in embryos coexpressing BMP7 together with BMP4 (Fig. 3F, nonreducing, lane 3; note that in this experiment, BMP7 is present in excess, most likely due to an RNA pipetting error and thus BMP7 homodimers are overrepresented), whereas in embryos coexpressing equal amounts of BMP7 together with BMP7–4, only a small amount of BMP4/7 heterodimer and BMP4 homodimer was detected, whereas BMP7 homodimers were the dominant species detected in four independent experiments (lane 5). Two bands that migrate with a mobility intermediate to BMP4/7 heterodimers and BMP7 homodimers were also reproducibly detected in embryos coexpressing BMP7 together with BMP7–4 (Fig. 3F, asterisks). These bands may represent partially misfolded heterodimers, because they are detected only when the two proteins are coexpressed, and only under nonreducing, but not under reducing conditions. Collectively, these results show that the BMP7 prodomain is sufficient to direct formation of stable BMP4 homodimers, but these ligands are less active than those generated from native precursors. By contrast, the BMP7 prodomain is not sufficient to enable stable BMP4/7 heterodimers to form, but instead favors BMP7 homodimer formation.
The BMP4 Prodomain Is Necessary and Sufficient to Generate Heterodimeric BMP4/7 Ligands with Enhanced Activity and to Generate BMP7 Homodimers with a Novel Activity.
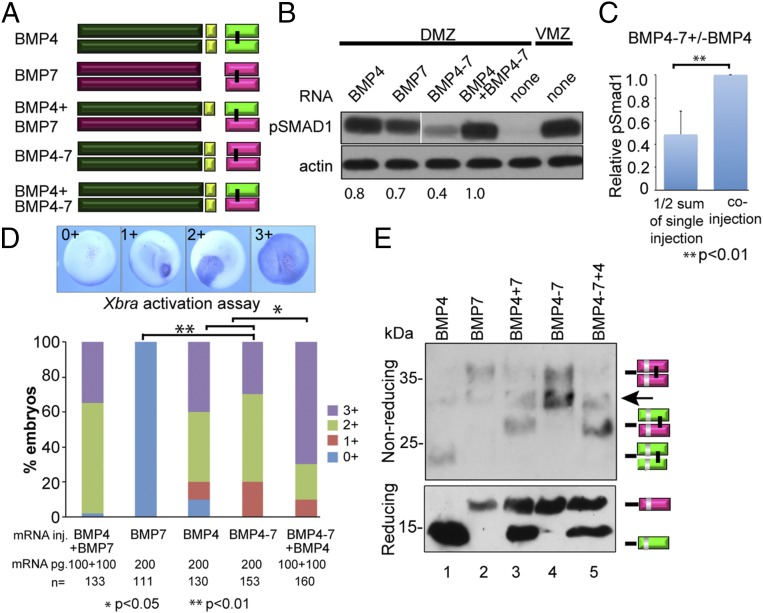
To ask whether the BMP4 prodomain is essential for heterodimer activity, Xenopus embryos were injected with RNA encoding a previously characterized chimeric protein consisting of the BMP4 prodomain fused to the BMP7 mature domain (BMP4–7) (27) (Fig. 4A) either alone or together with RNA encoding BMP4. Coinjection of RNA encoding BMP4–7 together with BMP4 is predicted to generate dimeric precursors containing two copies of the BMP4 prodomain, but upon cleavage, they will generate the same heterodimeric ligand (BMP4/7) that would be derived from coinjection of RNA encoding native BMP4 and BMP7 precursors (illustrated in Fig. 4A). Levels of pSMAD1 in DMZ explants expressing BMP4–7 alone were less than those in embryos made to express BMP7 alone in two experiments (Fig. 4B) and were relatively equivalent in a third replicate. In all three experiments, however, coexpression of BMP4–7 together with BMP4 led to a synergistic induction of pSMAD1 in DMZ explants (Fig. 4 B and C) and inhibited dorsal development to a greater degree (Fig. S3B) than was observed in embryos injected with twice as much RNA encoding either precursor protein alone.
Fig. 4.
The prodomain of BMP4 is necessary and sufficient to generate stable heterodimers with enhanced activity. (A) Schematic illustration of ligands generated from native and chimeric BMP precursor proteins. (B) BMP RNAs were injected individually (200 pg) or together (100 pg of each RNA) near the dorsal midline of four-cell embryos. DMZ and VMZ explants were isolated at stage 10 and pSMAD1 levels were analyzed by immunoblot. Actin levels were analyzed in duplicate samples as a loading control. The relative level of pSMAD1 induced by injection of BMP4-7 or BMP4 alone, normalized to actin and reported as a fraction of that induced by the two co-injected RNAs, is indicated below each lane. All lanes are from the same pSmad1 immunoblot, aligned following removal of an intervening lane (following the 2nd lane, marked by white bar) using Photoshop. The pSmad1 data shown in Figs. 2B (Lower Right), 3B, and 4B is all from a single injection experiment, run on the same gel and thus the BMP4 and BMP7 control lanes are the same in all three blots. Actin loading controls for these lanes are from duplicate samples that were rerun on different gels. (C) Normalized pSMAD1 levels in embryos injected with BMP4–7 or BMP4 alone were added together, divided by two, and reported as a fraction of pSMAD1 levels induced by the two coinjected RNAs. Data are from three independent experiments (mean ± SD). (D) BMP RNAs were injected along with β-galactosidase RNA into one animal pole blastomere of 8- to 16-cell embryos. Embryos were stained for β-galactosidase activity at stage 11 and then assayed for expression of Xbra by in situ hybridization. The spread of Xbra signal outside of cells that express the precursor proteins was scored as 0+ to 3+ as illustrated. A total of 30–50 embryos were injected with each RNA per experiment and results are pooled from a minimum of three experiments. (E) Protein present in blastocele fluid from the same number of embryos in each group was deglycosylated, resolved by SDS/PAGE under reducing or nonreducing conditions as indicated, and immunoblots were probed with antibodies specific for the myc epitope in the ligand domain. Bands corresponding to each ligand dimer or monomer are indicated to the right of each gel. The arrow marks a nonspecific band due to cross-reaction of the myc antibody with PNGase.
Unexpectedly, injection of RNA encoding BMP4–7 alone led to robust induction of Xbra expression in ectodermal cells (Fig. 4D). This was a surprising finding, given that recombinant BMP7 homodimers (7, 9) or homodimers generated by injection of BMP7 RNA into embryos (Fig. 4D and Fig. S2A) are unable to induce Xbra expression in ectodermal cells. Thus, whereas BMP7 is not normally capable of activating a signal transduction cascade leading to Xbra expression in ectodermal cells, the presence of the BMP4 prodomain enables this ligand to do so. Furthermore, coexpression of BMP4–7 together with BMP4 led to a synergistic induction of Xbra in ectodermal cells that was greater than that observed in embryos injected with twice as much RNA encoding either precursor protein alone (Fig. 4D). These data demonstrate that the BMP4 prodomain is sufficient for maximal BMP4/7 heterodimer activity in both mesodermal and ectodermal cells and can enable BMP7 homodimers to acquire a new signaling activity in ectodermal cells.
Relatively equivalent levels of mature BMP7 monomers (Fig. 4E, reducing) and homodimers (nonreducing) were detected in embryos expressing BMP7 and BMP4–7 (compare lanes 2 and 4; note that on the reducing gel the BMP7 monomer signal is partially masked in lane 2 and the left half of lane 3 due to an air bubble). Thus, the ability of mature BMP7 to signal in ectoderm when expressed in the context of the BMP4 prodomain is not due to enhanced dimerization or stability of the ligand. BMP4/7 heterodimers were the predominant species detected in embryos coexpressing BMP7 or BMP4–7 together with BMP4 (Fig. 4E, lanes 3 and 5), indicating that the BMP4 prodomain is sufficient to preferentially drive formation of stable heterodimers.
As a control to demonstrate that both of the chimeric precursors are able to support ligand activity, we injected RNA encoding BMP7–4 or BMP4–7 either alone or together into ectodermal or dorsal mesodermal cells of Xenopus embryos. Coinjection of these two RNAs will generate dimeric precursors carrying both the BMP7 and the BMP4 prodomain and mature domain, in trans, and are thus predicted to generate the same heterodimeric ligand (BMP4/7) that would be derived from coinjection of RNA encoding native BMP4 and BMP7 precursors (Fig. S4A). Coinjection of RNA encoding the two chimeric precursors (100 pg each) led to a synergistic induction of Xbra when targeted to ectodermal cells and to enhanced loss of dorsal structures when targeted to mesodermal cells that was greater than that observed in embryos injected with 200 pg of RNA encoding either precursor protein alone (Fig. S4 B and C).
BMP4 and BMP7 Prodomains Remain Associated with Mature BMP4/7 Heterodimers.
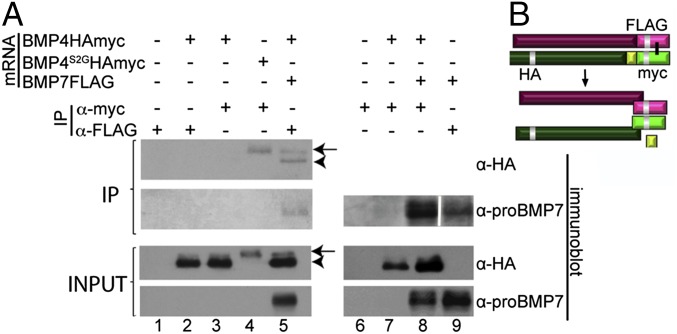
Given that noncovalent association of the prodomain with the cleaved ligand can affect receptor binding and ligand stability (20, 31), it was of interest to know whether one or both prodomains remain associated with mature BMP4/7 heterodimers, and we tested this using coimmunoprecipitation assays. HEK cells were transiently transfected with cDNAs encoding epitope-tagged BMP4HAmyc or BMP7Flag (illustrated in Fig. 5B) or both. Mature ligands were immunoprecipitated from the culture media using antibodies against the myc or Flag tag in the mature domain of BMP4 or BMP7. The ability of each prodomain to copurify with homodimeric or heterodimeric ligands was assayed by probing immunoblots of immunoprecipitates with antibodies specific for the HA tag in the prodomain of BMP4, or using an antibody directed against the prodomain of BMP7 (α-proBMP7). Consistent with published studies, the prodomain of BMP7 coimmunoprecipitated with mature BMP7 homodimers (Fig. 5, lane 9) (19). Also consistent with published studies (20), the S1 only cleaved prodomain from BMP4S2G coimmunoprecipitated with mature BMP4 homodimers (lane 4) whereas the S1 and S2 cleaved prodomain from wild-type BMP4 did not (lane 3), thus providing internal positive and negative controls for these experiments. When mature BMP4/7 heterodimers were immunoprecipitated from the culture media using antibodies specific for the myc tag in BMP4, the BMP7 prodomain copurified with the heterodimeric ligand (lane 8). Conversely, when mature heterodimers were immunoprecipitated from culture media using antibodies specific for the Flag-tag in BMP7, the S1 and S2 cleaved prodomain of BMP4 copurified with the heterodimeric ligand (lane 5, IP, arrowhead), although this same fragment does not copurify with mature BMP4 homodimers (lane 3). Notably, a faint signal corresponding to S1-only cleaved prodomain also copurified with mature heterodimers (lane 5, IP, arrow), consistent with the observation that a minor fraction of BMP4 present in heterodimers is cleaved at the S1 site alone in HEK cells (lane 5, input, arrow) and with previous studies showing that this fragment has high affinity for mature BMP4 (20). Collectively, these data show that proteolytic processing of heterodimeric BMP4/7 precursor proteins generates a complex consisting of the heterodimeric mature ligand noncovalently associated with the S1 and S2 cleaved BMP4 prodomain as well as the BMP7 prodomain (illustrated in Fig. 5B).
Fig. 5.
BMP4 and BMP7 prodomains remain associated with mature BMP4/7 heterodimers. (A) HEK cells were transfected with cDNAs encoding epitope-tagged BMP4 or BMP7 precursor proteins alone or together. Mature ligand fragments were immunoprecipitated (IP) from the cell media using antibodies specific for Flag or myc tags as indicated above each lane, and prodomain fragments were detected by probing immunoblots of immunoprecipitates or total protein (input) with antibodies specific for the BMP7 prodomain or the HA-epitope as indicated to the right of each gel. The position of the S1 only (arrow) or S1 and S2 cleaved BMP4 prodomain (arrowhead) is indicated. In each panel, samples were run on the same gel. An intervening lane was removed using Photoshop (between lane 8 and 9, marked by white bar) in the upper Right panel. Results were reproduced in three independent experiments. (B) Schematic illustration of the final product generated by proteolytic maturation of BMP4/7 heterodimers.
Discussion
Previous studies have shown that BMP heterodimers have a higher specific activity than homodimers but it is not known whether one or both prodomains contribute to the enhanced activity of the heterodimeric ligand. The current studies demonstrate that both prodomains remain complexed with the mature heterodimeric ligand following cleavage. Furthermore, we discovered that the BMP4 prodomain, but not the BMP7 prodomain is necessary and sufficient to promote formation of stable heterodimeric ligands that show enhanced activity in vivo.
To the best of our knowledge, our studies are the first to show that BMP4 and BMP7 preferentially form heterodimers when coexpressed at equivalent levels in vivo, albeit in an overexpression system. These findings suggest that when BMP4 and BMP7 precursor proteins are coexpressed in the same cell type in vivo, they preferentially form heterodimers rather than either homodimer. Because expression of Bmp4 and Bmp7 overlap extensively throughout development (32), it is likely that they signal as heterodimers in at least some contexts. However, the degree of heterodimer formation will be dependent on the relative level of expression of BMP class I and class II precursor proteins in a given cell type.
BMP4 must be cleaved at both the S1 and the S2 sites to achieve optimal signaling range and stability of homodimers (20, 27), but surprisingly cleavage of either site is sufficient for enhanced heterodimer activity. Our previous results showed that failure to cleave the S2 site causes shuttling of BMP4 homodimers to the lysosome and rapid protein turnover (20), whereas failure to cleave the S1 site may cause misfolding of the ligand domain (27). The formation of heterodimers that show enhanced bioactivity even when BMP4 is cleaved at only the S1 or the S2 site suggests that something intrinsic to the heterodimeric complex prevents misfolding or degradation of the BMP4/7 ligand.
Seen through the context of tissue-specific cleavage of the S2 site of BMP4, our results have broader implications in development. Analysis of mice carrying a knock-in mutation that prevents cleavage at the S2 site (Bmp4S2G), as well as studies in Drosophila, show that cleavage at the S2 site is essential for normal development of some, but not all tissues (17, 26). Interestingly, a DPP variant that can be cleaved only at the S2 site is fully functional in supporting wing development in dpp mutant rescue assays (16), whereas analogous cleavage variants of BMP2/BMP4 generate either dominant mutant (28, 33) or nonfunctional ligands (27) when overexpressed. The ability of BMP4 variants that can be cleaved at only the S1 or the S2 site to function in specific tissues when expressed from the endogenous locus in vivo, but not when overexpressed, could be explained in the context of our data showing that these BMP4 variants are fully functional as heterodimers. Specifically, whereas assays in which a single BMP is overexpressed inherently test the function of homodimers, BMP4 may normally form heterodimers when expressed from the endogenous Bmp4 locus, and these heterodimers would retain full activity regardless of whether one or both sites of BMP4 were cleaved.
Our finding that the BMP4 prodomain facilitates formation of stable heterodimers in vivo, whereas the BMP7 prodomain is neither necessary nor sufficient to do so can explain the difference in activity we observe following coexpression of BMP4 and BMP7 ligand domains together with only the BMP7 or the BMP4 prodomain. Our results cannot distinguish whether the BMP4 prodomain preferentially drives formation of heterodimeric precursor proteins, or whether the prodomain is required to stabilize the heterodimeric ligand following cleavage. When BMP7 and BMP4 ligand domains are expressed in the context of the BMP4 prodomain alone, high steady-state levels of heterodimeric ligand are present, whereas very little of either homodimer is observed. By contrast, when BMP7 and BMP4 ligand domains are expressed in the context of the BMP7 prodomain alone, very little heterodimeric ligand is present, whereas high levels of BMP7 (but not BMP4) homodimers are present. These findings support a model in which the BMP7 prodomain preferentially drives homodimerization of BMP7 precursor proteins, whereas the BMP4 prodomain preferentially drives BMP4 and BMP7 precursor proteins to heterodimerize. Further structural analysis will be required to test this model. Regardless of which model is correct, it is likely that the enhanced activity of heterodimers is intrinsic to the ligand once proteolytic activation has occurred. Consistent with this idea, Escherichia coli-derived recombinant mature BMP2/7 or BMP2/6 heterodimers are more active than either homodimer (10, 34), despite being synthesized in the absence of their respective prodomains.
Besides promoting stable heterodimer formation, the BMP4 prodomain may play an additional role in relieving the inhibitory activity of the BMP7 prodomain. This is possible given our results showing that both prodomains remain noncovalently associated with the heterodimeric ligand, and given previous studies showing that the BMP7 prodomain competes with the BMP7 ligand for binding to type II receptors (31). Analysis of the crystal structure of mature TGFβ as a latent complex with its prodomain reveals that each growth factor monomer interacts with the opposite prodomain (35). By analogy, it is possible that the BMP4 prodomain envelops the BMP7 ligand and vice versa in mature heterodimeric complexes. If the BMP4 prodomain has a higher affinity for mature BMP7 than for mature BMP4, this would explain why it remains complexed with mature heterodimers, but not homodimers. Furthermore, because the relative affinities of mature TGFβ family ligands for prodomain versus receptor binding can determine whether a prodomain/growth factor complex is latent or active (36), this might contribute to the inability of the BMP7 prodomain to support active homodimer or heterodimer signaling. BMP heterodimers engage a unique receptor complex (10, 34) and it is possible that the relative affinity of this complex for the heterodimeric ligand is sufficient to displace two BMP4 prodomains or one of each prodomain but not two copies of the BMP7 prodomain.
In addition to being necessary and sufficient to support heterodimer activity, the prodomain of BMP4 can enable BMP homodimers to signal in a context in which they normally lack activity. BMP4 homodimers generated by injection of synthetic RNA that includes the BMP4 prodomain can induce expression of Xbra and other ventral mesodermal markers in ectodermal explants, whereas recombinant mature BMP4 homodimers cannot (7, 37, 38). Notably, we show that BMP7 homodimers generated by injection of a chimeric RNA containing the BMP4 prodomain can also induce expression of Xbra in ectodermal cells, whereas recombinant mature BMP7 homodimers (7, 9) or homodimers generated from a precursor containing the BMP7 prodomain (these studies) cannot. Collectively, these findings demonstrate that the prodomain is not merely a chaperone that promotes dimer formation and folding, but that it can directly modulate the ability of mature BMPs to activate signaling cascades. The ability of the BMP4 prodomain to modulate ligand function is context specific because it is required for BMP7 and BMP4 homodimer activity in ectodermal cells but not in mesodermal cells (Fig. S2).
One way that the BMP4 prodomain might modulate ligand function in a cell-type–specific fashion is by targeting it to specific ECM binding partners. By analogy, the structurally related ligand TGFβ remains noncovalently associated with its prodomain following cleavage and is targeted to the ECM via covalent interactions between the prodomain and the ECM proteins’ latent TGFβ binding protein (LTBP) and fibrillin (39). The ligand/prodomain/LTBP complex forms intracellularly (40) and then binds to fibrillin following secretion. In the case of TGFβ, the mature ligand remains inactive until it is released from the ECM by proteolysis or mechanical stress (39). The prodomain of several BMPs, including BMP4 and BMP7, bind to the ECM protein fibrillin (31), whereas the prodomain of the BMP family member, GDF8, binds to perlecan (41). Additional ECM components can positively or negatively modulate BMP signaling, in some cases via direct interactions with the ligand. For example, members of the glypican family of heparin sulfate proteoglycans (HSPGs) have been shown to stabilize DPP (42), to facilitate movement of DPP in the extracellular space (43) and/or to function as BMP coreceptors (44). HSPGs can also bind to BMP7 and either promote or inhibit signaling (45–47). Other ECM components or ECM-bound proteins, such as fibronectin, collagen, and members of the crossveinless family, promote BMP signaling through diverse and context-dependent mechanisms (48–52). The composition of the ECM varies between different tissues and cell types and thus it is feasible that the BMP4 prodomain might be required to facilitate interactions with obligate ECM binding partners present on ectodermal, but not mesodermal cells.
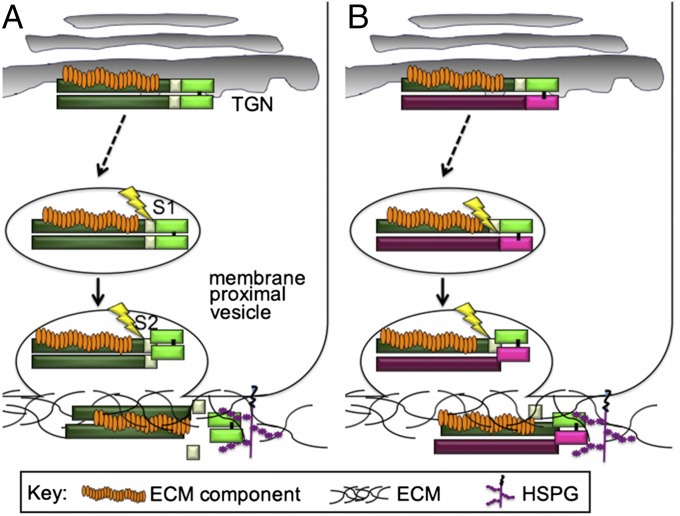
Our recent studies support a role for the BMP4 prodomain in targeting the cleaved homodimeric ligand to ECM binding partners and we propose that it plays a similar role in promoting heterodimer activity. Specifically, we have shown that mice carrying a knock-in mutation that causes mature BMP4 to be released prematurely from the prodomain die during embryogenesis due to destabilization of the cleaved ligand (53). Our findings support a model in which the transient prodomain/mature ligand complex that forms during sequential cleavage targets mature BMP4 to binding partners in the ECM that protect the ligand from degradation and thus promote signaling (Fig. 6A). Specifically, we have shown that BMP4 is sequentially cleaved at the S1 and S2 sites in a post-trans-Golgi network (TGN) vesicle and that cleavage is closely coupled to secretion (53) (Fig. 6A). We propose that one or more ECM components bind to the BMP4 prodomain intracellularly, analogous to the intracellular binding of LTBP to the TGFβ prodomain (40), and anchor the S1 cleaved prodomain/ligand complex in the ECM. This enables mature BMP4 to be passed off to HSPGs or other binding partners coincident with S2 cleavage. Our current data showing that the prodomain of BMP4 is necessary and sufficient for maximal BMP4/7 heterodimer activity, and that the prodomain remains associated with the heterodimer following cleavage support a model in which the BMP4 prodomain functions in a similar manner to target mature BMP4/7 heterodimers to HSPGs or other binding partners located in the ECM (Fig. 6B). Specifically, we propose that the BMP4 prodomain, but not the BMP7 prodomain, interacts with ECM components that are required to anchor the complex in the matrix, and/or to enable the ligand to interact with additional binding partners. These binding partners may stabilize the protein, facilitate interactions with the receptor complex, or otherwise support the intrinsic activity of the mature heterodimer. Additional studies will be required to test this model and to identify the specific ECM proteins that interact with each fragment of the cleaved precursor protein.
Fig. 6.
Hypothetical model for the role of the prodomain in promoting homodimer and heterodimer activity. (A) One or more ECM proteins bind to the prodomain of BMP4 intracellularly and traffic with the precursor to the cell surface where BMP4 is sequentially cleaved in a membrane proximal compartment. The transient ECM protein/prodomain/mature ligand complex that forms after S1 cleavage ensures that mature BMP4 is deposited in the ECM coincident with S2 cleavage, thereby facilitating association of the homodimeric ligand with HSPGs or other ECM proteins that are required for ligand stability. (B) The BMP4 prodomain, unlike the BMP7 prodomain, is sufficient to enable stable heterodimers to form. Following cleavage, the BMP4 and BMP7 prodomains remain associated with the heterodimeric ligand. The BMP4 prodomain binds to one or more ECM components, thereby anchoring the signaling complex in the matrix and/or presenting the heterodimeric ligand to HSPGs or other binding partners that facilitate signaling.
Materials and Methods
Xenopus Embryo Culture and Manipulation.
Embryos were obtained, microinjected, and cultured as described (54). Embryonic stages are according to Nieuwkoop and Faber (55). Embryos were stained for β-galactosidase activity using Red-gal (Research Organics, Inc.), and processed for in situ hybridization as described (56).
Immunoblots of Xenopus Extracts and Blastocele Fluid.
Proteins were harvested from 10 pooled DMZ explants by freon extraction as described previously (57). Fluid was aspirated and pooled from the blastocele of 10–20 gastrula (stage 10) embryos in each RNA injection group, as described (58). Proteins present in DMZs from two embryo equivalents, or blastocele fluid aspirated from the same number of embryos per experimental group (ranging from 10 to 15 in different experiments) were resolved by SDS/PAGE under reducing or nonreducing conditions and immunoblotting was performed as described (29). Proteins present in blastocele fluid were denatured by boiling in the presence of 0.5% SDS with or without 1% β-mercaptoethanol followed by deglycosylation with peptide-N-glycosidase F (PNGase F). Proteins were resolved by 15% SDS/PAGE and transferred onto PVDF membranes. Membranes were probed with anti-phosphoSmad1/5/8 (1:1,000, Cell Signaling), anti-actin (1:5,000, Sigma), anti-myc (71D10 Cell Signaling, 1:1,000), anti-HA (3F10, 1:1,000; Roche), anti-Flag (M2, 1:1,000; Sigma) antibodies. Immunoreactive proteins were detected using Enhanced Chemiluminescence reagent (Pierce). Images were scanned and relative band density was quantitated using ImageJ.
Transient Transfection, Coimmunoprecipitation and Immunoblot Analysis.
HEK293 cells were transiently transfected with expression plasmids encoding mouse BMP4 and/or BMP7 (250 ng of BMP4 or BMP7 alone or 125 ng of BMP4 plus 125 ng of BMP7) using Lipofectamine 2000 (Invitrogen) and cultured in OptiMEM-I (Invitrogen) for 20–24 h. The total amount of DNA transfected in each experiment was normalized to 2 µg by the addition of pCS2 + DNA. For coimmunoprecipitation assays, cleavage products were immunoprecipitated from 600 µL of conditioned media by overnight incubation with rabbit anti-myc (71D10, Cell Signaling) or anti-Flag (2368, Cell Signaling) antibodies and protein A-conjugated beads. Conjugates were washed successively with 700 µL of 500 mM NaCl, 50 mM Tris pH 8.0, 0.1% Nonidet P-40, 1 mM EDTA, 0.25% gelatin, 0.02% Na azide, then 700 µL of 50 mM Tris pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.02% Na azide, 0.1% SDS, 0.5% deoxycholic acid, and finally with 700 µL of 10 mM Tris pH 7.5, 0.1% Nonidet P-40. Proteins were precipitated from the remaining 250 µL of conditioned medium using 10% TCA. Immunoprecipitated and TCA precipitated (input) proteins were deglycosylated with PNGase F, separated by SDS/PAGE on 12% (wt/vol) acrylamide gels, and transferred onto PVDF membrane. Membranes were probed with antibodies specific for HA (3F10, Roche) or the BMP7 prodomain (mAb2) (18) and immunoreactive proteins were detected using Enhanced Chemiluminescence reagent (Pierce).
Statistical Analysis.
A student's t test was used to compare differences in DAI or pSMAD1 levels between two groups. Differences in Xbra induction were analyzed using GraphPad software to conduct two-way ANOVA followed by a Bonferroni multiple comparisons test. Differences with P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Lynn Sakai for providing BMP7 prodomain antibody, Megan Williams and Mary Mullins for helpful comments on the manuscript, and Chris Gregg for advice on statistical analysis. This work was supported in part by NIH Grant R01HD037976 (to J.L.C.) and by NIH Postdoctoral Fellowship 5T32DK007115-37 (to J.M.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501449112/-/DCSupplemental.
References
- 1.Bragdon B, et al. Bone morphogenetic proteins: A critical review. Cell Signal. 2011;23(4):609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84(12):1093–1103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- 3.Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012;23(1-2):61–67. doi: 10.1016/j.cytogfr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Aono A, et al. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210(3):670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 5.Hazama M, Aono A, Ueno N, Fujisawa Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209(3):859–866. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- 6.Israel DI, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13(3-4):291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 7.Nishimatsu S, Thomsen GH. Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech Dev. 1998;74(1-2):75–88. doi: 10.1016/s0925-4773(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 8.Schmid B, et al. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127(5):957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun. 1997;232(1):153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- 10.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11(5):637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120(6):873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho AM, et al. Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol. 2008;8:35. doi: 10.1186/1471-213X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JT, et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet. 1997;17(1):58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs MJ, et al. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol. 2010;24(7):1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, et al. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev. 2001;15(21):2797–2802. doi: 10.1101/gad.940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Künnapuu J, Björkgren I, Shimmi O. The Drosophila DPP signal is produced by cleavage of its proprotein at evolutionary diversified furin-recognition sites. Proc Natl Acad Sci USA. 2009;106(21):8501–8506. doi: 10.1073/pnas.0809885106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sopory S, Kwon S, Wehrli M, Christian JL. Regulation of Dpp activity by tissue-specific cleavage of an upstream site within the prodomain. Dev Biol. 2010;346(1):102–112. doi: 10.1016/j.ydbio.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory KE, et al. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem. 2005;280(30):27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- 19.Jones WK, et al. Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors. 1994;11(3):215–225. doi: 10.3109/08977199409046919. [DOI] [PubMed] [Google Scholar]
- 20.Degnin C, Jean F, Thomas G, Christian JL. Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol Biol Cell. 2004;15(11):5012–5020. doi: 10.1091/mbc.E04-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray AM, Mason AJ. Requirement for activin A and transforming growth factor—beta 1 pro-regions in homodimer assembly. Science. 1990;247(4948):1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- 22.Constam DB, Robertson EJ. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol. 1999;144(1):139–149. doi: 10.1083/jcb.144.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammonds RG, Jr, et al. Bone-inducing activity of mature BMP-2b produced from a hybrid BMP-2a/2b precursor. Mol Endocrinol. 1991;5(1):149–155. doi: 10.1210/mend-5-1-149. [DOI] [PubMed] [Google Scholar]
- 24.Groppe J, et al. Biochemical and biophysical characterization of refolded Drosophila DPP, a homolog of bone morphogenetic proteins 2 and 4. J Biol Chem. 1998;273(44):29052–29065. doi: 10.1074/jbc.273.44.29052. [DOI] [PubMed] [Google Scholar]
- 25.Sengle G, Ono RN, Lyons KM, Bächinger HP, Sakai LY. A new model for growth factor activation: Type II receptors compete with the prodomain for BMP-7. J Mol Biol. 2008;381(4):1025–1039. doi: 10.1016/j.jmb.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman DC, et al. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133(10):1933–1942. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- 27.Sopory S, Nelsen SM, Degnin C, Wong C, Christian JL. Regulation of bone morphogenetic protein-4 activity by sequence elements within the prodomain. J Biol Chem. 2006;281(45):34021–34031. doi: 10.1074/jbc.M605330200. [DOI] [PubMed] [Google Scholar]
- 28.Hawley SH, et al. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9(23):2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- 29.Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. 2009;284(40):27157–27166. doi: 10.1074/jbc.M109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17(16):4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengle G, et al. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283(20):13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns. 2009;9(5):255–265. doi: 10.1016/j.gep.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki A, Kaneko E, Ueno N, Hemmati-Brivanlou A. Regulation of epidermal induction by BMP2 and BMP7 signaling. Dev Biol. 1997;189(1):112–122. doi: 10.1006/dbio.1997.8652. [DOI] [PubMed] [Google Scholar]
- 34.Valera E, Isaacs MJ, Kawakami Y, Izpisúa Belmonte JC, Choe S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE. 2010;5(6):e11167. doi: 10.1371/journal.pone.0011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi M, et al. Latent TGF-β structure and activation. Nature. 2011;474(7351):343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. 2011;29(5):174–186. doi: 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- 37.Jones CM, Lyons KM, Lapan PM, Wright CV, Hogan BL. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- 38.Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: A ventralizing factor in early Xenopus development. Development. 1992;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez F, Rifkin DB. Extracellular microfibrils: Contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21(5):616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10(5):1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: Extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286(7):5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama T, et al. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol. 2008;313(1):408–419. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belenkaya TY, et al. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119(2):231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Kuo WJ, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21(22):4028–4041. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Midorikawa Y, et al. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103(4):455–465. doi: 10.1002/ijc.10856. [DOI] [PubMed] [Google Scholar]
- 46.Irie A, Habuchi H, Kimata K, Sanai Y. Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem Biophys Res Commun. 2003;308(4):858–865. doi: 10.1016/s0006-291x(03)01500-6. [DOI] [PubMed] [Google Scholar]
- 47.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol. 2001;231(1):31–46. doi: 10.1006/dbio.2000.0127. [DOI] [PubMed] [Google Scholar]
- 48.Serpe M, et al. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14(6):940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenny AP, et al. Sizzled-tolloid interactions maintain foregut progenitors by regulating fibronectin-dependent BMP signaling. Dev Cell. 2012;23(2):292–304. doi: 10.1016/j.devcel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley R, et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184(4):597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeya M, et al. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133(22):4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- 52.Harris RE, Ashe HL. Cease and desist: Modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12(6):519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilak A, et al. Simultaneous rather than ordered cleavage of two sites within the BMP4 prodomain leads to loss of ligand in mice. Development. 2014;141(15):3062–3071. doi: 10.1242/dev.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mimoto MS, Christian JL. Manipulation of gene function in Xenopus laevis. Methods Mol Biol. 2011;770:55–75. doi: 10.1007/978-1-61779-210-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. North Holland Publishing Co.; Amsterdam: 1967. [Google Scholar]
- 56.Nakayama T, et al. Xenopus Smad8 acts downstream of BMP-4 to modulate its activity during vertebrate embryonic patterning. Development. 1998;125(5):857–867. doi: 10.1242/dev.125.5.857. [DOI] [PubMed] [Google Scholar]
- 57.Moon RT, Christian JL. Microinjection and expression of synthetic mRNAs in Xenopus embryos. Technique. 1989;1:76–89. [Google Scholar]
- 58.Birsoy B, et al. XPACE4 is a localized pro-protein convertase required for mesoderm induction and the cleavage of specific TGFbeta proteins in Xenopus development. Development. 2005;132(3):591–602. doi: 10.1242/dev.01599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.