Significance
Regulation of differential gene expression by Wnt/glycogen synthase kinase 3 (GSK3) signaling plays a major role in development, but there is accumulating evidence for a transcription-independent function of this pathway. In mature oocytes and early embryos, transcription is temporarily silenced, providing an opportunity to study the nontranscriptional role of Wnt signaling. Our study shows that inhibition of Wnt signaling in frog oocytes leads to early cleavage arrest after fertilization and provides evidence that this process occurs by regulating proteins involved in mitosis. Our study indicates that control of early development is a prominent role for nontranscriptional, Wnt/GSK3 signaling.
Keywords: Wnt/STOP, Xenopus, mitosis, GSK3, proteolysis
Abstract
During Xenopus development, Wnt signaling is thought to function first after midblastula transition to regulate axial patterning via β-catenin–mediated transcription. Here, we report that Wnt/glycogen synthase kinase 3 (GSK3) signaling functions posttranscriptionally already in mature oocytes via Wnt/stabilization of proteins (STOP) signaling. Wnt signaling is induced in oocytes after their entry into meiotic metaphase II and declines again upon exit into interphase. Wnt signaling inhibits Gsk3 and thereby protects proteins from polyubiquitination and degradation in mature oocytes. In a protein array screen, we identify a cluster of mitotic effector proteins that are polyubiquitinated in a Gsk3-dependent manner in Xenopus. Consequently inhibition of maternal Wnt/STOP signaling, but not β-catenin signaling, leads to early cleavage arrest after fertilization. The results support a novel role for Wnt signaling in cell cycle progression independent of β-catenin.
Wnt/glycogen synthase kinase 3 (GSK3) signaling is a pathway thought to act essentially by regulating differential gene expression via β-catenin. In unstimulated cells, β-catenin is phosphorylated by GSK3, then polyubiquitinated by beta-transducin repeat containing protein 1 (β-TrCP), and thereby targeted to proteasomal degradation (1, 2). Upon Wnt stimulation, Wnt ligands form a ternary complex with Frizzled (Fzd) and low density lipoprotein receptor-related protein 6 (LRP6) coreceptors, which cluster with Disheveled to form endocytic LRP6 signalosomes (3–5). Clustered LRP6 becomes phosphorylated, which triggers the inhibition of GSK3 by various mechanisms (6–11), thereby preventing β-catenin from proteolysis and promoting signaling transduction.
However, GSK3 is a promiscuous protein kinase, and there is evidence for significant signaling through a Wnt cascade that bifurcates at the level of GSK3 to stabilize proteins other than β-catenin (10, 12–14). Notably, GSK3 inhibition by Wnt peaks in mitosis because cyclin Y (CCNY) and its cyclin dependent kinase 14 (CDK14) activate the Wnt coreceptor LRP6 in a cell cycle-dependent manner (15). However, during mitosis, transcription is silenced, suggesting that mitotic Wnt signaling has β-catenin–independent functions. Following the discovery of De Robertis and colleagues that Wnt signaling stabilizes potentially hundreds of proteins other than β-catenin (10), we have recently introduced β-catenin–independent Wnt/STOP (Wnt-dependent stabilization of proteins) signaling and provided evidence that it serves to slow down protein degradation as cells prepare to divide (12).
In this study, we sought to provide in vivo evidence for Wnt/STOP signaling under conditions where β-catenin–mediated transcription can be ruled out. In most animals, including Xenopus laevis, transcription is silenced during oocyte maturation (16). This transcriptional arrest is maintained after fertilization until midblastula transition (17). Moreover, in Xenopus oocytes and eggs, Wnt11 signals through the canonical Wnt pathway (18). Finally, Xenopus oocytes and eggs are naturally arrested at defined cell cycle phases, which avoids pharmacological synchronization normally required for biochemical cell cycle analysis. We found that Wnt signaling is induced during oocyte maturation and inhibits Gsk3, thereby preventing protein polyubiquitination. We identified a cluster of mitotic effectors that are polyubiquitinated in a Gsk3-dependent manner and found that, consistently, inhibition of maternal Wnt/STOP signaling led to cleavage arrest in early embryos. Thus, maternal Wnt/GSK3 signaling functions β-catenin–independently in cell cycle progression.
Results
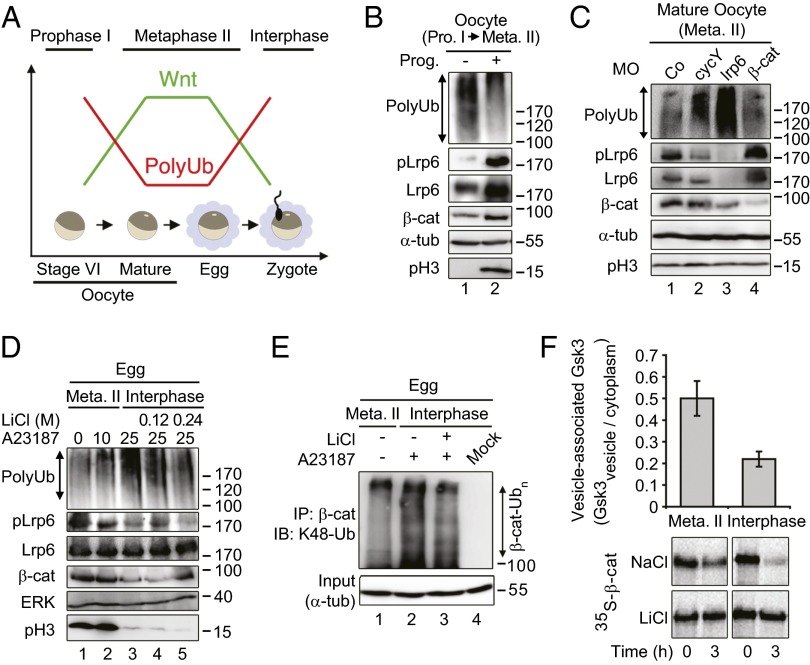
Wnt/STOP Signaling Operates in X. laevis Oocytes and Eggs.
Fully grown Xenopus oocytes (stage VI) are naturally arrested in meiotic prophase I. Exposure to progesterone, their physiological mitogen, induces oocyte maturation and arrest in meiotic metaphase II, akin to mitosis in somatic cells (Fig. 1A). Upon ovulation and egg fertilization, metaphase II arrest is released, and the zygotes enter into interphase. Because Wnt11 signaling is active in oocytes (19), we predicted that Wnt/STOP signaling peaks upon maturation in metaphase II (Fig. 1A). Indeed, we found that progesterone treatment of oocytes induces Lrp6 phosphorylation (pLrp6), reduces protein polyubiquitination (PolyUb), and stabilizes β-catenin (Fig. 1B). Moreover, inhibition of Wnt signaling upstream of Gsk3 by knocking down cyclin Y and lrp6 using previously established antisense Morpholinos (15, 20) strongly induced polyubiquitination in these metaphase II-arrested oocytes (Fig. 1C). In contrast, knock down of β-catenin with an established antisense Morpholino (21) had little effect.
Fig. 1.
Wnt/STOP operates in Xenopus oocytes and eggs in metaphase II. (A) Scheme of proposed Wnt signaling and protein polyubiquitination state in Xenopus oocytes and eggs (ovulated mature oocytes) as revealed in this study. Stage VI oocytes are arrested in prophase I (Pro. I). Progesterone (Prog.) matured oocytes and eggs are arrested in metaphase II (Meta. II). Fertilized or Ca2+ ionophore-released eggs progress to interphase. (B) Western blots of indicated proteins from Xenopus stage VI and progesterone-matured oocytes. α-tub, α-tubulin (loading control); β-cat, β-catenin; pH3, phosphorylated histone 3; pLrp6, phosphorylated Lrp6; PolyUb, polyubiquitination. (C) Western blots of indicated proteins from Xenopus matured oocytes injected with the indicated antisense Morpholinos (MO). α-tub, α-tubulin (loading control); β-cat, β-catenin; Co, control; cycY, cyclin Y; pH3, phosphorylated histone 3; pLrp6, phosphorylated Lrp6; PolyUb, polyubiquitination. (D and E) Western blots of indicated proteins from Xenopus eggs (naturally arrested in metaphase II) and treated with the calcium ionophore A23187 for the indicated times (minutes; D), or 25 min (E), in the presence of LiCl or NaCl (Control). (E) Analysis of the ubiquitination state of the β-catenin via immunoprecipitation (IP) followed by Western blotting (IB). α-tub, α-tubulin (loading control); β-cat, β-catenin; pH3, phosphorylated histone 3; pLrp6, phosphorylated Lrp6; PolyUb, polyubiquitination. (F) Gsk3 localization and activity in metaphase II and interphase eggs. (Top) Vesicular and cytoplasmic fractions were obtained from egg extracts, as described in Methods. Gsk3β protein levels were determined by Western blot, and the ratio (vesicle/cytoplasm) is indicated. (Bottom) In vitro-translated 35S-β-catenin was added to metaphase II extract and interphase extract at 0 h (input), and its degradation was monitored after 3 h, in the presence (LiCl) or absence (NaCl) of Gsk3 inhibitor. Note that higher vesicle-associated Gsk3 levels in metaphase II extracts are accompanied by greater β-catenin stability.
Because entry of oocytes into metaphase II via progesterone induced Wnt/STOP signaling and reduced polyubiquitination, we asked whether exit from metaphase II would have the opposite effect. Release of eggs into interphase by Ca2+ ionophore (A23187), which mimics fertilization, induced Histone 3 dephosphorylation after 25 min, indicating exit from mitosis (Fig. 1D). In agreement with our model, Lrp6 phosphorylation and β-catenin levels decreased upon mitotic exit (Fig. 1D, lanes 2 and 3). This reduction was accompanied by a strong increase in total polyubiquitination level, which was reversed by blocking Gsk3 with LiCl (Fig. 1D, lanes 4 and 5). Similarly, β-catenin polyubiquitination was increased upon mitotic exit, which was also reversed by LiCl (Fig. 1E).
Activation of Wnt signaling leads to the recruitment of GSK3 to membrane-associated LRP6 signalosomes (3, 10). Consistent with higher Wnt activity (Fig. 1A), extracts from metaphase II eggs showed a higher fraction of membrane-associated Gsk3 than interphase extracts (Fig. 1F, Top). Consequently, β-catenin was more stable in metaphase II compared with interphase extracts (Fig. 1F, Bottom).
Collectively, these results indicate that Wnt/STOP signaling operates in Xenopus mature oocytes and eggs.
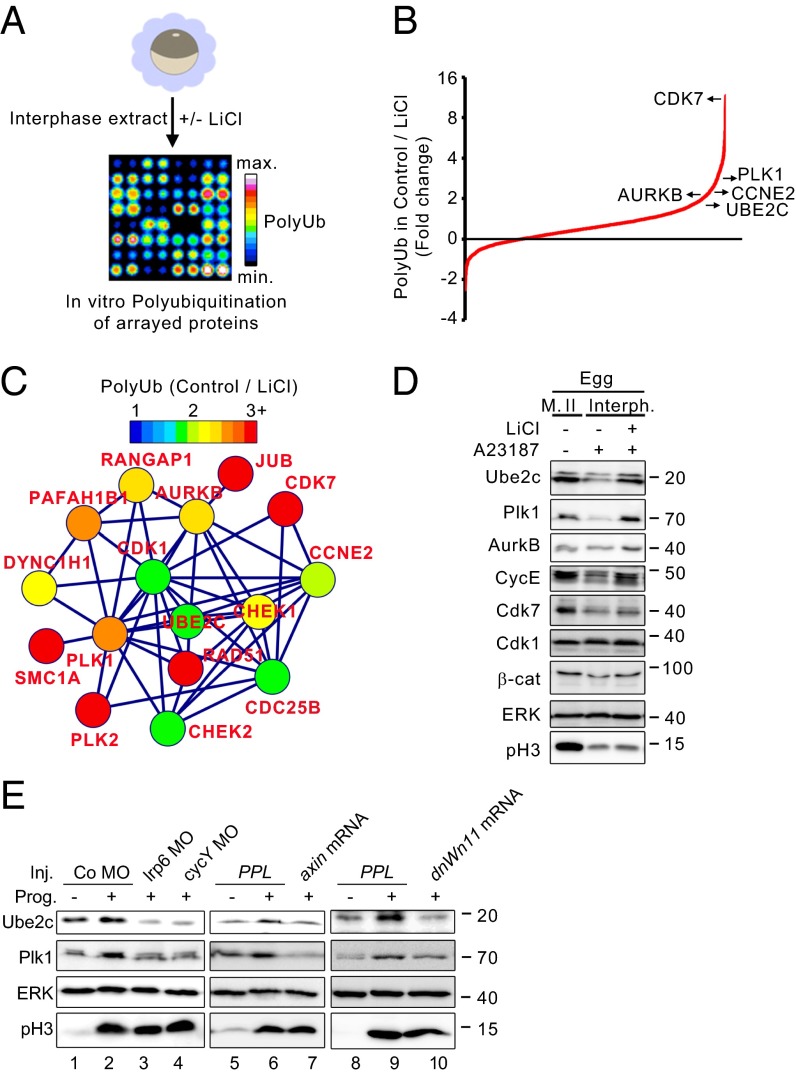
Gsk3 Negatively Regulates Mitotic Effectors in Xenopus.
Because Wnt/STOP signaling stabilizes proteins by inhibiting Gsk3, we sought to identify potential Gsk3 targets stabilized in Xenopus. We used interphase egg extracts to carry out in vitro polyubiquitination assays on commercial protein arrays containing ∼9,000 proteins (Fig. 2A) (12, 22). A total of 3,873 arrayed proteins (∼40%) were significantly polyubiquitinated (Dataset S1 and Methods), exceeding previously reported levels of around 10% (12, 22), likely because of the higher protein concentration obtainable in Xenopus egg extracts. Inhibition of Gsk3 in the egg extracts by LiCl increased polyubiquitination of only 22 proteins >1.8-fold (Dataset S1). In stark contrast, Gsk3 inhibition reduced the polyubiquitination of 864 (22.3%) of the proteins more than 1.8-fold (Fig. 2B). Among these putative Gsk3 targets, we identified a cluster of mitosis-associated proteins (Fig. 2C), which function in cell cycle progression of fertilized embryos during the first synchronous cleavages: Cyclin E, a known Gsk3 target (23), and its positive regulators Cdk7 and Plk2 (24); Cdk1, required for mitotic entry, and its activators Cdc25 and Cdk7 (24, 25); Ube2C/UbcH10, a component of the Anaphase promoting complex (APC/C) (26), and its positive regulator Plk1 (27); Cohesin (Scm1), required for chromosomal integrity during mitosis; Chk1/2 and Rad51, components of the DNA damage response checkpoint; and Aurora kinase B (Aurkb), acting in the spindle assembly checkpoint (28).
Fig. 2.
Gsk3 negatively regulates mitotic effectors in Xenopus extracts. (A) Scheme of the protein microarray experiment to assess Gsk3-dependent polyubiquitination. Interphase egg extracts were supplemented with ubiquitin, pretreated with LiCl or NaCl (Control), and then applied on protein arrays containing ∼9,000 proteins. Polyubiquitination of arrayed proteins (PolyUb) was monitored using anti-polyubiquitin antibodies and immunofluorescence detection using an array scanner. Signal intensities were transformed and are displayed as heat-map. (B) Distribution plot of polyubiquitination changes of 3,873 in vitro polyubiquitinated proteins after Gsk3 inhibition by LiCl (Log2[Control/LiCl]). Arrays were treated with 30 mM LiCl or 30 mM NaCl (Control). LiCl reduced polyubiquitination of 864 proteins >1.8-fold. Some candidates, associated with cell cycle progression and selected for further validation are indicated in the plot. (C) Cluster of mitotic associated proteins that are significantly regulated by LiCl in B. The functional network was determined using String 9.1 and color-coded based on the fold change in the microarray (Dataset S1). (D) Western blots of indicated proteins from Xenopus eggs (naturally arrested in metaphase II) and released into interphase with the Ca2+ ionophore A23187 for 25 min, treated with LiCl, or mock treated (NaCl). β-cat, β-catenin; cycE, cyclin E; Interph., interphase; M. II, metaphase II; pH3, phosphorylated histone 3. (E) Western blots of stage VI oocytes in Prophase I and Progesterone-matured (Prog.) oocytes in metaphase II. Oocytes were injected (Inj.) with Morpholinos (MO) or mRNA as indicated. Co, control; dnWnt11, dominant negative Wnt11; pH3, phosphorylated histone 3; PPL, preprolactin (negative control).
To further validate these putative Gsk3 target proteins, we selected several candidates based on antibody availability and cross-reactivity with Xenopus, including Ube2c, Plk1, Aurkb, Cyclin E, Cdk7, and Cdk1 (29–31). We analyzed whether their protein levels decrease upon egg activation (transition from metaphase II to interphase) and whether they are stabilized by LiCl (Fig. 2D and Fig. S1A). This pattern was observed for Ube2c, Plk1, and Cyclin E. Little stabilization was observed for Cdk7 and β-catenin whereas no stabilization was observed for Cdk1. Consistent with being Wnt/STOP targets, Ube2c and Plk1 protein levels decreased in mature oocytes after cyclin Y and lrp6 knock down (Fig. 2E, Left and Fig. S1B). Furthermore, inhibition of Wnt signaling with axin mRNA and dominant negative wnt11 (dnWnt11) also reduced the protein levels of Ube2c and Plk1 (Fig. 2E, Middle and Right and Fig. S1B).
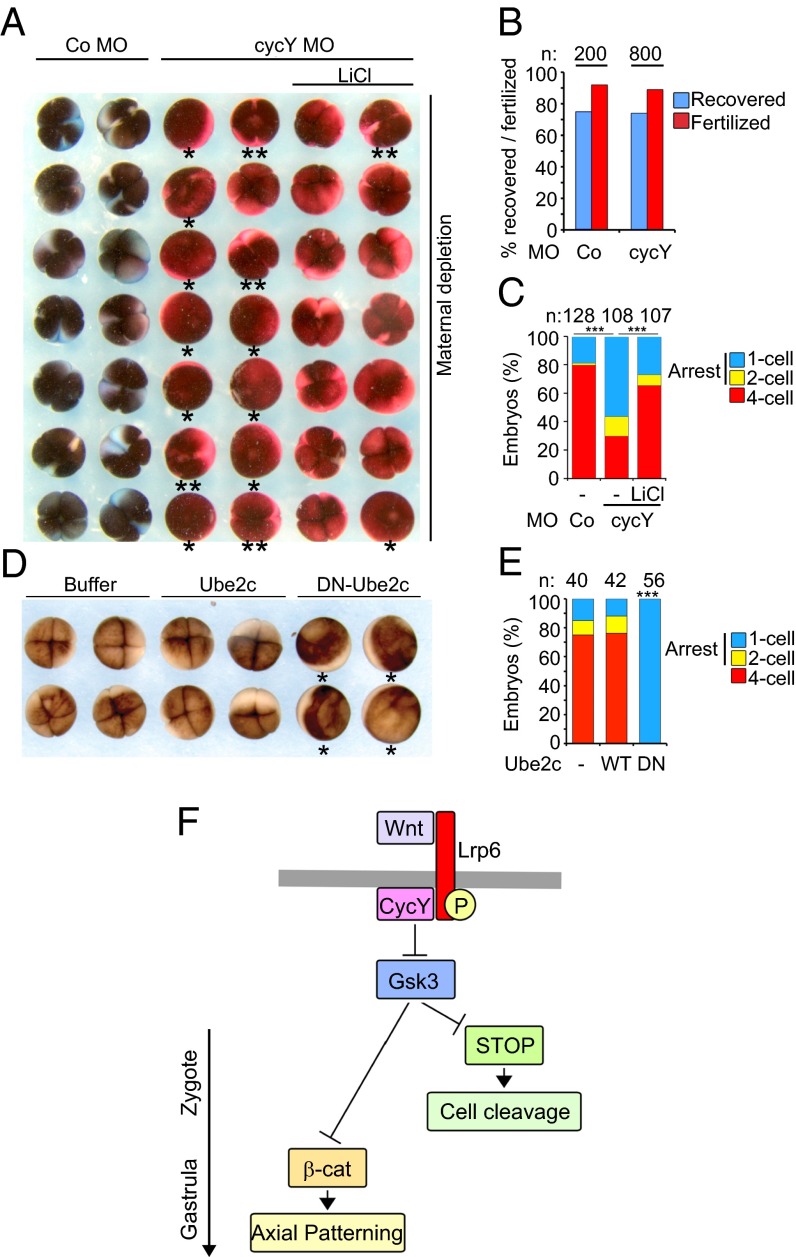
Inhibiting Maternal Wnt/STOP Signaling Induces Cleavage Arrest in Xenopus Embryos.
Stabilization of mitotic regulators by maternal Wnt/STOP signaling may prepare eggs for the rapid sequence of cell divisions ensuing after fertilization. We tested this hypothesis by inhibiting maternal Wnt/STOP signaling and analyzing Xenopus early development. Oocytes were depleted of Cyclin Y, Lrp6, or β-catenin using antisense Morpholinos (Fig. 3B). After in vitro maturation and host transfer (32), eggs were fertilized (Fig. 3A). There was no difference between morphants in egg recovery or fertilization rate (Fig. 3C). Importantly, 60–80% of cyclin Y and lrp6 morphants failed to cleave during first or second cell division (Fig. 3 D and E). Those cyclin Y and lrp6 morphants that managed to cleave did so at the same time as control morphants (Fig. 3F), indicating that cell cycle time was unaffected but rather cell division proper was affected.
Fig. 3.
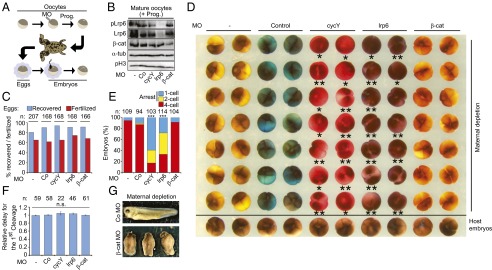
Inhibiting Wnt/STOP induces cleavage arrest in Xenopus embryos. (A–F) Cell cycle progression in early Xenopus embryos upon maternal depletion of Wnt components. Co, control; cycY, cyclin Y; β-cat, β-catenin. (A) Experimental scheme of the Host Transfer experiment. Xenopus oocytes were Morpholino (MO) depleted as indicated, matured by progesterone and foster-mother transfer, and fertilized in vitro. Prog., progesterone. (B) Western blots of mature oocytes injected with the indicated Morpholinos. α-tub, α-tubulin (loading control); pH3, phosphorylated histone 3; pLrp6, phosphorylated Lrp6. (C) Proportion of the indicated morphant eggs recovered and fertilized after host transfer in D, respectively. n, number of initial mature oocytes. (D) Cell cycle progression in early Xenopus embryos upon maternal depletion of Wnt components. Fertilized eggs were filmed until four-cell stage, and the last frame is shown. Embryos arrested before the first or the second cleavage are marked with one or two asterisks, respectively. Eggs were labeled with a neutral color additive for identification before transfer in a foster mother. (E) Quantification of cleaving morphants from D. n, number of embryos. (F) Time required for the indicated morphants to reach the first cleavage. Note that many lrp6 and cycY morphants failed to cleave; thus, only the escapers were measured. n, number of embryos; n.s., not significant. (G) Control (Co) and β-catenin (β-cat) morphants at tadpole stage upon maternal depletion.
This early cleavage arrest phenotype is a surprising finding because Wnt signaling is thought to regulate embryonic patterning via β-catenin–mediated transcription only after midblastula transition (18, 33). Indeed, β-catenin morphants expectedly showed dorso-ventral patterning defects at late embryonic stages (Fig. 3G), but, unlike cyclin Y morphants, they cleaved normally (Fig. 3 D and E). Previous maternal depletion studies of Wnt components (e.g., wnt11, lrp6, and cyclin Y) did not report cleavage arrest, likely because attention was focused on the axial patterning defects after midblastula transition (15, 18).
Importantly, the cleavage defect in cyclin Y morphants was rescued by inhibiting Gsk3 with LiCl (Fig. 4 A–C). This key control experiment corroborates the specificity of the cleavage arrest phenotype and confirms that Cyclin Y acts via Gsk3 to promote early embryonic cell cycle progression.
Fig. 4.
Inhibiting Wnt/STOP induces cleavage arrest through Gsk3 in Xenopus embryos. (A–C) Cleavage arrest in early Xenopus embryos depleted of Cyclin Y (cycY) can be rescued by Gsk3 inhibition. (A) Oocytes, eggs, and embryos were handled as described in Fig. 3D and treated with 60 mM LiCl to inhibit Gsk3 or control treated (NaCl) after fertilization. Fertilized eggs were filmed until four-cell stage, and the last frame is shown. (B) Fraction of the indicated morphant eggs recovered and fertilized after host transfer in A. n, number of initial mature oocytes. (C) Quantification of cleaving morphants from A. n, number of embryos. (D) Dominant negative Ube2c arrests cell cycle progression in early Xenopus embryos. Embryos were injected at one-cell stage with the indicated recombinant proteins and recorded at the four-cell stage. Embryos arrested before the first cleavage are marked with an asterisk. (E) Quantification of cleaving embryos from D. DN, DN-Ube2c; n, number of embryos; WT, Ube2c. (F) Wnt/β-catenin and Wnt/STOP signaling in Xenopus.
Cleavage arrest is also observed upon depletion of the Cyclin B/Cdk1 or components of APC/C (34). Among these components, the E2 ligase of APC/C, Ube2c, was detected in our screen for Gsk3 targets (Fig. 2 B–E). Injection of dominant negative Ube2c protein (DN-Ube2c) (35), but not WT Ube2c, phenocopied cleavage arrest observed in cyclin Y and lrp6 morphants (Fig. 4 D and E). These results suggest that Ube2c is one of several mitotic effectors that need to be stabilized by maternal Wnt signaling in preparation for embryonic cell cycle progression.
Discussion
There is accumulating evidence that the widely held notion whereby the output of Wnt/GSK3 signaling is essentially β-catenin–dependent transcription is at best incomplete (10, 12, 14, 36–38). The results presented provide, to our knowledge, the first in vivo evidence for a role of Wnt/STOP signaling, which so far has been demonstrated only in cultured cells (10, 12). Our study establishes Xenopus oocytes and zygotes as a model that allows uncoupling the well-established transcriptional roles of β-catenin from the posttranscriptional effects mediated by Wnt/STOP.
The surprising conclusion is that, in Xenopus embryogenesis, Wnt/GSK3 signaling functions β-catenin–independently before axial patterning to promote cell cleavage (Fig. 4F). This result corroborates that one function of mitotic Wnt signaling is to stabilize Gsk3 target proteins in preparation for cell division (12). Gsk3 regulates a battery of mitotic effectors, which are required for embryonic cleavage after fertilization. Together with a body of evidence that Wnt pathway components regulate the mitotic spindle (37, 39, 40), our study supports the proposition that Wnt signaling orchestrates a transcription-independent mitotic program (13). It therefore seems fruitful to investigate also in somatic cells whether Wnt/STOP signaling not only regulates cell size and chromosome segregation as reported (12, 40), but in addition stabilizes mitotic regulators to regulate cell cycle progression.
Methods
Antibodies.
Rabbit polyclonal anti-cyclin Y (CycY) and anti–Sp1490-LRP6 (pLrp6) antibodies were as described (15). Other antibodies used were as follows: anti–α-tubulin (α-tub), anti-β-catenin (β-cat), and anti-ERK (Sigma); anti-LRP6 (C5C7) (Cell Signaling); anti–phospho-Histone 3-Ser10 (pH3) (Abcam); anti–polyubiquitin FK-1 (PolyUb) (ENZO Life Sciences); anti-Ube2c and anti-Cdk7 (Santa Cruz Biotechnology); and anti-Lys48–linked ubiquitin (anti-K48-Ub) (Millipore). Anti-Xenopus Aurora B (Aurkb) and anti-Xenopus Plk1/Plx1 (Plk1) were a kind gift from H. Funabiki, Rockefeller University, New York (31, 41). Anti-Xenopus Cyclin E was a kind gift from C. Bonne-Andrea, Centre de Recherche de Biochimie Macromoléculaire (CRBM), Montpellier, France (29). For Western blot, antibodies were diluted in Tris buffered saline with Tween 20 containing 5% (wt/vol) BSA and 1 mM EDTA.
Anti-PolyUb recognizes all ubiquitin chains. Anti–K48-Ub binds specifically to lysine-48–linked ubiquitin chains, which are the most common label for proteasomal degradation.
X. laevis Methods.
In vitro fertilization, embryo culture, staging, and microinjection were carried out as described (42). Morpholinos against ccny plus ccnyl1 (cycY), lrp6, and β-catenin have been described previously (15, 20, 21).
Stage 6 oocytes were manually defolliculated, injected equatorially with Morpholinos (MOs), and cultured a total of 48–72 h at 18 °C in oocyte culture medium (OCM) (18, 33). Oocyte maturation was stimulated with 1 μM progesterone in OCM for 12 h. Where indicated, oocytes were injected with MOs after defolliculation and fertilized using the host transfer technique (18). Fertilized eggs (showing a perivitelline space and no germinal vesicle spot) were selected and imaged under a Zeiss SteREO Discovery V20 microscope every minute until four-cell stage. To monitor a possible cleavage delay in experimental embryos, 10 host embryos were used as reference, and their average time point of first cleavage was set to 1. In Fig. 4 A–C, fertilized eggs were treated with 60 mM LiCl or mock treated (NaCl) for 1 h right after dejellying. In Fig. 2E, Middle and Right, oocytes were injected with preprolactin (PPL, as negative control), axin or dominant negative wnt11 (dnwnt11) mRNA and maturated with 1 μM progesterone in OCM for 12 h. In Fig. 4 D and E, one-cell-stage embryos were injected with recombinant dominant negative Ube2c protein (DN-Ube2C) (35), WT Ube2c, or buffer.
For Western blot analysis, whole Xenopus embryos, eggs, or oocytes were homogenized in Triton lysis buffer [1% Triton X-100, 50 mM Tris⋅HCl, pH 7.0, 150 mM NaCl, 10 mM NaF, 5 mM Na3VO4, 1 mM PMSF, 20 mM N-ethylmaleimide, and protease inhibitor mixture tablet (Roche)] at one embryo or oocyte per 10 μL, cleared with FREON followed by centrifugation (17,000 × g, 3 min at 4 °C), heated at 95 °C for 3 min with SDS loading buffer, and analyzed by SDS/PAGE. Eggs (Fig. 1 D and E) were treated with 5 μM A23187 in the presence of 100 µg/mL cycloheximide (Sigma) in combination with LiCl or NaCl (0.24 M unless otherwise indicated).
In Fig. 1E, for analysis of β-catenin polyubiquitination, eggs were lysed in lysis buffer II [30 mM Tris, pH 7.5, 150 mM NaCl, 10 mM NaF, 2 mM EDTA, 1 mM β-mercaptoethanol, 1× protease inhibitor mixture tablet (Roche), 20 μM MG-132, 10 mM N-ethylmaleimide, 0.8% Nonidet P-40, 6 M Urea] for 15 min at 4 °C. Lysates were cleared and diluted sixfold in IP Buffer [20 mM Tris, pH 7.5, 150 mM NaCl, 50 mM NaF, 1× protease inhibitor mixture tablet (Roche), 25 μM MG-132, 10 mM N-ethylmaleimide, and 0.8% Nonidet P-40]. Proteins were immunoprecipitated using β-catenin antibody followed by Western blotting using anti-K48–linked ubiquitin antibody. Ubiquitination and proteasomal degradation in Xenopus oocytes have been previously reported (43).
In Fig. 1F, crude metaphase II and interphase egg extracts were prepared essentially as described (44). Briefly, Xenopus eggs were collected overnight in 1× Marc´s Modified Ringer´s solution (MMR, 5 mM Hepes, pH 7.5, 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EGTA), dejellied in 2% cysteine solution [2% (wt/vol) l-Cysteine, 100 mM KCl, 1 mM MgCl2, 100 μM CaCl2, pH 7.8], washed extensively with XB buffer (100 mM KCl, 1 mM MgCl2, 100 μM CaCl2, 50 mM sucrose, and 10 mM Hepes, pH 7.5). To maintain eggs in metaphase II, XB buffer was supplemented with 5 mM EGTA and 1 mM MgCl2. To release eggs into interphase, they were activated with A23187 Ca2+ ionophore (2 μg/mL, 5 min) before the washing steps. Washed eggs were packed [SW41 Ti rotor (Beckman), 2,000 rpm, 2 min, 16 °C] and then crushed [SW41 Ti rotor (Beckman), 11,000 rpm, 20 min at 4 °C]. The crude cytoplasm beneath the lipid plug was collected with an 18G needle, supplemented with protease inhibitors (Roche), 1 μg/mL cytochalasin D, 1 mM ATP, and 1 mM MgCl2.
For in vitro β-catenin degradation (Fig. 1F, Bottom), interphase or metaphase II crude extracts were supplemented with 0.5 mg/mL recombinant ubiquitin (BostonBiochem), 100 μg/mL cycloheximide, in vitro-translated 35S-Methionine–labeled β-catenin [Promega TNT(R) Coupled Reticulocyte Lysate kit; 1:10 dilution in extract], and 25 mM LiCl or NaCl (mock treatment). Destabilization of in vitro-translated 35S-β-catenin in the metaphase II extract and the interphase extract was monitored at 0 h (input) and 3 h, in the presence (LiCl) or absence (NaCl) of Gsk3 inhibitor.
For Gsk3β quantification (Fig. 1F, Top), extracts were diluted 5× in their respective extraction buffers and centrifuged at 200,000 × g (Beckman, TLA200.2 rotor) for 2 h. Top cytosolic fraction was collected for Western blot analysis. Light and heavy membrane fractions, sandwiched between the cytoplasm and the glycogen pellet, were collected and passed through a sucrose cushion (extraction buffer containing 250 mM sucrose at 4 °C) to obtain the vesicular fraction and collected for Western blot analysis. Gsk3β protein levels in the cytosolic and vesicular fractions were quantified in triplicate in an LAS-3000 (Fujifilm).
Protein Array.
Eggs were collected overnight in 1× MMR solution. High quality eggs were pooled and washed in 0.5× MMR and dejellied in 2% cysteine solution (2% wt/vol l-Cysteine HCl in H2O, pH 7.8). Eggs were washed once with 0.5× MMR followed by several washes in Lysis Buffer (LB, 10 mM Hepes, pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 50 mM sucrose, 50 μg/mL cycloheximide and 1 mM DTT). Washed eggs were first packed in the presence of 1 μg/mL cytochalasin D and protease inhibitors [SW41 Ti rotor (Beckman), 2,000 rpm, 2 min, 16 °C], followed by a crushing step [SW41 Ti rotor (Beckman), 11,000 rpm, 20 min at 16 °C to 4 °C]. Crude extracts were cooled on ice and supplemented with protease inhibitors, 1 μg/mL cytochalasin D, 100 μg/mL cycloheximide and an energy-regenerating mix giving 1 mM ATP, 1 mM MgCl2, 7.5 mM creatine phosphate, and 30 μg/mL creatine phosphokinase. To obtain high-speed cytoplasm, crude extract was subjected to 2 h centrifugation at 200,000 × g, 4 °C. High speed extracts were further supplemented with 150 μM MG132, 1 μM ubiquitin-aldehyde (Santa Cruz), and 0.5 mg/mL ubiquitin. Extract dilution was roughly 75% and contained ∼11 mg/mL protein. Interphase was assessed by sperm nuclei swelling in crude extracts.
ProtoArray Human protein microarrays (ProtoArray 5.0 platform; Invitrogen) containing more than 9,000 proteins, were handled essentially as described (22). Briefly, the arrays were washed in TBST and blocked for 4 h at 4 °C using Microarray Blocking solution (Arrayit), followed by a wash in lysis buffer. Extracts (0.6 mL) were prewarmed to room temperature, treated with 120 mM LiCl or NaCl (Control), and incubated on the microarrays under coverslips at room temperature for 1 h. Microarrays were washed with TBST and incubated overnight at 4 °C with anti-polyubiquitin antibody FK-1 (4 μg/mL) (ENZO Life Sciences) diluted in TBST, washed again, and incubated with Alexa Fluor 680 anti-mouse antibody (Invitrogen) for 4 h. The microarrays were washed three times in TBST and two times in water, dried, and scanned in an Odyssey scanner (LI-COR) using the following parameters: channel 700; grid = 8 × 3, 21.17; intensity = 5; off-set = 0. ProtoArrays were analyzed using GenePix Pro (version 5.0.0.49; Molecular Devices), preprocessed following the manufacturer’s recommendations, and processed using a noise floor of 250 RFU and a cutoff of 512 relative fluorescence units. “Polyubiquitination hits” were defined as proteins whose anti-polyubiquitin immunofluorescence signal intensity showed a Z-factor above 0.2, replicate spot coefficient of variation (CV) below 0.5, and mean signal above the background noise floor. Retained for analysis were 3,873 proteins (Dataset S1). Candidates in which Gsk3 inhibition by LiCl reduced polyubiquitination in more than 1.8-fold (864) were further analyzed using STRING (string-db.org/) for known or inferred protein–protein interactions. The most prominent cluster, formed by mitotic effectors (blue lines), was depicted together with the fold change in polyubiquitination of its members, and indicated as a heat map.
Statistics.
For Western blots and embryonic cell cleavages, experiments were replicated at least three times, and representative images are shown. Figs. 3E and 4 C and E were analyzed with a Fisher’s exact test. Protein arrays in Fig. 2B were analyzed as described in Protein Array. Differences were considered to be statistically significant for P values of <0.05 (*), <0.01 (**), or <0.005 (***).
Supplementary Material
Acknowledgments
We thank J. Herbst, N. Kirsch, and P. Stannek for expert technical help and the German Cancer Research Center microarray, flow cytometry, and microscopy core facilities for expert technological support. We thank G. Roth (ASKA Pharmaceutical), O. Gruss, E. Lee, C. Bonne-Andrea, and H. Funabiki for reagents. We thank S. Koch for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423533112/-/DCSupplemental.
References
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 3.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316(5831):1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 4.Cruciat CM, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz-Romond T, et al. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14(6):484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 6.Kim NG, Xu C, Gumbiner BM. Identification of targets of the Wnt pathway destruction complex in addition to beta-catenin. Proc Natl Acad Sci USA. 2009;106(13):5165–5170. doi: 10.1073/pnas.0810185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li VS, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe C, Mendoza-Topaz C, Mieszczanek J, Bienz M. Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J Cell Sci. 2010;123(Pt 9):1588–1599. doi: 10.1242/jcs.067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piao S, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS ONE. 2008;3(12):e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinyoles M, et al. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol Cell. 2014;53(3):444–457. doi: 10.1016/j.molcel.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Acebron SP, Karaulanov E, Berger BS, Huang YL, Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol Cell. 2014;54(4):663–674. doi: 10.1016/j.molcel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. EMBO J. 2012;31(12):2705–2713. doi: 10.1038/emboj.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8(24):4032–4039. doi: 10.4161/cc.8.24.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson G, et al. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17(6):788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Davidson EH. Gene Activity in Early Development. 3rd Ed Academic; Orlando, FL: 1986. [Google Scholar]
- 17.Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 18.Tao Q, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120(6):857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Kofron M, et al. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134(3):503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- 20.Hassler C, et al. Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development. 2007;134(23):4255–4263. doi: 10.1242/dev.005942. [DOI] [PubMed] [Google Scholar]
- 21.Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: A novel antisense approach. Dev Biol. 2000;222(1):124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- 22.Merbl Y, Kirschner MW. Large-scale detection of ubiquitination substrates using cell extracts and protein microarrays. Proc Natl Acad Sci USA. 2009;106(8):2543–2548. doi: 10.1073/pnas.0812892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welcker M, et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12(2):381–392. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 24.Fesquet D, Morin N, Doree M, Devault A. Is Cdk7/cyclin H/MAT1 the genuine cdk activating kinase in cycling Xenopus egg extracts? Oncogene. 1997;15(11):1303–1307. doi: 10.1038/sj.onc.1201300. [DOI] [PubMed] [Google Scholar]
- 25.Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 26.Summers MK, Pan B, Mukhyala K, Jackson PK. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell. 2008;31(4):544–556. doi: 10.1016/j.molcel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt A, et al. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005;19(4):502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002;12(11):900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 29.Cueille N, Nougarede R, Mechali F, Philippe M, Bonne-Andrea C. Functional interaction between the bovine papillomavirus virus type 1 replicative helicase E1 and cyclin E-Cdk2. J Virol. 1998;72(9):7255–7262. doi: 10.1128/jvi.72.9.7255-7262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier J, et al. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- 31.Kelly AE, et al. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12(1):31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mir A, Heasman J. How the mother can help: Studying maternal Wnt signaling by anti-sense-mediated depletion of maternal mRNAs and the host transfer technique. Methods Mol Biol. 2008;469:417–429. doi: 10.1007/978-1-60327-469-2_26. [DOI] [PubMed] [Google Scholar]
- 33.White JA, Heasman J. Maternal control of pattern formation in Xenopus laevis. J Exp Zoolog B Mol Dev Evol. 2008;310(1):73–84. doi: 10.1002/jez.b.21153. [DOI] [PubMed] [Google Scholar]
- 34.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 35.Townsley FM, Aristarkhov A, Beck S, Hershko A, Ruderman JV. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc Natl Acad Sci USA. 1997;94(6):2362–2367. doi: 10.1073/pnas.94.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi K, Niikura Y, Kitagawa K, Kikuchi A. Dishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1. EMBO J. 2010;29(20):3470–3483. doi: 10.1038/emboj.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111(Pt 10):1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 39.Habib SJ, et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339(6126):1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stolz A, Neufeld K, Ertych N, Bastians H. Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep. 2015;16(4):490–499. doi: 10.15252/embr.201439410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghenoiu C, Wheelock MS, Funabiki H. Autoinhibition and Polo-dependent multisite phosphorylation restrict activity of the histone H3 kinase Haspin to mitosis. Mol Cell. 2013;52(5):734–745. doi: 10.1016/j.molcel.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. Antagonizing the Spemann organizer: Role of the homeobox gene Xvent-1. EMBO J. 1995;14(24):6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. The ‘second-codon rule’ and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J. 1992;11(7):2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.