Significance
Recent reports of widespread drought-induced plant die-back have enhanced the need to understand variation in plant hydraulic strategies and to determine which species are susceptible to drought-induced mortality. Finding meaningful ways to describe the many different types of responses plants might demonstrate in biodiverse regions can be extremely challenging. Here, we present the architecture in which functional traits can be incorporated into a known hydraulic framework to provide additional insight into the range of hydraulic strategies present in diverse communities. Merging stomatal regulation strategies that represent an index of water use behavior with xylem hydraulic strategies that represent water transport and vulnerability to water deficit behavior facilitates a more comprehensive framework to characterize plant response to drought.
Keywords: drought, functional traits, plant hydraulics, xylem vulnerability, stomatal response
Abstract
Attempts to understand mechanisms underlying plant mortality during drought have led to the emergence of a hydraulic framework describing distinct hydraulic strategies among coexisting species. This framework distinguishes species that rapidly decrease stomatal conductance (gs), thereby maintaining high water potential (Px; isohydric), from those species that maintain relatively high gs at low Px, thereby maintaining carbon assimilation, albeit at the cost of loss of hydraulic conductivity (anisohydric). This framework is yet to be tested in biodiverse communities, potentially due to a lack of standardized reference values upon which hydraulic strategies can be defined. We developed a system of quantifying hydraulic strategy using indices from vulnerability curves and stomatal dehydration response curves and tested it in a speciose community from South Africa’s Cape Floristic Region. Degree of stomatal regulation over cavitation was defined as the margin between Px at stomatal closure (Pg12) and Px at 50% loss of conductivity. To assess relationships between hydraulic strategy and mortality mechanisms, we developed proxies for carbon limitation and hydraulic failure using time since Pg12 and loss of conductivity at minimum seasonal Px, respectively. Our approach captured continuous variation along an isohydry/anisohydry axis and showed that this variation was linearly related to xylem safety margin. Degree of isohydry/anisohydry was associated with contrasting predictions for mortality during drought. Merging stomatal regulation strategies that represent an index of water use behavior with xylem vulnerability facilitates a more comprehensive framework with which to characterize plant response to drought, thus opening up an avenue for predicting the response of diverse communities to future droughts.
Increased frequency of plant mortality is often predicted in response to global climate change-induced drought events (1). Such a threat has resulted in attempts to understand the precise mechanisms underlying plant mortality and to determine which species are more vulnerable than others (2, 3). One of the most promising avenues is a hydraulic framework describing the influence during drought periods of distinct hydraulic regulation strategies on carbon balance and the integrity of the soil-plant-atmosphere hydraulic pathway (2, 4, 5). The framework’s success has come with the realization that two major mechanisms of mortality, carbon starvation and hydraulic failure, are intimately linked (2, 6, 7). In land plants, water loss and CO2 uptake are closely coupled, because the vast majority of gaseous exchange occurs through a single pathway: stomatal pores on the leaf surface. This coupling sets up a long-recognized carbon assimilation vs. water conservation trade-off (8). To achieve adequate rates of assimilation and to avoid becoming carbon-limited, plants need to maintain a favorable water status that, in turn, begets high stomatal conductance (gs) (9). High gs also promotes high transpiration rates, which, for a given level of whole-plant hydraulic conductance, generate increasingly negative leaf and stem water potential (Px) and greater tension in the water column, as posited by the cohesion-tension theory of sap ascent (10). Increasing tension in the water column may eventually lead to cavitation within the xylem by promoting the seeding of air bubbles into water-filled conduits (5, 11). Because cavitation reduces total plant hydraulic conductance, it limits the ability of a plant to transport water and nutrients to sites of growth and productivity, and ultimately may lead to death (2, 6).
As one might expect, attempts have been made to categorize plant species’ responses according to which process, carbon uptake or water conservation, is favored. Frequently, hydraulic strategies are classified along a continuous axis from drought avoidance (or isohydric Px regulation) to drought tolerance (or anisohydric Px regulation). Isohydric species respond to dehydration by rapidly decreasing gs, thereby limiting excessive water loss and maintaining high plant Px (12). Such drought avoiders may prevent cavitation occurring in xylem conduits, albeit at the cost of carbon assimilation during dry periods. Anisohydric species maintain relatively high gs at low Px, thereby maintaining CO2 uptake, but endure the cost of possible formation of embolism within conduits (2, 12) or the cost of constructing xylem that can resist negative Pxs (13).
A major drawback of the current hydraulic framework is that it has yet to be thoroughly examined in highly diverse communities, where it is unclear to what extent species separate out into the isohydric-anisohydric dichotomy or form a continuum of hydraulic strategies. In functionally diverse communities, significant variability in key hydraulic functional traits, such as rooting depth (14), may produce a high diversity of drought response strategies. Much of the limitation in our understanding can be traced back to the model systems explored in the original hydraulic framework studies. These systems were often composed of relatively few codominant species, which made detailed, long-term monitoring tractable (2, 15–18). To address this shortcoming, we aimed to develop a functional traits-based framework that can be applied to the world’s biodiversity hotspots, where knowledge of the vulnerability to future drought events might be particularly urgent. Functional traits are often well-coupled to environmental stresses, are less time-consuming to measure, and have been used widely in plant ecology to explore functional strategies of different species (19–22). Provided that we can develop a system whereby plant functional traits capture a hydraulic strategy of a species, they may also afford rapid comparisons between multiple species (21). Here, we propose a framework that uses several commonly measured traits to define a hydraulic response and show that it provides insight into potential mechanisms of mortality within diverse communities.
To define a hydraulic strategy, it is necessary to include some measure of xylem vulnerability (2, 21). The resistance of a plant to embolism propagation is quantified by describing the loss of conductivity with declining Px: so-called vulnerability curves (4, 23). Although several standardized indices of vulnerability can be taken from these curves, the most commonly used is the Px at 50% loss of conductivity (P50). Species have been shown to vary considerably in P50, ranging from −0.18 MPa (highly vulnerable) to −14.1 MPa (highly resistant) (24). Much of this variation has been explained by a safety-efficiency trade-off, because xylem conduits (mostly vessels in angiosperms or tracheids in conifers) with greater resistance to embolism and implosion usually [but not always (25)] have lower maximum hydraulic conductance (24, 26–28). However, in isolation, xylem vulnerability to cavitation does not provide a robust measure of hydraulic conservatism or drought tolerance, as shown by the lack of a clear relationship between P50 and aridity (19, 24). As previously mentioned, it is essential to account for the CO2 uptake-water loss trade-off, in addition to the safety-efficiency trade-off of xylem conduits, to define a hydraulic strategy (29–32).
A way to quantify these trade-offs emerges from the concept of safety margins, usually described as the difference between a critical point on the vulnerability curve and the minimum seasonal Px, referred to as Pmin. Safety margins are used to describe the degree of conservatism in a plant’s hydraulic strategy (19, 30, 33). In this context, Pmin is often assumed to provide a measure of stomatal regulation of Px, where large positive differences imply a relatively conservative response and small differences (or even negative differences) suggest a hydraulically risky response (19). Although this assumption is true for many species, many other traits may also influence Pmin, causing it to either underestimate or overestimate the influence of stomatal regulation on Px. For example, shallow-rooted species that close stomata early in response to drought to avoid hydraulic failure may still reach low Px as the soil dries out. Other species are able to maintain high Px independent of stomatal regulation, such as through access to permanent (deep) moisture sources (14, 34, 35) or regular uptake of atmospheric moisture (36, 37). These factors make Pmin a potentially unreliable measure of stomatal regulation of Px.
To assess stomatal response directly during desiccation, we propose defining standardized critical points from Pg Px response curves (Fig. 1A). One such point, which we term Pg12, is particularly useful as a proxy of the point of stomatal closure (Fig. 1A). Pg12 is defined as the Px at which the tangent to the response curve at 50% of maximum stomatal conductance (gmax) intersects with the Px axis (Fig. 1A). The term “Pg12” is derived from the observation that the gs at this Px is ∼12% of gmax (Fig. 1A and Methods). The difference between Pg12 and P50 (Fig. 1B) can then be used as a safety margin for determining stomatal regulation strategy (Fig. 1C), because neither point in isolation provides a reliable measure of hydraulic conservatism or drought tolerance. Large positive differences (e.g., the point labeled “2 & 3” in Fig. 1C) indicate that stomatal closure occurs before large losses of conductivity, whereas negative differences (e.g., the point labeled “1 & 4” in Fig. 1C) indicate stomatal closure subsequent to P50. We used these safety margins as a proxy for the degree of hydricity of a species, with positive values being defined as isohydric and negative values being defined as anisohydric. By defining a measure of hydricity, we were then able to test hypotheses relating to the trade-offs between CO2 uptake and water loss. In particular, we hypothesized that if a trade-off exists between CO2 uptake and water loss, we might expect isohydric species (drought avoiders) to have greater xylem safety margins in comparison to anisohydric species (drought tolerators).
Fig. 1.
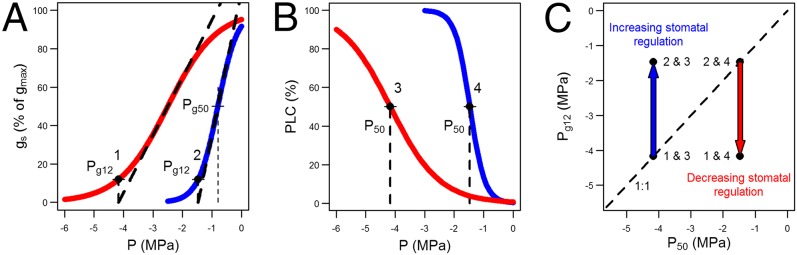
Functional traits can be used to describe the hydraulic regulation strategy of a species. (A) gs response curves provide a measure of the sensitivity of stomata to desiccation: Pg12. (B) Vulnerability curves provide a measure of xylem resistance to embolism: P50. The colored curves illustrate two extreme responses for each: early (blue) and late (red) stomatal response in A and vulnerable (blue) and resistant (red) xylem in B. (C) These traits can be combined to define a safety margin capturing the degree to which stomatal closure regulates cavitation.
An appreciation of why knowledge of the hydraulic strategy of a species is essential emerges from the logical conclusion to the CO2 uptake-water loss trade-off. Under severe drought events, plants may die from two mechanisms that are not mutually exclusive: carbon starvation or hydraulic failure (38). Although the precise details of these mechanisms are intensely debated (6, 39), there is evidence that both have contributed to recent mortality events (16, 39, 40). The intensity and duration of a drought are critical to understanding which species are likely to succumb to either hydraulic failure or carbon starvation (2, 16, 40). Isohydric species are more likely to succumb to carbon starvation during protracted mild drought, whereas anisohydric species are more likely to die from hydraulic failure during short but severe droughts (2, 40). A further test of our traits-based approach is whether it fits into this predictive framework: Can we use our assessment of hydraulic strategy to assess the likely mechanism of drought-induced mortality for each species in a diverse community?
To answer this question, we developed proxies for the likelihood of suffering carbon starvation or hydraulic failure using combinations of functional traits. We used the number of days since Px in the field surpassed the point of stomatal closure (Pg12) as a novel proxy for the degree of carbon limitation. We reasoned that the longer a species spends with its stomata closed, the more likely it is to incur leaf-level carbon limitation, because assimilation rate is linearly related to gs (9, 41). To assess the degree to which a species suffers hydraulic failure during drought conditions, we calculated the percent loss of conductivity (PLC) at Px. The two proxies can also be envisaged as corresponding to axes of drought intensity and duration, which are considered crucial to predictions of drought mortality of distinct hydraulic strategies (2). We then hypothesized that species defined as more isohydric by our traits-based system (i.e., species with more positive Pg12 − P50 values) would tend to be more carbon-limited during a drought, whereas anisohydric species would tend to incur hydraulic failure.
We tested our standardizable traits-based framework system in highly diverse mountain fynbos communities located within South Africa’s Cape Floristic Region (CFR). The CFR presents an excellent system in which to explore these questions and examine the suitability of the hydraulic framework in understanding how complex communities might respond to drought events. Much of the CFR is exposed to hot, dry summers typical of Mediterranean-type climate regions, ensuring that the plants are regularly exposed to drought events (42). In addition, strong associations between climatic variables and diversity within the region suggest that climate might be an important driver of functional diversity (14, 43). Although previous long-term rainfall manipulation or in situ studies have shown the presence of contrasting drought response strategies within mountain fynbos communities, these studies have only been able to capture the response of a handful of species (14, 44, 45). Given the exceptionally diverse nature of the flora (42), a more rapid assessment of the response of a wider range of species is desirable. This capacity may be particularly pressing, considering that the CFR is predicted to become hotter and drier with future climate change (46, 47). We quantified the response of gs to changes in Px and related the point of stomatal closure to standardized measures of vulnerability for a wide range of species. Here, we show that our traits-based framework assesses the degree of stomatal regulation of cavitation of a species and allows direct comparison between species within this global biodiversity hotspot. We also show how this system can be extended beyond the CFR to allow predictions about likely mechanisms of mortality within highly diverse communities elsewhere.
Results and Discussion
Here, we have extended previous research by presenting the architecture in which functional traits can be incorporated into a known hydraulic framework to provide additional insight into the range of hydraulic strategies present in diverse communities (Fig. 1). The ability to quantify the range of hydraulic regulation strategies rapidly within a single, highly diverse ecosystem is critically important in being able to assess vulnerability to future drought (22). Merging stomatal regulation strategies, which represent an index of water use behavior, with xylem hydraulic strategies, which represent water transport and vulnerability to water deficit behavior, facilitates a more comprehensive framework with which to characterize plant response to drought. This architecture may prove to be most valuable in biodiverse regions, where finding meaningful ways to determine the diversity of plant responses to drought can be extremely challenging.
The value of direct quantification of stomatal closure dynamics is shown by a comparison of the relationships between P50 and Pg12, P50 and Pmin, and Pg12 and Pmin (Fig. 2). Although the range of Px over which stomata closed was less than the range of P50, it was large enough in magnitude to separate the different species. Some species displayed stomatal closure at Px higher than −1 MPa, and others displayed stomatal closure at Px at lower than −3 MPa (Fig. 2A). This variation of 2 MPa shows that a varied, continuous range of stomatal responses to declining Px can exist within species-rich communities. However, unlike the relationship between Pmin and P50 (Fig. 2B), Pg12 and P50 were not linearly related (Fig. 2A). There exist at least two possible alternative explanations for this observation. On the one hand, stomatal closure might be entirely unrelated to xylem vulnerability. This explanation is contrary to a well-supported functional role for stomatal closure in avoiding xylem cavitation and is also inconsistent with several recent findings showing coordination between stomatal closure and xylem vulnerability for a range of species (28, 48, 49). A more plausible alternative explanation is that there exists a nonlinear relationship between these two variables that is influenced by multiple trade-offs (28, 32). As we show below, species that close their stomata relatively early (in terms of P50) might well be favoring CO2 uptake during wet conditions while avoiding loss of hydraulic conductivity during dry periods (hydraulic plasticity). Conversely, species that close their stomata relatively late might be playing a riskier hydraulic game to maintain carbon uptake later into a drought.
Fig. 2.
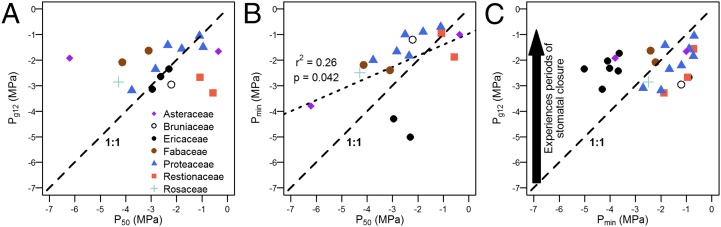
Relationships between xylem vulnerability (P50) and Px at stomatal closure (A; Pg12) and minimum Px (B; Pmin), and between Pg12 and Pmin for fynbos species (C). These data highlight the inadequacy of Pmin for capturing the point of stomatal closure.
Comparison of Pg12 and Pmin allowed us to identify species in which Pmin overestimated or underestimated stomatal response to declining Px (Fig. 2C). Over 90% of the species in which Pmin was lower than Pg12 were ericoid (i.e., small- to medium-sized shrubs with small leaves). Species of this functional type occurring in mountain fynbos generally have shallow rooting depths (14), which, although promoting the uptake of episodic rainfall, may also expose them to low soil Pxs during seasonal drought periods (11). Our results are consistent with the notion that reduced leaf size may be adaptive under these hot, dry conditions, because it decreases the boundary layer thickness, promoting convective heat loss without a further loss of water (50). The majority of species that experienced higher Pmin than Pg12 were from the Proteaceae and Restionaceae (Fig. 2C). The Proteaceae within the CFR are commonly deep-rooted (51), consistent with the notion that they are able to avoid desiccation partly by having access to moist soil layers (14). However, this explanation does not fit with the Restionaceae, which are composed of shallow-rooted, reed-like species. Here, low Pg12 and high Pmin are consistent with previous suggestions that species of this functional type are able to take up cloud moisture or dew (14, 52). Taken together, these findings suggest an additional utility of our traits-based approach: It highlights unusual combinations of functional traits and reveals alternative physiological strategies to coping with drought stress. Applying our system within diverse biomes should produce testable hypotheses regarding the functionality of species.
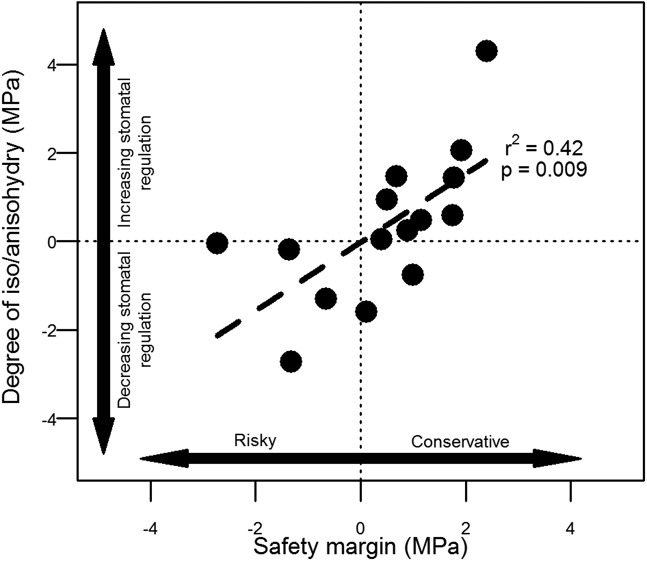
Returning to the question of potential trade-offs captured by the relationship between P50 and Pg12, it is significant that species within mountain fynbos communities separated out along a continuous range of stomatal regulation strategies or degree of isohydry/anisohydry (Fig. 3). The large amount of variation in Pg12 − P50 is consistent with a recent global comparison of xylem safety margins (measured as Pmin − P50), which revealed that species within Mediterranean and woodland biomes displayed the highest range of values (19). Given a potential trade-off between carbon assimilation and hydraulic safety, one might propose that these species are occupying distinct water regulation niches. We provide further support for this notion by showing a linear relationship between degree of isohydry/anisohydry (Pg12 − P50) and xylem safety margin (Pmin − P50) (Fig. 4). Species that displayed a conservative xylem safety margin (i.e., had a low risk of experiencing major xylem cavitation in situ) were also those species that closed their stomata relatively early in response to desiccation (Fig. 4). On the other hand, those species that closed their stomata relatively late were also those species that displayed a relatively risky xylem safety margin (Fig. 4). Our interpretation of these results is that isohydric species are more likely to maximize carbon uptake when conditions are favorable, but to do so and to avoid hydraulic failure, they also need to maintain larger safety margins. Anisohydric species, on the other hand, tend to favor lower carbon uptake over longer periods, but with lower rates of water loss. As a result, these species can afford to have smaller safety margins. Our interpretation is consistent with previous observations showing that species with higher maximum gs displayed greater sensitivity and an increased rate of stomatal closure in response to changes in vapor pressure deficit (53). It is also supported by observations that isohydric species are able to recover gas exchange rates more rapidly following a drought compared with anisohydric species (18, 54).
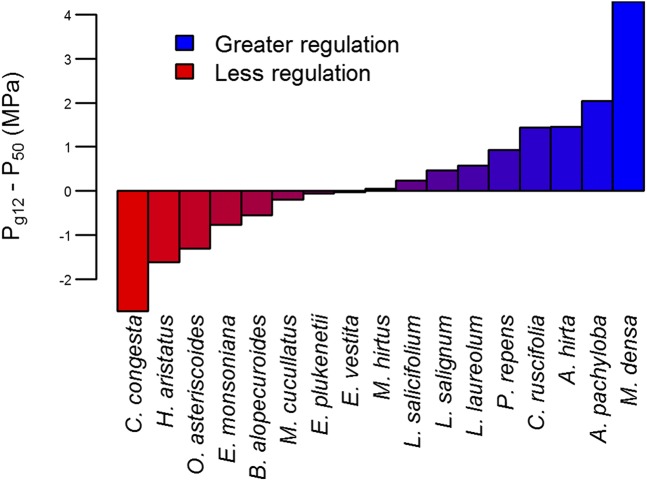
Fig. 3.
Stomatal safety margin (Pg12 − P50) of species from mountain fynbos showing the range of stomatal regulation strategies within a single community. We propose that Pg12 − P50 provides a proxy for degree of isohydry/anisohydry. The full names for all species shown are as follows: Cannomois congesta, Hypodiscus aristatus, Osmitopsis asteriscoides, Erica monsoniana, Brunia alopecuroides, Mimetes cucullatus, Erica plukenetii, Erica vestita, Mimetes hirtus, Leucadendron salicifolium, Leucadendron salignum, Leucadendron laureolum, Protea repens, Cliffortia ruscifolia, Aspalathus hirta, Aspalathus pachyloba, Metalasia densa.
Fig. 4.
Degree of isohydry/anisohydry (defined as the degree of stomatal regulation of cavitation or Pg12 − P50) varies with the stem-level hydraulic safety margin (Pmin − P50) within mountain fynbos communities. More isohydric species tend to have larger safety margins, whereas more anisohydric species tend to have smaller (or negative) safety margins.
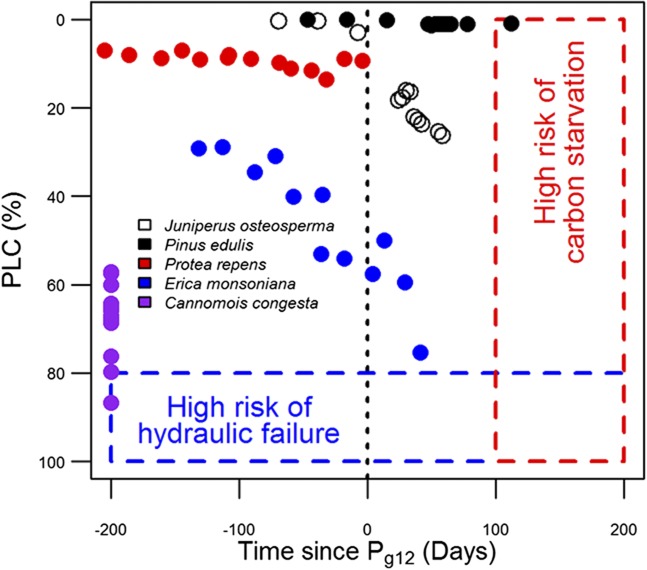
Combinations of functional traits also allowed us to explore how potential mechanisms of mortality might differ between species (Fig. 5). We selected three fynbos species with contrasting hydraulic regulation strategies for which we could obtain long-term in situ Px data from a previously published source (44). Protea repens, Erica monsoniana, and Cannomois congesta displayed significant variation in the degree to which stomata regulate xylem cavitation (measured by Pg12 − P50 as shown in Fig. 3), and thus are likely to have contrasting hydraulic strategies. These species displayed significant variation in the degree to which they were likely to suffer carbon limitation and hydraulic failure during drought periods in the field (Fig. 5). Significantly, there appeared to be a strong association between hydraulic strategy and likely mechanism of mortality. Of the three species selected, P. repens had the most conservative stomatal response, closing rapidly in response to desiccation (Fig. 3). In our predictive framework, the trajectory that P. repens displayed indicated that it was likely to suffer from carbon limitation if the drought persisted. In contrast, C. congesta displayed a relatively risky stomatal strategy (Fig. 3), and the trajectory of this species indicated that it was likely to suffer from hydraulic failure (Fig. 5). The degree of stomatal regulation of cavitation in E. monsoniana was intermediate between the previous two species (Fig. 3). Remarkably, E. monsoniana appears to be vulnerable to both carbon starvation and hydraulic failure (Fig. 5), making this species highly susceptible to drought mortality.
Fig. 5.
Likely mechanism of mortality experienced by a species can be assessed by plotting seasonal PLC against the time since stomatal closure. Time since stomatal closure (defined as time since Pg12) provides an indication of the duration of the drought, and hence the degree of carbon limitation that a species endures. Negative values are produced when Px is less negative than Pg12 (i.e., stomata remain open) and the plant is unlikely to be carbon-limited. Positive values are produced when Px is below Pg12 and plants are more likely to be carbon-limited. PLC is used as a proxy for risk of hydraulic failure. Trajectories for all species were created using previously published seasonal Px measurements and vulnerability curves (17, 44, 55).
These results are consistent with previous findings in fynbos communities (14), which have shown Erica species to be particularly sensitive to drought. In a rainfall exclusion study, anisohydric Erica species displayed the most amount of drought-induced die-back (14). Deep-rooted proteoid species displayed little drought mortality and were considered to be isohydric (14). Restioids (i.e., Cannomois) also exhibited patchy drought-induced die-back within individuals, consistent with hydraulic failure (14).
As an independent test of our framework, we explored how well we could categorize the well-documented drought responses of piñon and juniper from the southwestern United States. Using data from a previous study (17), we were able to show that our trait combinations predicted outcomes consistent with the reported mechanisms of drought mortality in these species. The isohydric Pinus edulis incurred increasing carbon limitation during a seasonal drought period (Fig. 5), consistent with the hypothesis that this species suffers drought-mediated carbon starvation in the field (17, 18, 38, 40, 55). The anisohydric Juniperus osteosperma appeared to incur increasing hydraulic failure, as well as a protracted period of stomatal closure (Fig. 5), consistent with field observations (43) and the hypothesis that this species is likely to incur hydraulic failure resulting from declining Px during intense drought (2). Taken together, the findings presented here suggest that expanding upon previous research that contrasted isohydric (avoidance) and anisohydric (tolerance) hydraulic strategies in plants by including additional traits (Pg12, Px, and Pg12 − P50) has substantial merit because using our trait combinations has allowed us to predict the likely mechanism of mortality within biodiverse regions.
The approach of using functional traits to infer the hydraulic strategy of a species has tremendous potential. Our system provides a standardizable means of identifying variation in hydraulic regulation strategies based on trait combinations and enables comparison both within and between highly diverse communities. Our examples emphasize the importance of explicitly assessing coordination between stomatal and xylem traits, as well as Pmin, and the benefit of standardized measures for comparison between multiple species. We have also shown how variation in trait combinations may assist in explaining potential mechanisms of mortality. This approach may prove valuable for rapid assessment of the vulnerability of communities to future drought events, particularly in species-rich communities where detailed, long-term physiological studies are unfeasible.
Methods
Study Sites and Species.
Species were sampled from mountain fynbos communities located within the southwest CFR (a full list of species is provided in Table S1). Specific sites were located at Jonaskop in the Riviersonderend mountain range (lat 33° 15′ 48.34′′ S, long 19° 31′ 33.97′′ E, elevation of 981 m), the Kogelberg Biosphere (lat 34° 19′ 02.57′′ S, long 18° 57′ 39.74′′ E, elevation of 61 m), and the Silvermine section of the Table Mountain National Park (lat 34° 06′ 26.45′′ S, long 18° 26′ 31.37′′ E, elevation of 375 m). These study sites were chosen because they contain particularly diverse floral communities that have been the focus of previous ecophysiological studies, including assessments of the variation in vulnerability of stem xylem to cavitation and long-term drought response investigations (14, 56–58).
Stomatal Response to Dehydration.
The relationship between gs, and Px was examined using a benchtop dehydration method. Stems or branches were cut under water in the field and subsequently transported back to the laboratory in water. Each stem was placed on a benchtop in a temperature- and light-controlled chamber and allowed to dry to a range of Px values. We measured gs using an IR gas analyzer (IRGA; Li-Cor 6400) and expressed per leaf area. Light intensity was kept constant at 1,500 μmol⋅m−2⋅s−1, and humidity was maintained slightly below ambient using a desiccant. Immediately following the gs measurement, the target shoot was cut and Px was measured using a Scholander pressure chamber (PMS Instrument Company). A Weibull function was fitted to the gs response curve for each species (Fig. 1A):
| [1] |
where a is a fitted parameter describing the slope of the curve and b is the Px at 50% loss of gmax (Pg50). Similar to the analysis of Domec and Gartner (59), we parameterized this model and extracted several standardized values describing the sensitivity of gs to Px. Specifically, we found the derivative of Eq. 1:
| [2] |
which provides the slope of the tangential line to Eq. 1 at each Px. We used this derivative to find the slope of the tangential line at Pg50 (Fig. 1A). We then calculated the Px intercept of this tangential line, which we termed Pg12, using the following equation:
| [3] |
We used Pg12 as a proxy for the Px at stomatal closure (points 1 and 2 in Fig. 1A).
Gas Exchange and Px of Plants in Situ.
To assess the efficacy of our reasonably simple benchtop dehydration technique, we compared laboratory-based measurements of Px and gs with those measurements taken in the field for six species over a period of three consecutive years as part of two separate long-term monitoring studies. We will briefly report the important details of these measurement campaigns as they have been reported elsewhere (14, 44). The gs was measured using an IRGA (Li-Cor 6400) at midday during spring and late summer over 3 y. Light intensity in the cuvette was set at 1,500 μmol⋅m−2⋅s−1, humidity was maintained just below ambient, and CO2 was held constant at 400 ppm. The gs was expressed on a per-leaf-area basis. The Px of the same shoot was measured immediately after gas exchange using a Scholander pressure chamber. There was generally very good agreement between gs response curves obtained using the benchtop dehydration method and data collected from long-term field measurements (Fig. S1).
Vulnerability Curves.
We obtained the PLC in response to declining Px (i.e., vulnerability curves) for 18 species (Table S1). To obtain vulnerability curves for as many species as possible, we measured vulnerability curves using both the benchtop dehydration method (60) and the air injection method (61), and we also gathered data from published studies (the studies in which vulnerability curve data were presented are listed in Table S1). We assessed the similarity of these measures by comparing the vulnerability curve of P. repens obtained using the benchtop dehydration method with the vulnerability curve of P. repens obtained using the air injection method and found strong agreement.
We used the benchtop dehydration method for P. repens, E. monsoniana, and C. congesta. For each species, we first determined the maximum vessel length to inform the minimum stem length needed to avoid any open vessels (62). To do this measurement, we cut five stems under water in the field and then placed tubing connected to an air tank on one end and made successive cuts from the opposite end. The mean length at which bubbles first emerged was taken as the maximum vessel length for each species. For the vulnerability curves, branches were cut under water in the field and subsequently dried to a range of Px values. Branches were then sealed in plastic bags for at least 1 h to equilibrate. The Px was measured from a shoot from each stem using a Scholander pressure chamber. The remaining branch was placed under water and cut into an unbranched segment. Several cuts were made at each end to ensure that the xylem tension relaxed (63). Native flow rate was measured by connecting each stem to tubing attached to a pressure head containing KCl solution (20 mM). Water was collected from the distal end of the stem and weighed on a balance. Once a consistent flow rate had been achieved and measured, the stems were removed and immediately connected to tubing connected to a high-pressure, filtered KCl solution for at least 1 h to remove any embolism. After flushing, the stems were reconnected to the pressure head and the flow rate was remeasured. Native stem-specific conductivity (Kn) and flushed stem-specific conductivity (Kmax) were calculated by expressing flow rate per pressure head per stem length per second. The PLC at each Px value was calculated as:
| [4] |
We quantified vulnerability curves of two species, Hypodiscus aristatus and Leucadendron laureolum, using the air injection method (60). Stems of these two species were collected in the field and flushed in the laboratory with filtered 20 mM KCl solution using high pressure (150 kPa) to remove any native embolism. Flushed stems were subsequently placed in air sleeves connected to a pressure tank after being scored slightly with a razor blade. The distal end of each stem was connected to tubing connected to a pressure head containing filtered 20 mM KCl solution. Flow rate was measured by periodically measuring the weight of vials connected to the axial end of the stem. Embolism was induced in the stem by applying different pressures to the sleeve for 10 min at a time. After each pressure treatment, the pressure was released and the stable flow rate was determined. The PLC was calculated as before using Eq. 4.
Vulnerability curves were modeled for each species using the Weibull function:
| [5] |
where a and b were parameters found using least-squares regression analysis.
Pmin.
The Pmin in the field was collected for nine species during summer dry periods. Because these data were from long-term datasets, we have confidence that they represent close to actual Pmin values in the absence of extreme drought. For each species, predawn and midday shoot water potential (Pshoot) of at least five individuals was sampled using a Scholander pressure chamber. For the remaining species, we extracted Pmin values from published sources (17, 19, 56, 58). A potential limitation of using midday Pshoot values to estimate PLC is that midday Pshoot will be more negative than stem Px, resulting in an overestimation of the amount of cavitation in stems (64). To reduce this error, we estimated PLC based on predawn Pshoot as opposed to midday values. Because the difference between stem Px and leaf Px is expected to be greatest under high transpiration rates, predawn measures will tend to reduce any difference between Px and Pshoot. Second, segmentation of xylem vulnerability often tends to make shoots more vulnerable than stems (33), which might counteract the difference created by sampling Pshoot.
Supplementary Material
Acknowledgments
Field access was kindly provided by the Du Plessis brothers and South African National Parks. Field assistance was provided by J. Nel, R. Karpul, M. Gibson, and A. Roddy. We thank the University of Cape Town, the A. W. Mellon Foundation, South African Environmental Observation Network, and the National Research Foundation for their financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503376112/-/DCSupplemental.
References
- 1.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259(4):660–684. [Google Scholar]
- 2.McDowell N, et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008;178(4):719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 3.Choat B. Predicting thresholds of drought-induced mortality in woody plant species. Tree Physiol. 2013;33(7):669–671. doi: 10.1093/treephys/tpt046. [DOI] [PubMed] [Google Scholar]
- 4.Tyree MT, Ewers FW. Tansley review no. 34. The hydraulic architecture of trees and other woody plants. New Phytol. 1991;119(3):345–360. [Google Scholar]
- 5.Zimmermann MH. Xylem Structure and the Ascent of Sap. Springer; Berlin: 1983. [DOI] [PubMed] [Google Scholar]
- 6.Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010;186(2):274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- 7.McDowell NG, et al. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol. 2011;26(10):523–532. doi: 10.1016/j.tree.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Cowan IR. Stomatal behaviour and environment. Adv Bot Res. 1977;4:117–118. [Google Scholar]
- 9.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33(1):317–345. [Google Scholar]
- 10.Dixon HH, Joly J. On the ascent of sap. Philos Trans R Soc London [Biol] 1895;186:563–576. [Google Scholar]
- 11.Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002;25(2):251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 12.Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot. 1998;49(Special Issue):419–432. [Google Scholar]
- 13.Hacke UG, Sperry JS. Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst. 2001;4(2):97–115. [Google Scholar]
- 14.West AG, et al. Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol. 2012;195(2):396–407. doi: 10.1111/j.1469-8137.2012.04170.x. [DOI] [PubMed] [Google Scholar]
- 15.Plaut JA, et al. Hydraulic limits preceding mortality in a piñon-juniper woodland under experimental drought. Plant Cell Environ. 2012;35(9):1601–1617. doi: 10.1111/j.1365-3040.2012.02512.x. [DOI] [PubMed] [Google Scholar]
- 16.Breshears DD, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA. 2005;102(42):15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West AG, Hultine KR, Jackson TL, Ehleringer JR. Differential summer water use by Pinus edulis and Juniperus osteosperma reflects contrasting hydraulic characteristics. Tree Physiol. 2007;27(12):1711–1720. doi: 10.1093/treephys/27.12.1711. [DOI] [PubMed] [Google Scholar]
- 18.West AG, Hultine KR, Burtch KG, Ehleringer JR. Seasonal variations in moisture use in a piñon-juniper woodland. Oecologia. 2007;153(4):787–798. doi: 10.1007/s00442-007-0777-0. [DOI] [PubMed] [Google Scholar]
- 19.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491(7426):752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 20.Wright IJ, et al. Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr. 2005;14(5):411–421. [Google Scholar]
- 21.Urli M, et al. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013;33(7):672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai T, Porporato A. Strategies of a Bornean tropical rainforest water use as a function of rainfall regime: Isohydric or anisohydric? Plant Cell Environ. 2012;35(1):61–71. doi: 10.1111/j.1365-3040.2011.02428.x. [DOI] [PubMed] [Google Scholar]
- 23.Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress?: Answers from a model. Plant Physiol. 1988;88(3):574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85(8):2184–2199. [Google Scholar]
- 25.Burgess SSO, Pittermann J, Dawson TE. Hydraulic efficiency and safety of branch xylem increases with height in Sequoia sempervirens (D. Don) crowns. Plant Cell Environ. 2006;29(2):229–239. doi: 10.1111/j.1365-3040.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 26.Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126(1):457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- 27.Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006;26(6):689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- 28.Manzoni S, et al. Hydraulic limits on maximum plant transpiration and the emergence of the safety-efficiency trade-off. New Phytol. 2013;198(1):169–178. doi: 10.1111/nph.12126. [DOI] [PubMed] [Google Scholar]
- 29.Brodribb TJ, Jordan GJ. Internal coordination between hydraulics and stomatal control in leaves. Plant Cell Environ. 2008;31(11):1557–1564. doi: 10.1111/j.1365-3040.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 30.Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol. 2009;23(5):922–930. [Google Scholar]
- 31.Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: A comparison of coexisting ferns and angiosperms. New Phytol. 2004;162(3):663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- 32.Manzoni S, Vico G, Porporato A, Katul G. Biological constraints on water transport in the soil-plant-atmosphere system. Adv Water Resour. 2013;51:292–304. [Google Scholar]
- 33.Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM. Hydraulic patterns and safety margins, from stem to stomata, in three eastern U.S. tree species. Tree Physiol. 2011;31(6):659–668. doi: 10.1093/treephys/tpr050. [DOI] [PubMed] [Google Scholar]
- 34.Dawson TE. Hydraulic lift and water use by plants: Implications for water balance, performance and plant-plant interactions. Oecologia. 1993;95(4):565–574. doi: 10.1007/BF00317442. [DOI] [PubMed] [Google Scholar]
- 35.Burgess SSO, Adams MA, Turner NC, Ong CK. The redistribution of soil water by tree root systems. Oecologia. 1998;115(3):306–311. doi: 10.1007/s004420050521. [DOI] [PubMed] [Google Scholar]
- 36.Goldsmith GR, Matzke NJ, Dawson TE. The incidence and implications of clouds for cloud forest plant water relations. Ecol Lett. 2013;16(3):307–314. doi: 10.1111/ele.12039. [DOI] [PubMed] [Google Scholar]
- 37.Gotsch SG, et al. Foggy days and dry nights determine crown-level water balance in a seasonal tropical Montane cloud forest. Plant Cell Environ. 2014;37(1):261–272. doi: 10.1111/pce.12151. [DOI] [PubMed] [Google Scholar]
- 38.Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014;37(1):153–161. doi: 10.1111/pce.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDowell NG. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011;155(3):1051–1059. doi: 10.1104/pp.110.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breshears DD, et al. Tree die-off in response to global change-type drought: Mortality insights from a decade of plant water potential measurements. Front Ecol Environ. 2009;7(4):185–189. [Google Scholar]
- 41.Wong SC, Cowan IR, Farquhar GD. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282(5737):424–426. [Google Scholar]
- 42.Linder HP. The radiation of the Cape flora, southern Africa. Biol Rev Camb Philos Soc. 2003;78(4):597–638. doi: 10.1017/s1464793103006171. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzler J, et al. Causes of plant diversification in the Cape biodiversity hotspot of South Africa. Syst Biol. 2011;60(3):343–357. doi: 10.1093/sysbio/syr006. [DOI] [PubMed] [Google Scholar]
- 44.Skelton RP. 2014. The role of hydraulic strategies in understanding the response of fynbos to drought. PhD dissertation (University of Cape Town, Cape Town, South Africa)
- 45.Skelton RP, West AG, Dawson TE, Leonard JM. External heat-pulse method allows comparative sapflow measurements in diverse functional types in a Mediterranean-type shrubland in South Africa. Funct Plant Biol. 2013;40(10):1076–1087. doi: 10.1071/FP12379. [DOI] [PubMed] [Google Scholar]
- 46.Hewitson BC, Crane RG. Consensus between GCM climate change projections with empirical downscaling: Precipitation downscaling over South Africa. Int J Climatol. 2006;26(10):1315–1337. [Google Scholar]
- 47.De Wit MJ, Stankiewicz J. Changes in surface water supply across Africa with predicted climate change. Science. 2006;311(5769):1917–1921. doi: 10.1126/science.1119929. [DOI] [PubMed] [Google Scholar]
- 48.Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003;132(4):2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003;26(3):443–450. [Google Scholar]
- 50.Yates M, Verboom GA, Rebelo AG, Cramer MD. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Funct Ecol. 2010;24(3):485–492. [Google Scholar]
- 51.Higgins KB, Lamb AJ, van Wilgen BW. Root systems of selected plant species in mesic mountain fynbos in the Jonkershoek Valley, south-western Cape Province. S Afr J Bot. 1987;3:249–257. [Google Scholar]
- 52.Marloth R. Results of experiments on Table Mountain for ascertaining the amount of moisture deposited from the south east clouds. Transactions of the South African Philosophical Society. 1903;14(1):403–408. [Google Scholar]
- 53.Oren R, et al. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999;22(12):1515–1526. [Google Scholar]
- 54.West AG, Hultine KR, Sperry JS, Bush SE, Ehleringer JR. Transpiration and hydraulic strategies in a piñon-juniper woodland. Ecol Appl. 2008;18(4):911–927. doi: 10.1890/06-2094.1. [DOI] [PubMed] [Google Scholar]
- 55.Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: Implications for drought tolerance and regulation of transpiration. Funct Ecol. 1998;12(6):906–911. [Google Scholar]
- 56.Jacobsen AL, et al. Xylem density, biomechanics and anatomical traits correlate with water stress in 17 evergreen shrub species of the Mediterranean-type climate region of South Africa. J Ecol. 2007;95(1):171–183. [Google Scholar]
- 57.Pratt RB, Jacobsen AL, Jacobs SM, Esler KJ. Xylem transport safety and efficiency differ among Fynbos shrub life history types and between two sites differing in mean rainfall. Int J Plant Sci. 2012;173(5):474–483. [Google Scholar]
- 58.Aston T. 2007. Geohydrological characteristics of Table Mountain Group aquifer-fed seeps and the plant ecophysiological consequences. M.Sc. dissertation (University of Cape Town, Cape Town, South Africa)
- 59.Domec J-C, Gartner BL. Cavitation and water storage capacity in bole xylem segments of mature and young Douglas-fir trees. Trees (Berl West) 2001;15(4):204–214. [Google Scholar]
- 60.Sperry JS, Saliendra NZ. Intra- and inter-plant variation in xyiem cavitation in Betula occidentalis. Plant Cell Environ. 1994;17(11):1233–1241. [Google Scholar]
- 61.Sperry JS, Ikeda T. Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol. 1997;17(4):275–280. doi: 10.1093/treephys/17.4.275. [DOI] [PubMed] [Google Scholar]
- 62.Cochard H, et al. Methods for measuring plant vulnerability to cavitation: A critical review. J Exp Bot. 2013;64(15):4779–4791. doi: 10.1093/jxb/ert193. [DOI] [PubMed] [Google Scholar]
- 63.Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ. 2013;36(11):1938–1949. doi: 10.1111/pce.12139. [DOI] [PubMed] [Google Scholar]
- 64.Melcher PJ, et al. Water relations of coastal and estuarine Rhizophora mangle: Xylem pressure potential and dynamics of embolism formation and repair. Oecologia. 2001;126(2):182–192. doi: 10.1007/s004420000519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.