Significance
Most ecosystem models used to predict changes in community composition with climate change assume species’ responses to environmental conditions are genetically fixed on the century scale, but this hypothesis has not been tested. Using an oceanographic time series with directional environmental changes, we show here that many phytoplankton species are able to track, on average, modest changes in temperature and irradiance, but not decreases in limiting nutrient concentrations, on decadal timescales. This result suggests that models that use genetically fixed traits may not provide reasonable projections for changes in biological communities in response to climate change over decadal to longer timescales.
Keywords: phytoplankton, realized niches, climate change, evolution
Abstract
Model projections indicate that climate change may dramatically restructure phytoplankton communities, with cascading consequences for marine food webs. It is currently not known whether evolutionary change is likely to be able to keep pace with the rate of climate change. For simplicity, and in the absence of evidence to the contrary, most model projections assume species have fixed environmental preferences and will not adapt to changing environmental conditions on the century scale. Using 15 y of observations from Station CARIACO (Carbon Retention in a Colored Ocean), we show that most of the dominant species from a marine phytoplankton community were able to adapt their realized niches to track average increases in water temperature and irradiance, but the majority of species exhibited a fixed niche for nitrate. We do not know the extent of this adaptive capacity, so we cannot conclude that phytoplankton will be able to adapt to the changes anticipated over the next century, but community ecosystem models can no longer assume that phytoplankton cannot adapt.
During the last several decades, global land temperature has increased by ∼0.3 °C per decade (1), and a further increase in global mean air temperatures of 1.1–6.4 °C is expected by 2100 (2). The warming of the oceans is resulting in spatially variable changes in sea surface temperature (3, 4), salinity, mixed-layer depth, and the distribution of nutrients. Ocean time series sampled on a monthly basis document intra- and interannual changes in physical forcing and biogeochemistry, providing crucial data for formulating ecosystem models and characterizing how ecosystems respond to climate change (5, 6). We have very high confidence that climate change during the last several decades has influenced the abundance, phenology, and geographic ranges for a wide assortment of species (7–10). Further increases in global temperature may result in significant and nonreversible changes to many populations and communities (11, 12). If dispersal rates are rapid relative to the rate of evolutionary adaptation, changes in climate will result in local species being displaced by nonresident species from a regional pool of species that are better adapted to the new conditions (13). When modelers project changes in biotic communities under climate change scenarios, they generally assume that each species has a genetically determined fixed environmental niche and that species’ spatial and temporal distributions will be determined by environmental conditions (14–17). A recent model of this type predicts a loss of a third of tropical phytoplankton strains by 2100 with a ∼2 °C increase in mean temperature (11); however, paleoecological studies indicate organisms may be much more resilient to climate change than these types of models suggest (18, 19).
Local populations may be able to acclimate physiologically and then adapt through evolutionary change to gradual climate shifts. We do not know the constraints or timescales required for phytoplankton to adapt to changes in environmental conditions anticipated over the next century. Phytoplankton species have short generation times and large population sizes, so they may be particularly able to adapt to rapid climate change (20, 21). In addition, temperature response curves measured in the laboratory show that phytoplankton usually have the fastest growth rates at or slightly below the mean temperature of the environment they were isolated from, suggesting that natural populations are adapted to their local environment (15, 22), although some species have niches that do not reflect the environmental conditions from which they were isolated (23). Evolutionary experiments in the laboratory indicate that phytoplankton species have the capacity to evolve over hundreds to thousands of generations in response to single environmental factors; specifically, changes in CO2 concentration or temperature (24–29). Laboratory evolution experiments do not replicate either the highly dynamic marine environment or the trajectory of climate change, so it is necessary to look to see how phytoplankton evolve in the field. Theoretical studies show that species will evolve to maximize their geometric mean fitness in temporally varying environments, so evolutionary change is expected even if decadal-scale changes in average environmental conditions are smaller than interannual variation in those same conditions (29). Here we explicitly test whether phytoplankton species niches are stable or are able to adapt to simultaneous changes in several different environmental conditions over a decadal scale, using ocean time-series data. The answer to this question is essential for modelers attempting to predict biotic responses to changes in climate.
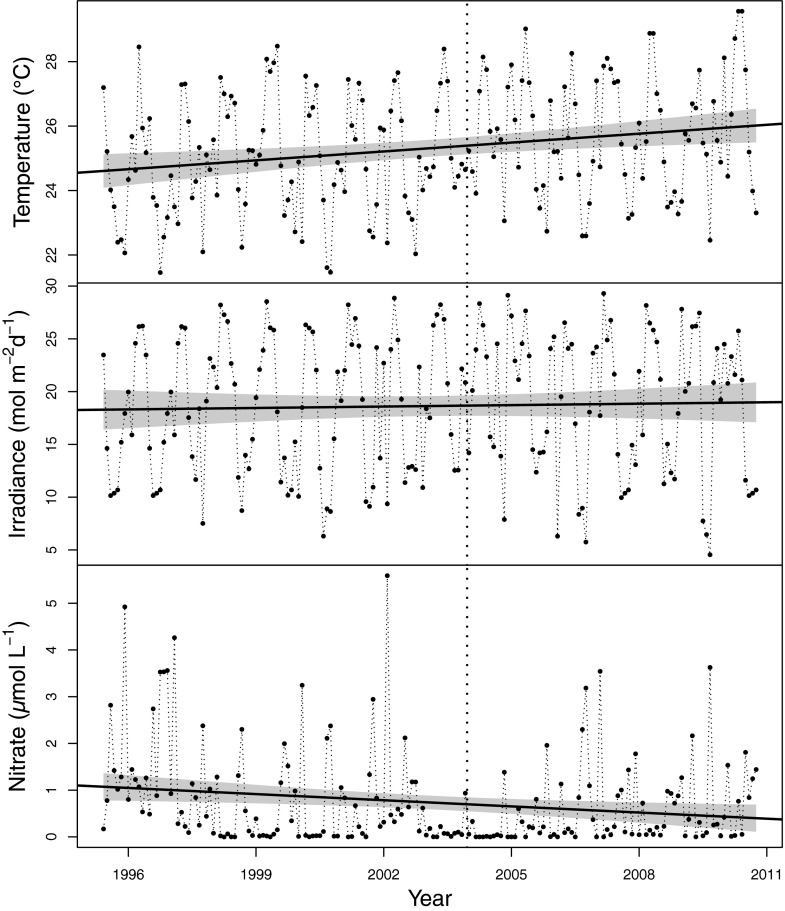
We quantify the realized niche for 67 dominant phytoplankton species (30) from Station CARIACO (Carbon Retention in a Colored Ocean) from the CARIACO Ocean Time-Series Program, using the MaxEnt method (31), which ignores species abundance and only relies on the conditions under which a species is present to describe the habitat of the species. We define the realized niche as the hypervolume of environmental conditions under which each species persists (32) and estimate the range of conditions for each species from a 15-y time series with monthly sampling. The MaxEnt method provides a robust estimate of the realized niche and is insensitive to the challenges posed by the detection of species at low abundance (33). During the 15 y from 1996 to 2011, there was a gradual warming of about 1 °C, an increase in average irradiance, and a decrease in nitrate concentration in the upper mixed layer (0–30 m) at Station CARIACO (34). These are regional changes resulting from the movement of the Inter-Tropical Convergence Zone (35). We divided the time series into an early, cooler period and a late, warmer period and examined the stability of the realized niches of phytoplankton species between these two periods (Fig. 1 and Table 1). It is challenging to compare niche hypervolumes for many species, so for convenience, we considered only one dimension of the realized niche at a time and summarized the realized niche for each axis by its mean. The weighted mean of the realized niche for each species and environmental variable was determined from the MaxEnt results, using the estimated probability of finding the species under each condition as the weights. Because the environmental conditions shifted slightly between the periods, the range of conditions common to both periods was used in determining the mean niche to avoid introducing a bias solely as a result of this change in the range of conditions present.
Fig. 1.
Monthly environmental conditions averaged over the upper mixed layer (1, 7, 15, and 25 m depth) from the CARIACO Ocean Time-Series Program: temperature (°C), irradiance (mol⋅m–2⋅d–1), and nitrate concentration (µmol⋅L–1). The vertical dotted line is drawn at the boundary (January 1, 2004) between the cool and warm periods. The straight lines are linear regressions: temperature = (24.6 ± 0.3) + (0.09 ± 0.03) t, R2 = 0.05, P < 0.005; irradiance = (18.1 ± 0.9) + (0.05 ± 0.11) t, R2= 0.001, P = 0.65; nitrate = (1.06 ± 0.14) – (0.045 ± 0.017) t, R2 = 0.04, P = 0.03, where t is time in years since January 1, 1996, errors are one SE, and the shaded region is the 95% confidence interval on the line. The R2 is very low because of the tremendous interannual variation relative to the trend.
Table 1.
Shift in mean niche tracks changes in environmental conditions
| Variable | Mean environment | Mean niche | ||||
| Early | Later | Change | Early | Later | Change | |
| Temperature, °C | 24.93 | 25.66 | +0.73* | 24.74 | 25.19 | +0.45* |
| Irradiance, mol m–2⋅d–1 | 18.20 | 18.77 | +0.57 | 15.87 | 16.45 | +0.58* |
| Nitrate, µmol⋅L–1 | 0.92 | 0.54 | −0.38* | 4.72 | 3.84 | −0.88* |
The mean environmental conditions in the upper mixed layer (0–30 m) and species' niches for the dominant phytoplankton at Station CARIACO were computed in the early, cooler (November 1995–December 2003) and later, warmer (January 2004–March 2011) periods. The change is the difference between values in the later and earlier periods. Sample sizes: 67 species total, of which 49 species were present in both periods, 12 were lost from the early period, and 6 gained in the later period.
Change is statistically significant at the 0.05 level, according to a t test.
Results and Discussion
On average, the species niches for temperature, irradiance, and nitrate concentration in the upper mixed layer are not stable over time, but shift significantly in the same direction and with comparable magnitude to the changes in the environmental conditions (Table 1 and Fig. 2). Most of the dominant species of phytoplankton in this community persist despite the environmental changes between the two periods. A small number of species are found in only in the cooler or warmer period (dark bars, Fig. 2), but there are too few of these species to conclude that their niches differ significantly from the niches of the species that are common to both periods. There are many possible explanations for the observed changes in species’ niches, including biotic interactions, substitution of cryptic species, or evolutionary change. The shift in mean niches is not an artifact of changing environmental conditions alone, as the niches were computed on the basis of environmental conditions common to both periods. We argue that changes in the distribution of environments and physiological acclimation are also unlikely explanations for the niche changes. A shift in a species’ niche cannot be attributed to a change in the probability distribution of environmental conditions because the probability a species is found in a particular environment does not depend on the frequency of occurrence of that environment. There is no reason to expect that the shift in niches is a result of physiological acclimation, as the time for physiological acclimation for most phytoplankton species is less than the month-long interval between samples (30, 36). We conclude that phytoplankton species niches are not stable but, instead, evolve in response to environmental pressures over the course of less than 15 y.
Fig. 2.
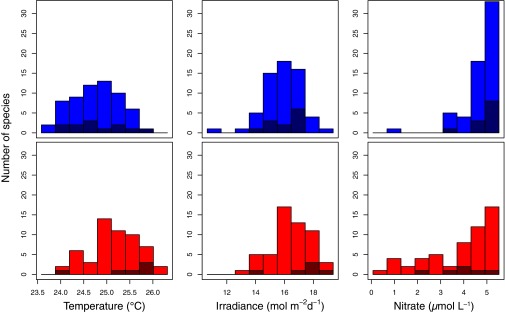
The change in the distribution of mean niches in response to warming for species before and after January 1, 2004, in the CARIACO Ocean Time-Series in pairs of panels: temperature, irradiance, and nitrate concentration. (Top) Mean niche before January 1, 2004, with species only observed in this early, cool period shown in dark blue. (Bottom) Mean niche after January 1, 2004, with species only observed in this later, warm period shown in dark red.
The structure in how species’ niches change between the two periods suggests selection is the primary driver of the niche changes observed. Species with the coldest niches in the earlier, cooler period increase their temperature niche more, on average, than species with warmer niches. In fact, there is a linear relationship between the change in temperature niche and initial temperature niche, with a slope of about −0.4, indicating that a species with a niche 1 °C lower than another tends to increase its niche by 0.4 °C more than the species with the warmer niche (Fig. 3). A similar result is found for irradiance, except the tracking is even stronger here: the mean irradiance niche increases the same amount as the mean environment, and a species with a niche 1 mol⋅m–2⋅d–1 lower than another increases its niche by 55% of this change between the colder and warmer periods. The situation for nitrate concentration is different. Most species change their nitrate niche very little (points near the dotted line in Fig. 3), whereas a minority of species are able to decrease their nitrate niches quite dramatically to take advantage of the increased frequency of low-nitrate environments in the later period. Because of a lack of ecophysiological information on the species, it is difficult to be certain why some species can and others cannot track changes in environmental concentrations (Fig. 3), but we speculate that the ability to adapt to decreasing nitrate concentration could be facilitated by associations with nitrogen fixers or flexibility in cell size or shape. Species are shifting their niches away from environments that are becoming less frequent with climate change, and the more extreme the initial niche compared with the average environmental conditions, the bigger the shift. We tested whether species with narrower initial niches shifted their mean niche more than species with wider niches, but our results were inconclusive because of a correlation between niche mean and niche width. This is likely partly an artifact: the realized niche of species with mean near the extremes of observed conditions is not fully observed, and so the niche width may be unreliable. There does not appear to be any reason to expect biotic interactions such as competition or grazing to cause the pattern observed here. This pattern is consistent with the hypothesis that phytoplankton are evolving to track changes in the environment, either through de novo genetic change or selection acting on existing genetic diversity and ecotypes (37).
Fig. 3.
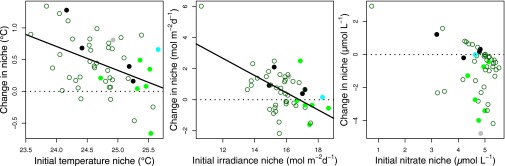
Change in mean niche for the 49 species observed in both the warmer and cooler periods as a function of the mean niche in the early, cooler period for temperature, irradiance, and nitrate concentration. There is an approximate linear relationship for temperature and irradiance indicated by the linear regressions for temperature [ΔT = (0.43 ± 0.06) – (0.38 ± 0.11) (Tearly – 24.74); R2 = 0.19; P < 0.002] and for irradiance [ΔE = (0.56 ± 0.16) – (0.55 ± 0.12)(Eearly –15.80); R2 = 0.30; P < 0.001, errors are one SE]. Tearly is the temperature niche from the early, cooler period with mean over species of 24.74 °C, and Eearly is the irradiance niche from the early, cooler period with mean over species of 15.87 mol⋅m–2⋅d–1 (see Table 1). The symbol color indicates the functional group of each species: diatom (green, open circles), dinoflagellate (dark green, filled circles), cyanobacteria (cyan), coccolithophorid (black), and silicoflagellate (gray). There are no significant differences in responses to changing conditions across the taxonomic groups.
Contrary to conventional expectations, we find that realized niches for many species of phytoplankton are not fixed on the decadal scale and are able to track changes in temperature and irradiance that are faster than the average changes we anticipate over the next century. In contrast, most, but not all, of the species we studied did not shift their nitrate niche in response to a depletion of this limiting resource. This may be a result of biophysical limits in the ability of some phytoplankton to adapt to low-resource environments. The evolutionary capacity of phytoplankton to adapt to changing climate may, on a decadal scale, be more predictive than short-term physiological responses in determining winners and losers in response to climate change. Because phytoplankton are limited by nitrate over vast regions of the ocean (38), we anticipate that the ability to shift nitrate niches may be a major factor driving the restructuring of phytoplankton communities during the next century. Models with fixed traits will likely miss the community restructuring made possible by evolutionary change. To advance our modeling of phytoplankton traits and niches for future climate scenarios, we need a better understanding of evolutionary capacity and dynamics in marine communities in response to changing environmental conditions.
Climate change scenarios over the next century project larger changes in mean conditions and the range of conditions than were observed in this 15-y time series. Our results cannot predict whether species will be able to adapt to these larger changes. For example, although many of the phytoplankton species in this study could adapt to a change of 1 °C over a decade, this result tells us very little about their ability to adapt to temperature changes of several degrees over many decades. Larger temperature changes may result in species reaching hard biochemical or physiological limits to the temperature adaptation that is achievable. To test our results and apply them to the next generation of models will require additional analyses of field data and a better understanding of the mechanisms controlling changes in realized niches in response to environmental change.
Methods
Monthly sampling at Station CARIACO recorded temperature, nitrate concentration, and the abundance of 67 dominant phytoplankton species (30, 35). Irradiance in the mixed layer was estimated from monthly SeaWiFS PAR and k490 data. We use the data from 178 sampling months during the 185 mo from November 1995 to March 2011 at four depths sampled in the upper mixed layer (1, 7, 15, and 25 m). We divided the time series at January 1, 2004, leaving 95 cruises in the early period, from November 1995 to December 2003, and 83 cruises in the later period, from January 2004 to March 2011. There is no abrupt change in any of the environmental data, so this is an arbitrary break chosen at a calendar year boundary near the middle of the time series. We only analyze species that were observed more than 10 times in at least one of the periods (39). The median number of observations per species per period was 56. After environmental forcing is accounted for, each monthly observation of phytoplankton community structure is essentially independent of both time of year and previous observations (30). Variables such as annual extreme values or amplitudes that capture changes in seasonality may influence community changes, but because of the short duration of the time series, we have few (15) observations of these data.
We used the MaxEnt method (31, 33, 39) to estimate the probability of finding each species as a function of each environmental variable, using presence-only data, meaning we use all of the observations of each species, but not the abundance data and without assuming zero abundance when a species is not detected. We permitted linear and quadratic features in the response curve and prohibited sudden jumps (threshold and hinge features). We constructed 95% confidence intervals on the mean niche, using 500 models for each species, using bootstrap resampling. The average width of the 95% confidence interval for species’ niches are 0.9 °C, 2.4 mol⋅m–2⋅d–1, and 2.0 µmol⋅L–1 for temperature, irradiance, and nitrate concentration, respectively. We define a mean niche that can be compared between periods as the probability-weighted mean environmental condition for each species restricted to the range of environmental conditions common to both periods. This ensures the niches do not drift simply because the range of environmental conditions has changed.
In 2005, there was a dramatic shift in the entire pelagic community at Station CARIACO. The phytoplankton community shifted to smaller cells not identified in this time series, and many species that were tracked dropped in abundance 50–300-fold. This change appears to be a result of a change in grazing rates (35) and is not linked to sudden changes in temperature or the availability of nutrients. Using presence data rather than abundance means our niche models were not affected by the change in species abundances. Niches inferred from abundance data would be affected by both the change in environmental conditions and the change in the food web, and thus would be more challenging to interpret.
Acknowledgments
We thank the captain and crew of the B/O Hermano Gines and the staff of the Estación de Investigaciones Marinas de Margarite, Fundación de la Salle de Cincias Naturales, Margarita Island, Venezuela, for their field assistance. Source data used in this study are available on the CARIACO website imars.marine.usf.edu/CAR/. We are especially grateful for the leadership and support provided by the Fundación La Salle de Ciencias Naturales e Venezuela in the CARIACO program. The CARIACO Time-Series Program was supported by Venezuela Fondo Nacional de Ciencia, Tecnología e Innovación (Awards 96280221 and 2000001702 to R. Varela and Y. Astor/Fundación La Salle de Ciencias Naturales de Venezuela) and the National Science Foundation (Grants OCE-0326268, OCE-0963028, and OCE-1259043 to F.E.M.-K.). A.J.I. and Z.V.F. were supported by the National Science and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Houghton JT, Jenkins GJ, Ephraums JJ. Climate Change: The IPCC Scientific Assessment. Cambridge Univ Press; Cambridge: 1996. [Google Scholar]
- 2.Meehl GA, et al. Global climate projections. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 3.Levitus S, Antonov J, Boyer T. 2005. Warming of the world ocean, 1955–2003. Geophys Res Lett 32(2):L12602. [DOI] [PubMed]
- 4.Latif M, et al. Reconstructing, monitoring, and predicting multidecadal-scale changes in the North Atlantic thermohaline circulation with sea surface temperature. J Clim. 2004;17(7):1605–1614. [Google Scholar]
- 5.Church MJ, Lomas MW, Muller-Karger F. 2013. Sea change: Charting the course for biogeochemical ocean time-series research in a new millennium. Deep Sea Res Part II Top Stud Oceanogr 93:2–15.
- 6.Karl D. Oceanic ecosystem time-series programs: Ten lessons learned. Oceanography. 2010;23(3):104–125. [Google Scholar]
- 7.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 8.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 9.Cimino MA, Fraser WR, Irwin AJ, Oliver MJ. Satellite data identify decadal trends in the quality of Pygoscelis penguin chick-rearing habitat. Glob Change Biol. 2013;19(1):136–148. doi: 10.1111/gcb.12016. [DOI] [PubMed] [Google Scholar]
- 10.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430(7002):881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 12.Harnik PG, et al. Extinctions in ancient and modern seas. Trends Ecol Evol. 2012;27(11):608–617. doi: 10.1016/j.tree.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Follows MJ, Dutkiewicz S, Grant S, Chisholm SW. Emergent biogeography of microbial communities in a model ocean. Science. 2007;315(5820):1843–1846. doi: 10.1126/science.1138544. [DOI] [PubMed] [Google Scholar]
- 14.Flombaum P, et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA. 2013;110(24):9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas MK, Kremer CT, Klausmeier CA, Litchman E. A global pattern of thermal adaptation in marine phytoplankton. Science. 2012;338(6110):1085–1088. doi: 10.1126/science.1224836. [DOI] [PubMed] [Google Scholar]
- 16.Tagliabue A, Bopp L, Gehlen M. 2011. The response of marine carbon and nutrient cycles to ocean acidification: Large uncertainties related to phytoplankton physiological assumptions. Glob Biogeochem Cycles 25(3):GB3017.
- 17.Dutkiewicz S, Scott JR, Follows MJ. Winners and losers: Ecological and biogeochemical changes in a warming ocean. Global Biogeochem Cycles. 2013;27(2):463–477. [Google Scholar]
- 18.Moritz C, Agudo R. The future of species under climate change: Resilience or decline? Science. 2013;341(6145):504–508. doi: 10.1126/science.1237190. [DOI] [PubMed] [Google Scholar]
- 19. Cermeño P. Marine planktonic microbes survived climatic instabilities in the past. Proc Biol Sci 2012;279(1728):474–479. [DOI] [PMC free article] [PubMed]
- 20.Collins S, Rost B, Rynearson TA. Evolutionary potential of marine phytoplankton under ocean acidification. Evol Appl. 2014;7(1):140–155. doi: 10.1111/eva.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellweger FL, van Sebille E, Fredrick ND. Biogeographic patterns in ocean microbes emerge in a neutral agent-based model. Science. 2014;345(6202):1346–1349. doi: 10.1126/science.1254421. [DOI] [PubMed] [Google Scholar]
- 22.Boyd PW, et al. Marine phytoplankton temperature versus growth responses from polar to tropical waters—outcome of a scientific community-wide study. PLoS ONE. 2013;8(5):e63091. doi: 10.1371/journal.pone.0063091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu F-X, et al. Differing responses of marine N2-fixers to warming and consequences for future diazotroph community structure. Aquat Microb Ecol. 2014;72:33–46. [Google Scholar]
- 24.Lohbeck KT, Riebesell U, Reusch TBH. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci. 2012;5(5):346–351. [Google Scholar]
- 25.Jin P, Gao K, Beardall J. Evolutionary responses of a coccolithophorid Gephyrocapsa oceanica to ocean acidification. Evolution. 2013;67(7):1869–1878. doi: 10.1111/evo.12112. [DOI] [PubMed] [Google Scholar]
- 26.Benner I, et al. Emiliania huxleyi increases calcification but not expression of calcification-related genes in long-term exposure to elevated temperature and pCO2. Philos Trans R Soc Lond B Biol Sci. 2013;368(1627):20130049. doi: 10.1098/rstb.2013.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins S, Bell G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431(7008):566–569. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- 28.Huertas IE, Rouco M, López-Rodas V, Costas E. Warming will affect phytoplankton differently: Evidence through a mechanistic approach. Proc Biol Sci. 2011;278(1724):3534–3543. doi: 10.1098/rspb.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank SA, Slatkin M. Evolution in a variable environment. Am Nat. 1990;136(2):244–260. [Google Scholar]
- 30.Mutshinda CM, Troccoli-Ghinaglia L, Finkel ZV, Müller-Karger FE, Irwin AJ. Environmental control of the dominant phytoplankton in the Cariaco basin: A hierarchical Bayesian approach. Mar Biol Res. 2013;9(3):246–260. [Google Scholar]
- 31.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography. 2008;31(2):161–175. [Google Scholar]
- 32.Hutchinson GE. 1957. The multivariate niche. Cold Spring Harbor Symp Quant Biol 22:415–427.
- 33.Elith J, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17(1):43–57. [Google Scholar]
- 34.Müller-Karger F, et al. Annual cycle of primary production in the Cariaco Basin: Response to upwelling and implications for vertical export. J Geophys Res. 2001;106(C3):4527–4542. [Google Scholar]
- 35.Taylor GT, et al. Ecosystem responses in the southern Caribbean Sea to global climate change. Proc Natl Acad Sci USA. 2012;109(47):19315–19320. doi: 10.1073/pnas.1207514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIntyre HL, Kana TM, Geider RJ. The effect of water motion on short-term rates of photosynthesis by marine phytoplankton. Trends Plant Sci. 2000;5(1):12–17. doi: 10.1016/s1360-1385(99)01504-6. [DOI] [PubMed] [Google Scholar]
- 37.Kashtan N, et al. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science. 2014;344(6182):416–420. doi: 10.1126/science.1248575. [DOI] [PubMed] [Google Scholar]
- 38.Moore JK, Doney SC, Glover DM, Fung IY. Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2001;49(1-3):463–507. [Google Scholar]
- 39.Irwin AJ, Nelles AM, Finkel ZV. Phytoplankton niches estimated from field data. Limnol Oceanogr. 2012;57(3):787–797. [Google Scholar]