Significance
International society has made a commitment to mainstreaming biodiversity conservation into broader socioeconomic development, but an incomplete theoretical basis translates into a lack of practical applications, especially regarding how individual plant productivity changes in response to the overall species loss. In this study, we developed niche–efficiency theory to address two mechanisms behind the effects of biodiversity on individual plant productivity. Supported by empirical evidence at the individual plant level, our theory facilitates adaptive biodiversity management implementation, and the straightforward mathematical formula facilitates the integration of biological conservation in our campaign against pressing global issues. Based on this theory, we developed relative productivity and productivity impact index to provide function-based tools for prioritizing biological conservation efforts.
Keywords: biodiversity loss, marginal productivity, productivity impact index, relative productivity, biological conservation
Abstract
The loss of biodiversity is threatening ecosystem productivity and services worldwide, spurring efforts to quantify its effects on the functioning of natural ecosystems. Previous research has focused on the positive role of biodiversity on resource acquisition (i.e., niche complementarity), but a lack of study on resource utilization efficiency, a link between resource and productivity, has rendered it difficult to quantify the biodiversity–ecosystem functioning relationship. Here we demonstrate that biodiversity loss reduces plant productivity, other things held constant, through theory, empirical evidence, and simulations under gradually relaxed assumptions. We developed a theoretical model named niche–efficiency to integrate niche complementarity and a heretofore-ignored mechanism of diminishing marginal productivity in quantifying the effects of biodiversity loss on plant productivity. Based on niche–efficiency, we created a relative productivity metric and a productivity impact index (PII) to assist in biological conservation and resource management. Relative productivity provides a standardized measure of the influence of biodiversity on individual productivity, and PII is a functionally based taxonomic index to assess individual species’ inherent value in maintaining current ecosystem productivity. Empirical evidence from the Alaska boreal forest suggests that every 1% reduction in overall plant diversity could render an average of 0.23% decline in individual tree productivity. Out of the 283 plant species of the region, we found that large woody plants generally have greater PII values than other species. This theoretical model would facilitate the integration of biological conservation in the international campaign against several pressing global issues involving energy use, climate change, and poverty.
Increasing extinction of species and simplification of communities may be impairing ecosystem productivity and services worldwide. Hence, better understanding and quantification of the effects of biodiversity loss on the functioning of natural ecosystems is becoming increasingly crucial (1). Amid the world’s struggle to reduce the loss of biodiversity, concern is mounting over the ongoing relationship between biological conservation and poverty (2), especially in rural areas where livelihoods depend heavily on ecosystem resources (3). International society has made a commitment to conserving biodiversity because of its importance to economic development and poverty relief (United Nations Resolution A/RES/65/161), but integrated strategies are rarely successful (2) in part because it remains difficult to assess how productivity of individual plants would respond to the loss of biodiversity.
Studies over the past 20 y have improved understanding of the consequences of biodiversity loss on ecosystems (1, 4). The preponderance of data, mostly from controlled field experiments with herbaceous species (4), indicate that ecosystem functioning may be impaired by species loss. The major mechanism behind it, besides a nonbiological sampling effect (5, 6), has been largely attributed to an increased total resource acquisition from positive feedbacks to resource supply (7, 8) and resource partitioning due to niche complementarity (see refs. 9–11 and references therein). However, a lack of study on resource utilization efficiency—a link between resource and productivity—has rendered it difficult (12) to quantify the effects of biodiversity loss on plant productivity.
Due in part to the biological and structural complexity and long life cycle of forests, limited studies on forested ecosystems have focused on tree species with little attention to other types of plants including shrub and nonwoody species (13–17). Understory vegetation has a significant effect on tree productivity, both by influencing tree seedling regeneration and by affecting belowground processes and soil nutrient buildup (18). Moreover, following habitat loss and fragmentation, understory biodiversity loss can be more pronounced than tree species loss (19, 20). It is therefore important to understand the consequences of biodiversity loss in all classes of plant species on forest productivity.
In this paper, we present a heretofore-ignored mechanism that is directly applicable at the individual plant level, which in conjunction with the previously discovered process of niche complementarity provides a complete explanation for common observations that plant productivity increases with biodiversity with a diminishing rate. We developed a theoretical model of niche–efficiency to quantify the effects of biodiversity loss (or gain) on individual plant productivity and a functionally based taxonomic index (for individual species) to assist in prioritizing biodiversity conservation efforts for nonendangered species.
Theoretical Model
We first define fij(rij) as the productivity of individual plant j of species i which is a twice-differentiable function of resource acquisition by that individual rij. To study the effect of biodiversity on individual plant productivity, we consider resource acquisition rij as a function of biodiversity (in the number of species or species richness, ω). We then define marginal biodiversity productivity as the ceteris paribus (i.e., while all other factors stay constant) change in individual plant productivity that corresponds to one unit change in biodiversity in its community. Marginal biodiversity productivity by definition is the first derivative of individual productivity with respect to biodiversity.
By the chain rule, marginal biodiversity productivity can be written as
| [1] |
According to this equation, marginal biodiversity productivity is a product of two factors (Fig. 1A). The factor r′ij(ω) represents niche complementarity (see refs. 9–11 and references therein). When other variables, including the number of individuals in the community, are kept constant, total resources acquired by a community increase asymptotically with biodiversity according to niche complementarity (12). Therefore, resource acquisition at the individual level also increases with biodiversity with a diminishing rate [r′ij(ω) ≥ 0 and r″ij(ω) ≤ 0].
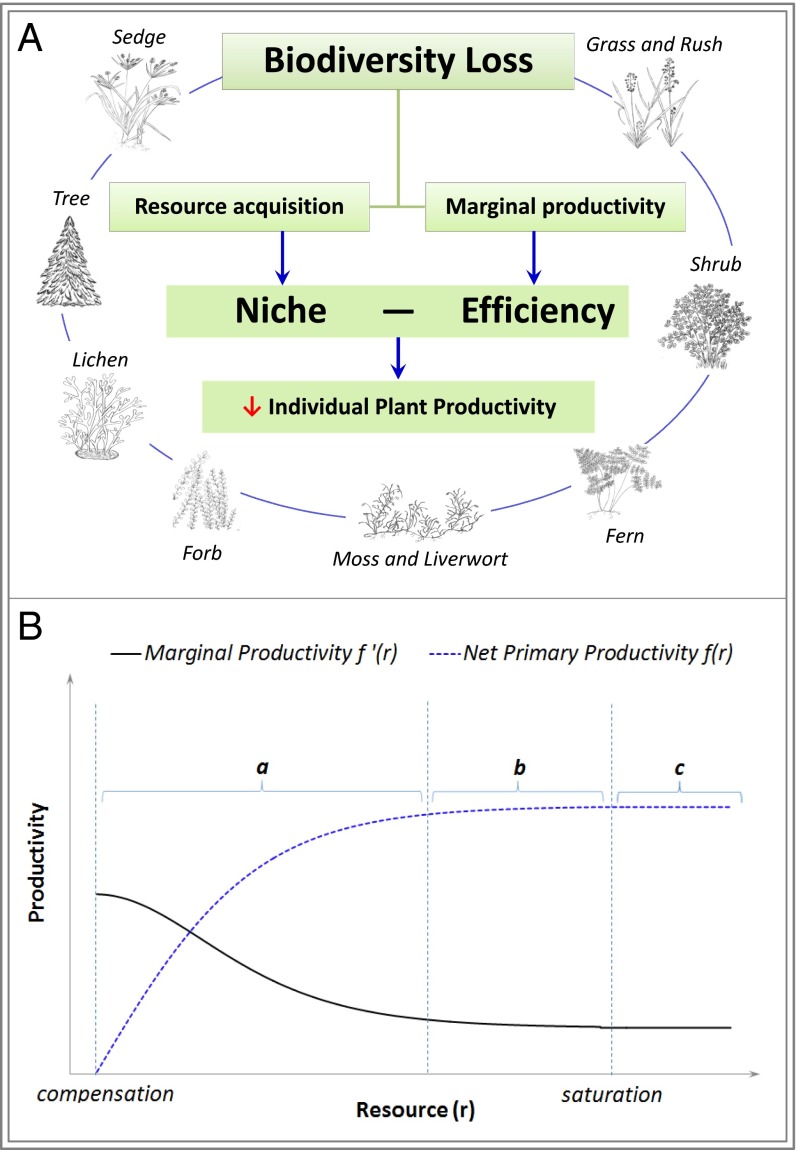
Fig. 1.
Conceptual diagram of niche–efficiency (A) and demonstration of diminishing marginal productivity (B). Loss in any type of the plant species (trees, grass, ferns, etc.) in forested ecosystems may reduce individual plant productivity by affecting both resource acquisition (through niche complementarity) and efficiency of resource utilization (through diminishing marginal productivity) (A). Based on this model, we developed the PII to quantify individual species’ inherent value in maintaining current ecosystem productivity. Empirical analysis shows that large woody species in general have greater PII values than any other plant species (Fig. 3). Redrawn from the observation relationship between net photosynthetic rate and light energy in a terrestrial ecosystem (23), diminishing marginal productivity means that marginal resource productivity decreases monotonically as resource abundance moves beyond the compensation point through the presaturation range (indicated by a), the resource saturation range (indicated by b), and the postsaturation range (indicated by c) (B).
The other factor, f′ij(rij), represents a heretofore-ignored mechanism behind the biodiversity–ecosystem functioning relationship. Based on the normal law of plant growth (21, 22), the first derivative of the individual productivity function with respect to resource is nonnegative (f′ij(rij) ≥ 0), and its second derivative is nonpositive (f″ij(rij) ≤ 0). Intuitively, the well-documented normal law of plant growth ensures that the productivity–resource relationship will be upper-bounded according to the photosynthetic capacity and growth efficiency of a plant species, even when resources are not limited. The concavity of plant growth functions is supported phenomenologically with respect to various topical biological resources, including light (23), CO2 (23), water (24), and nutrients (25). Because marginal resource productivity (i.e., the change in productivity resulting from one unit change in biologically essential resources) diminishes according to the normal law of plant growth (Fig. 1B), we name this mechanism after a parallel fundamental principal of economics called diminishing marginal productivity (DMP) (26). DMP has rarely been addressed in biology except for a study on ecosystem energy intensity (27).
Based on the above,
| [2] |
biodiversity increases individual plant productivity at a declining rate; ergo, individual plant productivity diminishes under biodiversity loss with an increasing rate. We name this dual-mechanism model niche–efficiency (N–E) because it consists of two related but distinct mechanisms on niche complementarity and efficiency of resource utilization.
Extension of Niche–Efficiency
To facilitate integration of N–E in resource management, we approximated the concave and asymptotic productivity function fij(ω) using the Dixit–Stiglitz–Ethier production function (28). As a standardized measure of the positive effect of biodiversity on individual productivity, the relative productivity (R) represents the ceteris paribus ratio of individual productivity in a diverse community to that in a monoculture:
| [3] |
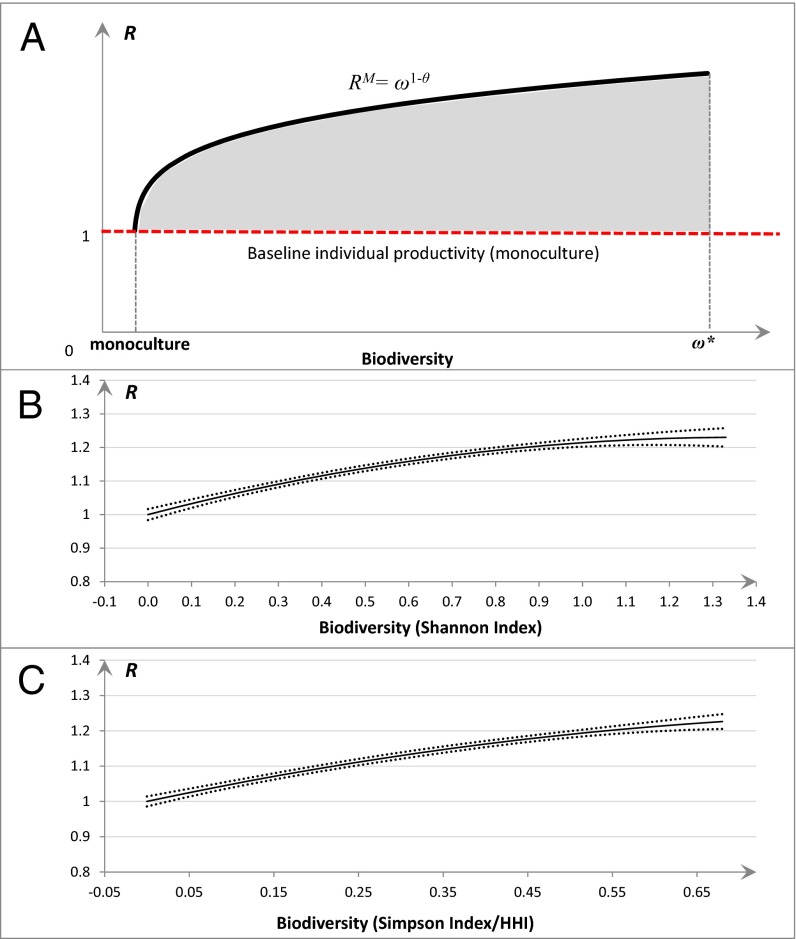
where ω stands for species richness but can also represent a continuous measure of biodiversity, such as common diversity indices. xi is the proportional biomass of a biodiversity unit (e.g., species) i in the population, and θi is the parameter governing the substitutability between species in maintaining the same value of fij(ω). The feasible region of the biodiversity−plant productivity relationship jointly defined by the maximal values (RM= ω1−θi) and the lower limit (R′ = 1) is in line with the preponderance of evidence from recent ecological studies highlighting a strong positive relationship between biodiversity and the productivity of ecological communities (see refs. 1, 4 and references therein). Species substitutability (θi), assumed to be constant between 0 and 1 to ensure concavity, can be obtained from controlled experiments in which communities (containing species i) of different levels of plant diversity are compared with the monoculture of species i.
Based on our theoretical model of niche efficiency, we developed the productivity impact index (PII) to quantify individual species’ inherent value in maintaining current ecosystem productivity based on its biodiversity elasticity of productivity, i.e., the percentage change in ecosystem productivity in response to a percentage change in the biomass of that species. PIIi is the negative natural logarithm of the ratio of the percent change in relative productivity (R) to the percent change in the standing biomass of a species i:
| [4] |
where f(ω) represents ecosystem/community productivity as a function of biodiversity and xi represents the proportional biomass of species i in the population.
Assuming independence of biomass across species (i.e., dxk/dxi = 0 for all i ≠ k), the derivative of f(ω) follows
| [5] |
Inserting Eq. 5 into Eq. 4 provides the final formula for PIIi:
| [6] |
For discrete measures of biodiversity ω, such as species richness, the formula for PIIi can be simplified as
| [7] |
PII is a function of a species’ current biomass (xi), species substitutability (θi), and relative productivity (R). PII is always a positive number, and as PII value declines, a species becomes more influential to the biodiversity-induced efficiency of resource utilization and therefore deserves more attention in biological conservation. PII, as (to our knowledge) the first function-based taxonomic index, reflects a species’ inherent value in maintaining ecosystem productivity. Because contribution to productivity alone does not fully represent the conservation priority of a species, PII should be used in conjunction with other conservation measures, such as species’ invasive and conservation status (e.g., the International Union for Conservation of Nature Red List of Threatened Species), to support decision making in biological conservation and natural resource management.
Empirical Evidence
The N–E model dictates that biodiversity improves individual plant productivity with a diminishing rate, other things being equal (Fig. 2A). Based on observational data from comprehensive vegetation censuses of the Alaska boreal forest (29), we developed a spatiotemporal model to test this hypothesis and investigate the ceteris paribus effect of plant diversity on individual tree aboveground net primary productivity (ANPP) (Materials and Methods).
Fig. 2.
Theoretical biodiversity–productivity relationship based on the niche–efficiency model (A) and empirical evidence drawn from the Alaska boreal forest (B and C). (A) The solid curve (black) represents the optimal relative productivity (RM = ω1 − θ) that can only be reached theoretically with an even distribution of biomass by species and a constant substitutability for all of the species, θ. The shaded area represents the feasible region of R that falls between the optimal individual productivity and the baseline individual productivity value of 1 (red broken line). ω* represents the highest possible degree of diversity. For empirical evidence, estimated mean (solid) R value against biodiversity in Shannon index (B) and in Simpson index/HHI (C) and associated 95% confidence interval bands (dots) are obtained using bootstrapping from the GLS model which controls for spatial and temporal autocorrelation and other exogenous factors (SI Appendix, Table S2).
When other factors, namely, the sampling effect, and spatial, temporal, and site conditions were accounted for and held constant at their sample means, lower values in both the Shannon index and Simpson index/HHI, viewed as surrogates for community simplification, corresponded to a drop in average individual tree ANPP from 38 kg·ha−1·y−1 in the most diverse stands to 31 kg·ha−1·y−1 in a tree monoculture, and relative productivity (R) decreased accordingly from 1.23 to 1.00 (Fig. 2). Empirical curves based on data observed from 440 permanent sample plots, which were consistent with the theoretical one (Fig. 2A), imply that greater plant diversity may increase individual tree productivity by improving both resource acquisition and resource utilization efficiency. Thus, a ceteris paribus decrease in biodiversity over its feasible range could lead to a 23% decline in tree NPP (Fig. 2 B and C). Because environmental, site, and physiographic factors have been accounted for, the diversity–productivity relationship discovered in this study is not confounded by these factors.
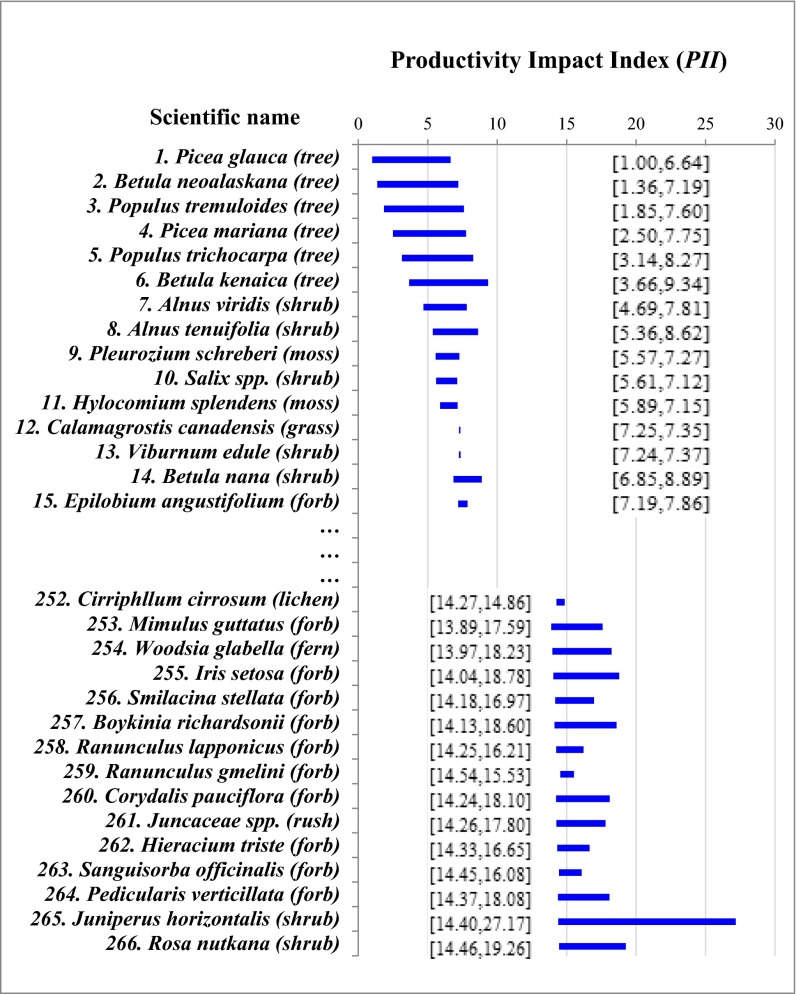
PII is jointly influenced by the relative productivity, percentage biomass, and substitutability of a species. Being unknown for most species in the Alaska boreal forest, species substitutability (θi) was assumed to be a random variable with a uniform distribution between 0 and 1 across all of the plots. Therefore, the mean PII score of a species was largely decided by the percentage biomass of that species, whereas the 95% confidence interval of the mean was associated with θi (Fig. 3). Among 283 plant species in the Alaska boreal forest, the top 15 with the greatest mean PII values consisted of 6 tree species, 5 shrub species, 2 moss species, 1 grass species, and 1 forb species. Picea glauca (white spruce) and Betula neoalaskana (Alaska birch) had the greatest average PII values. The top three shrub species in terms of PII were Alnus viridis (green alder), Alnus tenuifolia (mountain alder), and Salix (willow) species. The top two moss species were Pleurozium schreberi (Schreber’s moss) and Hylocomium splendens (glittering wood moss). The top grass and forb species were, respectively, Calamagrostis canadensis (bluejoint reedgrass) and Chamerion angustifolium (fireweed). It should be noted, however, that the rank of PII values is subject to change depending on the species-specific values of θi, but generally, the possibility of being the most influential species declines as it moves down the list (Fig. 3). These top species deserve close monitoring as they contribute (through N–E) to tree productivity more than other plant species in the Alaska boreal forest.
Fig. 3.
Rank of plant species in the Alaska boreal forest by the estimated average PII from the lowest to the highest. Horizontal bands and numbers in brackets represent the 95% confidence interval of the mean PII scores by species, assuming average species richness and a uniformly distributed random variable θ. Out of a total of 283 species studied in this paper, 17 have missing data. Therefore, the species with the lowest PII value is ranked 266 on the list.
Discussion
Niche Complementarity vs. Diminishing Marginal Productivity.
Although the positive biodiversity–plant productivity relationship is relatively easy to quantify using N–E, assessing the two underlying mechanisms as separate entities is challenging. Because DMP is a new theory, its model would remain hypothetical until a controlled experiment has been completed. Even for the niche complementarity theory, which has a longer history, mathematical models are still lacking (12). Therefore, it would be difficult to compare the contribution from niche complementarity and DMP to N–E, and partitioning the two underlying mechanisms can only be done empirically. For instance, in a monoculture context, the first derivative of the Dixit–Stiglitz–Ethier production function is equivalent to 1,
| [8] |
| [9] |
where r* represents total resource acquired by the species i. For simplicity, assuming species abundances are limited by two factors and the square habitat has a size of 1 by 1, according to a generalized niche model (12),
| [10] |
therefore, in this monoculture and hypothetical context, niche complementarity is 0.3500, and DMP is 1/0.3500 = 2.8574.
Species vs. Individual.
The nature of species is controversial in biology and philosophy. Biologists and philosophers disagree over the ontological status of species, and some suggest that species should be regarded as individuals (30). Here we demonstrated that compared with the positive effect of species richness, the number of individuals has a distinctive negative effect on individual productivity. Assuming the function of productivity–number of individuals is differentiable, marginal individual productivity (measured by the number of individuals, n) can be calculated as
| [11] |
where the first factor, f′(r), represents the same DMP mechanism as that in the N–E model. When other things, including species richness, remain constant, increasing number of individuals would reduce the amount of resources acquired by each individual, and hence, the second factor of marginal individual productivity, r′(n), the derivative of resources acquired by an individual with respect to the number of individuals, is apparently negative. Therefore, individual plant productivity declines with the number of individuals, ceteris paribus.
Implications at Community and Ecosystem Scales.
Due to the conflicting effects of species richness and number of individuals on productivity as demonstrated above, the number of individuals should be kept constant in drawing the implications of individual-based N–E effects on the relationship between biodiversity and productivity at the community scale. Under this assumption, the interaction between the two independent mechanisms can be illustrated graphically. First, assuming no effect of niche complementarity (i.e., under constant resource acquisition), an increase in biodiversity spreads the limited resource more thinly across a greater number of species (species richness or ω), rendering each functioning with fewer resources. As DMP dictates that the marginal productivity monotonically increases as resource declines, greater biodiversity would yield greater marginal productivity for all species (Fig. 4A). It can be further illustrated that when niche complementarity is considered (i.e., under variable resource acquisition), both marginal productivity and total community net primary productivity (NPP) still increase with ω and do so at a diminishing rate (Fig. 4 B and C). For example, when resources increase and all species share equally in them, the NPP curve as a function of ω shifts to a generally higher elevation, and the maximum ω increases (Fig. 4B). In a more realistic setting when species differ in resource acquisition as resource supply increases, with a smaller number capturing a greater fraction, the N–E model produces competitive exclusion, such that NPP increases as resources becomes higher at high ω, but the maximum ω is slightly reduced as a result (Fig. 4C), consistent with empirical observations (31).
Fig. 4.
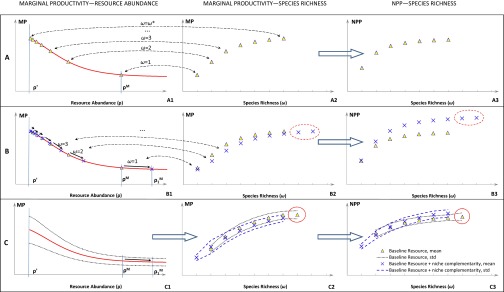
Individual-based niche–efficiency can aggregate in explaining the effect of biodiversity on plant productivity at the community level under gradually relaxed conditions (A → B → C). ρ denotes standardized resource abundance (ρε[0,1]), and ρ′ denotes the hypothetical minimum resource for a species to remain in a natural ecosystem. Biodiversity was represented by species richness (ω). (A) We first assumed (i) constant number of individual plants in the community, (ii) constant resource abundance, and (iii) all species share identical DMP curves and resource (ω−1). An increase in ω from 1 to 2, 3, … moves the accessible resource from the maximum value (ρM) toward ρM/2, ρM/3, and so on until the maximum species richness (ω* ≤ ρ′−1) is reached (A, 1). Corresponding marginal species productivity (MP) increased with ω with a diminishing rate (A, 2), and so did total community NPP (A, 3). (B) Then, we relaxed the assumption of constant resource level (assumption ii) by allowing the baseline accessible resource (ρM) to increase due to niche complementarity, other assumptions (assumptions i and iii) staying the same as A. With an increase in ρM by 30% to ρ1M (ρ1M = 1.3ρM), accessible resource at other species richness levels increased following the arrows, and hence, the site could in theory sustain a higher number of species (red dotted circle, B, 2, and B, 3), if all species share equally in resources and NPP and DMP functions. In this case, MP was similar between the two resource levels (B, 2), but total community NPP was higher for the increased resource level at every level of ω (B, 3). (C) Finally, we further relaxed previous assumptions (assumptions ii and iii) by allowing (i) DMP curves to randomly shift within ±30% of the original curve, (ii) random share of resources by different species, (iii) random increase of resources caused by niche complementarity to a maximum of 30% of the baseline resource level, (iv) resources to be distributed preferentially to species with the greatest share of resources, and (v) any species with a share of resources less than the threshold (ρ′) to die and leave the ecosystem (C, 1). Based on 10,000 bootstrap simulations, corresponding average marginal productivity per species increased with ω at a diminishing rate under both resource levels (C, 2). Total NPP also increased with ω at a diminishing rate, and the higher resource level made the NPP curve converge to a higher NPP at a slightly lower maximum species richness (red solid circles, C, 2, and C, 3).
Therefore, at the community and ecosystem levels, the individual-based N–E model under the foregoing conditions can in aggregate explain the positive and asymptotic effects of biodiversity on plant productivity derived from a majority of ecological experiments (4, 7–12). The number of individuals may have additional effects, and because it is not constrained to increase, remain stable, or decrease with changing species richness, it is advisable to account for this factor in all community- and ecosystem-scale analyses.
N–E should be applicable to different elements of biodiversity, including taxonomic, functional, phylogenetic, and genomic diversity, all of which have been found influential to plant productivity (1). For simplicity, we demonstrated the theoretical model using species richness as a measure of biodiversity, but it should be noted that resource utilization efficiencies are often associated more with traits and genotypes than with taxonomy. Nevertheless, because taxonomic diversity indirectly incorporates functional, phylogenetic, and genomic diversity, we believe that the results reported here likely reflect the importance of these elements of biodiversity and are consistent with mechanisms by which they act. Such a straightforward analysis also makes it easier to understand the taxonomic contribution to ecosystem functioning and the importance of preserving species diversity to biological conservation.
According to phenomenological studies, as resources saturate, DMP levels off at zero (range c in Fig. 1B). In this case, individual productivity will cease to increase with biodiversity (or decline with biodiversity loss) according to N–E, regardless of the niche complementarity effects. This explains why, in certain manipulated communities (e.g., monocultural plantations) where resources are made abundant, individual growth rates can be comparable to those in diverse communities. Therefore, the benefits of biological conservation on plant productivity are mostly applicable to natural ecosystems where resources are often limited. Recognizing this benefit and its limitation is essential for the integration of biological conservation into management strategies. As an important step in the international campaign against several pressing global issues, integrating biological conservation into resource management, due to the positive effect of biodiversity on the rate at which plants fix atmospheric carbon through photosynthesis, shows great potential for mitigating climate change, facilitating socioeconomic development, and maintaining the capability and sustainability of forested ecosystems to meet the increasing global demand for timber, fuelwood, and fiber.
Conclusion
Niche–efficiency (N–E) dictates that individual plant productivity diminishes under biodiversity loss with an increasing rate, everything else (including the number of individuals) being the same. Our theoretical model extends and expands understanding of the mechanistic underpinning of biodiversity–ecosystem function theory and implies a positive externality of biodiversity conservation; that is, conserving the diversity of plant species may help to maintain ecosystem services for current and future generations. Because of its explicit mathematical formula and direct applicability at individual plant level, N–E is useful to the development of integrated strategies for solving pressing global issues involving energy use, climate change, and poverty. Our theory also helps to explain the differences between individuals and species in terms of their contribution to ecosystem productivity (Species vs. Individual).
Materials and Methods
Data.
Empirical evidence supporting the N–E model was drawn from comprehensive vegetation surveys of the Alaska boreal forest (Cooperative Alaska Forest Inventory), consisting of ground-measured data of 283 plant species (64 woody, 119 forb, 12 grass and rush, 11 sedge and fern, and 77 nonvascular species) on 440 permanent sample plots. The sample area, stretching over 300,000 km2 in central and south-central Alaska, represents a wide range of species composition and physiographic conditions. A set of 440 permanent sample plots were used to capture the dynamics of 283 coexisting plant species in the region. The attributes measured include diameter at breast height, height, tree status, the percentage cover of nontree species, elevation, percentage slope, thickness of organic horizons, and number of snags. Based on these records, we calculated the ANPP of individual trees. To calculate plant diversity, we also estimated the aboveground biomass of shrub, herbaceous vascular, and nonvascular species based on the observed percentage cover and average height of that species using the species-specific biomass equations calibrated for interior Alaska (SI Appendix, section 1).
Statistical Analysis.
A spatiotemporal model was developed to investigate the ceteris paribus effect of plant diversity on individual tree ANPP across all of the sample plots, when other factors, namely, the sampling effect, and spatial, temporal, and site conditions have been controlled for. This model used the variation of plant diversity over space as a surrogate for temporal changes of plant diversity. Spatial autocorrelation was accounted for in this model to avoid biased or inconsistent coefficient estimates and significance levels (SI Appendix, section 2).
Supplementary Material
Acknowledgments
We thank Professor Emeritus Joseph Buongiorno and Professors Jean-Paul Chavas, Jun Zhu, and James Moseley for their comments on the economic, statistical, and mathematical analysis. We also thank Professor and guest editor Charles Perrings, the anonymous reviewers, and Professor Christa P. H. Mulder for their helpful comments on the entire manuscript. Plant drawings in Fig. 1 are courtesy of Rebekah K. Watson. This study is supported in parts by the Davis College of Agriculture, Natural Resources & Design, West Virginia University, under the US Department of Agriculture (USDA) McIntire–Stennis Funds WVA00104 and WVA00105; School of Natural Resources and Extension, University of Alaska Fairbanks; the Bonanza Creek Long-Term Ecological Research program funded jointly by the National Science Foundation (NSF) and the USDA Forest Service Pacific Northwest Research Station; the Cedar Creek Long-Term Ecological Research program funded by NSF; and the Wilderness Research Foundation and Institute of the Environment, University of Minnesota. This is scientific article no. 3246 of the West Virginia Agricultural and Forestry Experiment Station, Morgantown.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.P. is a guest editor invited by the Editorial Board.
Data deposition: The data are available on J.L.’s institutional website at jiliang.forestry.wvu.edu/research. In addition, a copy of the data has been submitted to PNAS.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409853112/-/DCSupplemental.
References
- 1.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336(6087):1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 2.Adams WM, et al. Biodiversity conservation and the eradication of poverty. Science. 2004;306(5699):1146–1149. doi: 10.1126/science.1097920. [DOI] [PubMed] [Google Scholar]
- 3.FAO UN . Assessing Forest Degradation: Towards the Development of Globally Applicable Guidelines. UN Food and Agric Organ; Rome: 2011. [Google Scholar]
- 4.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 5.Hooper DU, Vitousek PM. The effects of plant composition and diversity on ecosystem processes. Science. 1997;277(5330):1302–1305. [Google Scholar]
- 6.Wardle DA. Is “sampling effect” a problem for experiments investigating biodiversity-ecosystem function relationships? Oikos. 1999;87(2):403–407. [Google Scholar]
- 7.Cardinale BJ, et al. The functional role of producer diversity in ecosystems. Am J Bot. 2011;98(3):572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 8.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336(6081):589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 9.Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM. Declining biodiversity can alter the performance of ecosystems. Nature. 1994;368(6473):734–737. [Google Scholar]
- 10.Tilman D, et al. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277(5330):1300–1302. [Google Scholar]
- 11.Hector A. Ecology: Diversity favours productivity. Nature. 2011;472(7341):45–46. doi: 10.1038/472045a. [DOI] [PubMed] [Google Scholar]
- 12.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: Theoretical considerations. Proc Natl Acad Sci USA. 1997;94(5):1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chen HYH, Reich PB. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J Ecol. 2012;100(3):742–749. [Google Scholar]
- 14.Liang J, Buongiorno J, Monserud RA, Kruger EL, Zhou M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For Ecol Manage. 2007;243(1):116–127. [Google Scholar]
- 15.Gamfeldt L, et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat Commun. 2013;4:1340. doi: 10.1038/ncomms2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadrowski K, Wirth C, Scherer-Lorenzen M. Is forest diversity driving ecosystem function and service? Curr Opin Environ Sustainability. 2010;2(1-2):75–79. [Google Scholar]
- 17.Paquette A, Messier C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob Ecol Biogeogr. 2011;20(1):170–180. [Google Scholar]
- 18.Nilsson M-C, Wardle DA. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front Ecol Environ. 2005;3(8):421–428. [Google Scholar]
- 19.Gilliam FS. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57(10):845–858. [Google Scholar]
- 20.Woods KD, Hicks DJ, Schultz J. Losses in understory diversity over three decades in an old-growth cool-temperate forest in Michigan, USA. Can J For Res. 2012;42(3):532–549. [Google Scholar]
- 21.Van De Sande-Bakhuyzen HL. Studies on Wheat Grown Under Constant Conditions: A Monograph on Growth. Food Res Inst, Stanford University; Stanford, CA: 1937. [Google Scholar]
- 22.Sanderson FH. Methods of Crop Forecasting. Harvard Univ Press; Cambridge, MA: 1954. [Google Scholar]
- 23.Chapin FS, III, Matson PPA, Vitousek PM. Principles of Terrestrial Ecosystem Ecology. Springer; New York: 2011. [Google Scholar]
- 24.Huxman TE, et al. Convergence across biomes to a common rain-use efficiency. Nature. 2004;429(6992):651–654. doi: 10.1038/nature02561. [DOI] [PubMed] [Google Scholar]
- 25.Evans LT. The natural history of crop yield: A combination of improved varieties of crop plants and technological innovations continues to increase productivity, but the highest yields are approaching limits set by biological constraints. Am Sci. 1980;68(4):388–397. [Google Scholar]
- 26.Samuelson P, Nordhaus W. Microeconomics. McGraw–Hill; Oklahoma City, OK: 2001. [Google Scholar]
- 27.Hannon B. Marginal product pricing in the ecosystem. J Theor Biol. 1976;56(2):253–267. doi: 10.1016/s0022-5193(76)80073-2. [DOI] [PubMed] [Google Scholar]
- 28.Dixit AK, Stiglitz JE. Monopolistic competition and optimum product diversity. Am Econ Rev. 1977;67(3):297–308. [Google Scholar]
- 29.Malone T, Liang J, Packee EC. 2009. Cooperative Alaska Forest Inventory (Pac Northwest Res Stn, For Serv, US Dep of Agric, Portland, OR), Gen Tech Rep PNW-GTR-785.
- 30.Ereshefsky M. In: Species. The Stanford Encyclopedia of Philosophy. Zalta EN, editor. Stanford University; Stanford, CA: 2010. [Google Scholar]
- 31.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci USA. 2013;110(29):11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.