Significance
Disruptions in stress response system functioning are thought to be a central mechanism by which exposure to adverse early-life environments influences human development. Although rodent models support this possibility, results from human studies have been decidedly mixed. Using data from an experimental study examining whether random assignment to a caregiving environment alters development of the autonomic nervous system and hypothalamic–pituitary–adrenal axis in humans, we provide causal evidence for persistent effects of the early caregiving environment on stress response system functioning in humans with effects that differ markedly from those observed in rodent models. We also provide evidence of a sensitive period in human development during which the environment is particularly likely to alter stress response system development.
Keywords: childhood adversity, early-life stress, HPA axis, autonomic nervous system, stress reactivity
Abstract
Disruptions in stress response system functioning are thought to be a central mechanism by which exposure to adverse early-life environments influences human development. Although early-life adversity results in hyperreactivity of the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis in rodents, evidence from human studies is inconsistent. We present results from the Bucharest Early Intervention Project examining whether randomized placement into a family caregiving environment alters development of the autonomic nervous system and HPA axis in children exposed to early-life deprivation associated with institutional rearing. Electrocardiogram, impedance cardiograph, and neuroendocrine data were collected during laboratory-based challenge tasks from children (mean age = 12.9 y) raised in deprived institutional settings in Romania randomized to a high-quality foster care intervention (n = 48) or to remain in care as usual (n = 43) and a sample of typically developing Romanian children (n = 47). Children who remained in institutional care exhibited significantly blunted SNS and HPA axis responses to psychosocial stress compared with children randomized to foster care, whose stress responses approximated those of typically developing children. Intervention effects were evident for cortisol and parasympathetic nervous system reactivity only among children placed in foster care before age 24 and 18 months, respectively, providing experimental evidence of a sensitive period in humans during which the environment is particularly likely to alter stress response system development. We provide evidence for a causal link between the early caregiving environment and stress response system reactivity in humans with effects that differ markedly from those observed in rodent models.
Disruptions in stress response system functioning are thought to be a central mechanism by which exposure to adverse early-life environments influences human development. This idea is borne out in rodent models, where the effects of early-life adversity on the development of stress response systems have been well characterized. Exposure to early-life adversity—involving repeated and prolonged separation of a pup from its mother—results in hyperreactivity of the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenal (HPA) axis in adolescence and adulthood and elevations in anxiety, fearful behaviors, and hypervigilance (1–4). Stress exposure in mature rodents is associated with immediate, but not lasting, changes in stress response systems (5, 6), suggesting the presence of an early sensitive period when exposure to adverse environments results in long-term changes in physiological stress response system functioning.
A similar pattern of findings has been observed in some, but not all, studies of HPA axis development in nonhuman primates following early-life adversity. Rhesus and squirrel monkeys exposed to prolonged early-life maternal deprivation exhibit elevated basal levels of cortisol (7–9), enhanced glucocorticoid feedback sensitivity (10), and heightened cortisol reactivity to social stress in some studies (11, 12), but lower basal cortisol and reduced cortisol reactivity in others (9, 13, 14). The effect of maternal deprivation on SNS development in nonhuman primates has been studied infrequently.
Investigations of early-life adversity and stress response system reactivity in humans have produced decidedly mixed findings. Some studies document hyperreactivity of the SNS and HPA axis following early-life adversity (15–19) and others observe blunted HPA axis reactivity (20–22) or discordance between SNS and HPA axis responses (23). Reconciling these inconsistencies has proved challenging for several reasons. First, there is considerable heterogeneity in the type, frequency, severity, and co-occurrence of early-life adversities in human studies, including both abuse and neglect (15, 18, 19, 21, 22), poverty (24–26), institutional rearing followed by international adoption (17, 20, 27, 28), or accumulation of multiple adversities, ranging from marital conflict to parental psychopathology (29–31). These exposures not only differ widely from each other, they also vary in their resemblance to the exposure used in animal studies: maternal deprivation. Lower morning cortisol and blunted cortisol reactivity are the most commonly reported patterns in studies of maternal deprivation related to neglect or institutional rearing following by international adoption (20, 28, 32), although elevated basal cortisol and cortisol reactivity have also been observed (17, 27). Second, existing human research has been unable to identify causal effects of the rearing environment on stress response system development. In animals, physiological hyperreactivity induced by exposure to early-life adversity can be ameliorated by placement in an enriched environment during puberty (33), indicating that the neurobiological consequences of early-life adversity may be reversed, at least in part, through improvements to the environment. Although physiological reactivity in humans can be altered in the short term by psychosocial interventions (34, 35), including in children exposed to maternal deprivation (36, 37), we are unaware of experimental research examining whether random assignment to a caregiving environment alters patterns of physiological reactivity later in development. Finally, although a sensitive period exists during which the environment has particularly strong influences on stress response system development in animals, studies that can rigorously identify such a period in humans are lacking.
We present comprehensive data on autonomic nervous system (ANS) and HPA axis reactivity from the Bucharest Early Intervention Project (BEIP), the only randomized controlled trial of foster care as an alternative to institutional rearing for abandoned children, to address each of these challenges. First, the nature of early-life adversity in the BEIP closely parallels the type of adversity studied in the animal literature: psychosocial (including maternal) deprivation. Second, the experimental design of the BEIP allows us to identify causal effects of the caregiving environment on long-term development of the stress response system. Finally, the BEIP data are unique in having detailed information on the timing of exposure to adversity, which allows us to determine whether there is a sensitive period of stress response development in humans.
Results
Baseline Physiological Characteristics.
We examined five measures of ANS function, including three global measures [heart rate (HR) and systolic and diastolic blood pressure (SBP and DBP)], one measure of parasympathetic nervous system (PNS) function [respiratory sinus arrhythmia (RSA)], and one measure of SNS function [preejection period (PEP)], as well as two markers of HPA axis function [cortisol and dehydroepiandrosterone-sulfate (DHEA-S)].
Group differences were observed in baseline sympathetic tone, F = 9.94, P < 0.001. Elevated sympathetic tone (i.e., lower resting PEP) was observed among children in the care-as-usual group (CAUG) relative to the foster care group (FCG) and never institutionalized group (NIG) (see Table S1 for all values of ANS measures and Table S2 for all HPA axis measures). No group differences were found in baseline HR, SBP, DBP, RSA, cortisol, or DHEA-S.
Experimental Paradigm.
We examined changes in ANS and HPA axis measures during three tasks: two social stressors [the Trier Social Stress Test (TSST), which includes preparation, speech, and math portions; and a peer evaluation task], and one nonsocial stressor (a frustration task). In the entire sample, significant changes in all ANS measures were observed during the social tasks: HR, SBP, and DBP increased, and RSA and PEP decreased (indicating significant PNS withdrawal and SNS activation, respectively). ANS reactivity to the frustration task was less marked, with changes only in HR, DBP, and PEP, all of which increased. Cortisol levels changed significantly across the session, but no changes in DHEA-S were observed.
Causal Effects of the Caregiving Environment.
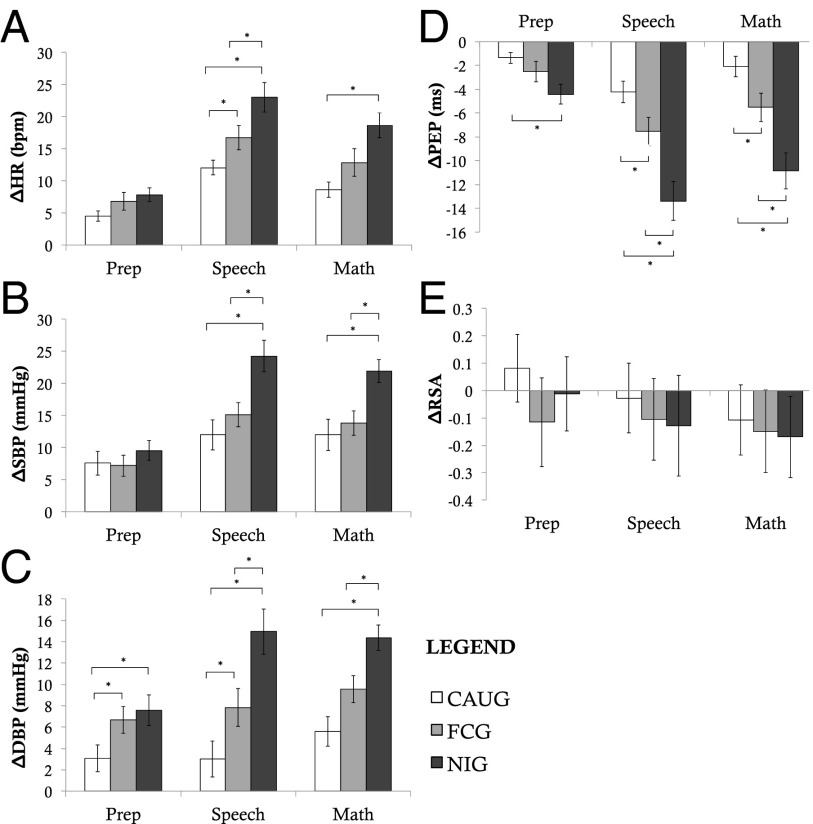
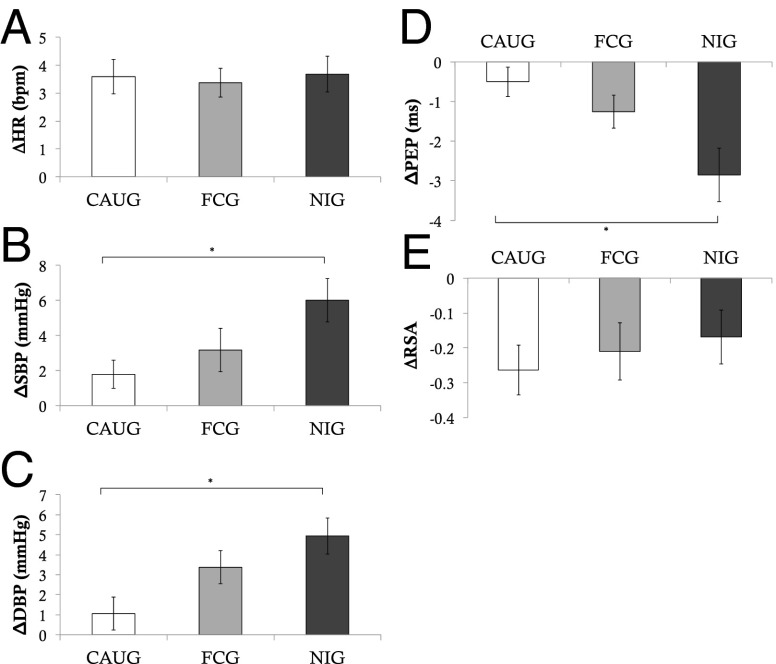
The experimental nature of the BEIP allows causal effects of the caregiving environment on physiological reactivity to be examined. We found strong evidence for causal effects of the caregiving environment on ANS reactivity to social stress. Children randomized to CAUG exhibited significantly blunted ANS responses compared with children randomized to FCG during the preparation, speech, and math portions of the TSST (Fig. 1 and Table S3). During the preparation period, these effects were present only for DBP (F = 3.95, P = 0.05), but were observed for HR, DBP, and PEP during the speech (F = 4.08–4.90, P = 0.030–0.047), and for DBP and PEP during math (F = 4.35–5.35, P = 0.040–0.023). DBP differences between the FCG and CAUG were marginal during the peer evaluation task, F = 3.83, P = 0.054 (Fig. 2). No intervention effects were observed for the frustration task.
Fig. 1.
Group differences in ANS reactivity to the TSST. Figure depicts changes in ANS measures from baseline to each portion of the TSST (speech preparation, speech, and math). A depicts changes in heart rate; B depicts changes in systolic blood pressure; C depicts changes in diastolic blood pressure; D depicts changes in pre-ejection period; and E depicts changes in respiratory sinus arrhythmia. *P < 0.05, two-sided test.
Fig. 2.
Group differences in ANS reactivity to peer evaluation. Figure depicts changes in ANS measures from baseline to the peer evaluation task. A depicts changes in heart rate; B depicts changes in systolic blood pressure; C depicts changes in diastolic blood pressure; D depicts changes in pre-ejection period; and E depicts changes in respiratory sinus arrhythmia. *P < 0.05, two-sided test.
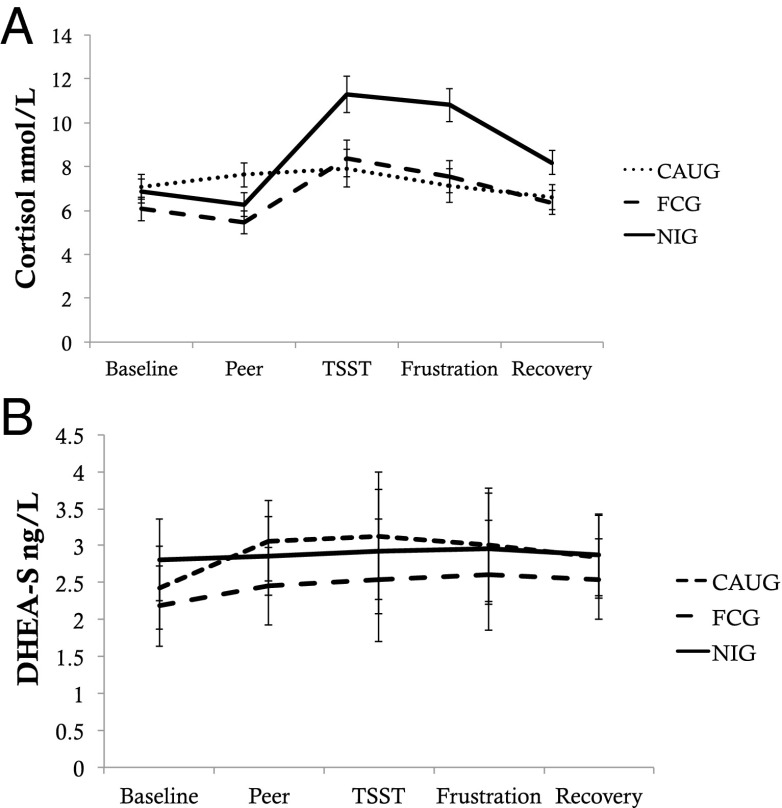
Significant intervention effects were also observed in cortisol reactivity, examined using multilevel modeling (Materials and Methods) (Fig. 3 and Table S4). The CAUG exhibited significantly blunted cortisol responses over the study session relative to the FCG, t = 2.43, P = 0.016. No intervention effects were observed for DHEA-S reactivity.
Fig. 3.
Group differences in HPA axis reactivity. Figure depicts changes in HPA axis measures across the study session. A depicts changes in cortisol; B depicts changes in DHEA-S.
Timing of Placement.
To evaluate the presence of a sensitive period, we examined whether the timing of placement influenced physiological reactivity among children in the FCG. First, we examined age of placement as a continuous predictor of reactivity. Earlier age of placement was significantly associated with cortisol reactivity, t = 2.41, P = 0.018, such that children placed earlier had an enhanced cortisol response. Earlier age of placement also predicted greater vagal engagement (i.e., RSA enhancement) during the preparation, β = −0.32, P = 0.048, speech, β = −0.38, P = 0.014, and math, β = −0.36, P = 0.020, portions of the TSST. In both cases, patterns among children placed earlier more closely resembled those in the typically developing NIG.
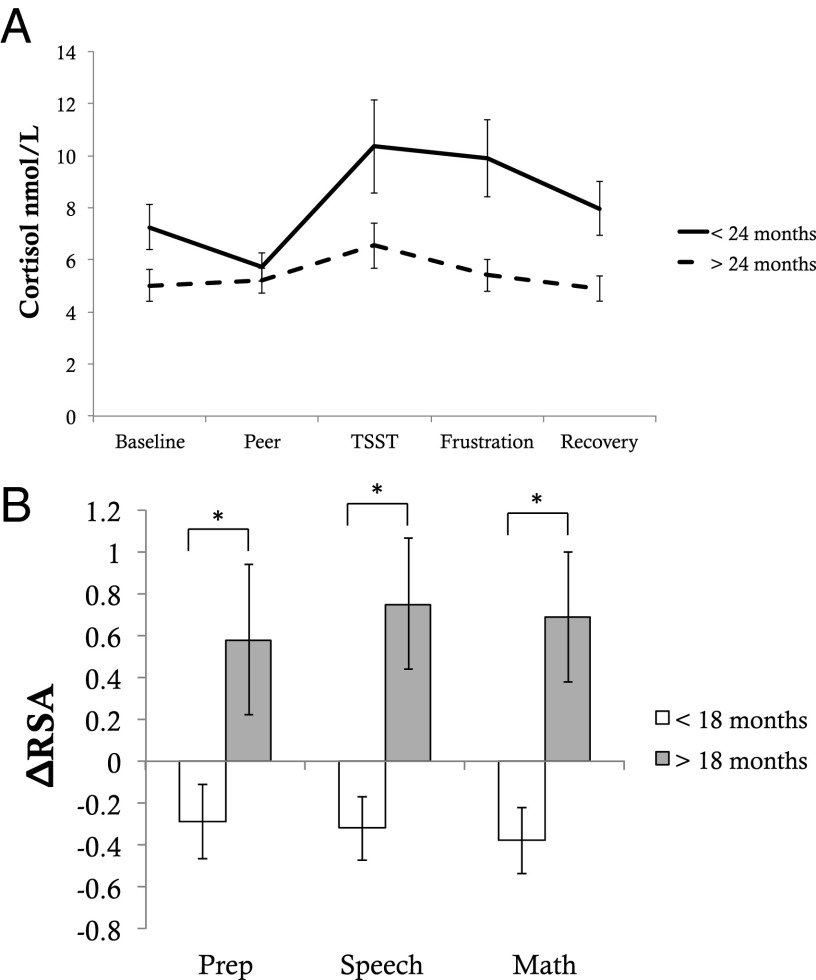
We also examined whether there was a discontinuity in the association of age of placement with cortisol and RSA reactivity. Children placed before 24 months had significantly greater cortisol response than children placed after 24 months, t = 3.22, P = 0.002 (Fig. 4), a trajectory that more closely resembled NIG. Placement before 18 months was associated with RSA enhancement during each portion of the TSST (preparation, F = 4.64, P = 0.037; speech, F = 9.38, P = 0.004; math, F = 9.15, P < 0.001).
Fig. 4.
Timing of placement and HPA axis and parasympathetic nervous system reactivity. Figure depicts changes in HPA axis and parasympathetic nervous system measures across the study session for children in the foster care group, separately for children placed into foster care before and after the age of 24 (A) or 18 months (B). A depicts changes in cortisol; B depicts changes in RSA during the TSST.
Persistent Alterations in ANS Reactivity Following Psychosocial Deprivation.
After establishing the presence of intervention and timing effects on ANS and HPA axis reactivity, we examined whether children exposed to institutional rearing differed from never institutionalized children (NIG). We were particularly interested in determining whether foster care placement resulted in reactivity patterns similar to the NIG.
Children in the CAUG exhibited a pervasive pattern of blunted SNS response to social tasks compared with the NIG, involving reduced SBP, DBP, and PEP reactivity during the TSST and peer evaluation tasks (see Figs. 1 and 2 and Table S3). The CAUG also had blunted vagal withdrawal to the frustration task compared with the NIG (Fig. S1). Blunted cortisol reactivity across the study session was also observed among the CAUG relative to the NIG (Fig. 3 and Table S4).
Although children randomized to foster care exhibited ANS and HPA axis reactivity patterns that resembled those in the NIG, some differences persisted. Children in the FCG had blunted SBP and PEP reactivity during the speech and math components of the TSST and reduced DBP reactivity during all portions of the TSST (Fig. 1 and Table S3), as well as blunted cortisol reactivity across the study session (Fig. 3 and Table S4).
Pubertal Development.
We conducted a sensitivity analysis to determine whether the observed group differences were explained by differences in pubertal development, assessed with a self-report questionnaire at the same age as physiological reactivity was assessed. Pubertal development was not associated with HR, SBP, PEP, or RSA reactivity and was associated with DBP reactivity in one of five comparisons (during the TSST speech only). Group differences remained unchanged when pubertal development was controlled in models examining reactivity during the TSST speech. Pubertal development was associated with overall cortisol level during the study session, but not cortisol reactivity. When pubertal development was controlled in our analysis of cortisol responses, we observed no change in the pattern of results regarding intervention effects or timing of placement in foster care.
Discussion
Exposure to adverse early-life environments is associated with a range of negative developmental outcomes, including poor mental and physical health and atypical social functioning. Alterations in the development of stress response systems are thought to be a central mechanism linking adverse environments to the onset of developmental problems (4, 38). Although research in rodents supports this hypothesis, human research has been hampered by inconsistency in the types of adverse early environments studied and an inability to identify causal effects. Here, we demonstrate causal effects of the caregiving environment on physiological reactivity in humans. Random assignment to high-quality family care following institutionalization mitigates otherwise persistent effects of early psychosocial deprivation on the functioning of stress response systems in children.
Normalization of ANS and HPA axis responses among children placed in foster care relative to those who remained in institutional care suggests plasticity in the ANS and HPA axis even after extreme psychosocial deprivation. Intervention effects on both the PNS and HPA axis were evident for children placed before 24 months of age, suggesting the possible presence of a sensitive period during which stress response systems are most strongly influenced by environmental inputs. Earlier age of placement into foster care was associated with normalization of cortisol reactivity and enhanced vagal engagement during social tasks, the latter of which likely reflects greater propensity for social engagement behaviors (39). Although one prior study observed an association between maternal depression during the first two years of life and child cortisol levels but no association between maternal depression in the third through seventh years of life and child cortisol (40), these findings provide the first experimental evidence in humans, to our knowledge, of a sensitive period with regard to stress response system development.
Lack of responsive, contingent, and sensitive care is one of the most prominent deviations from the expected environment for institutionalized children, and foster care placement in BEIP is associated with dramatic improvements in caregiving quality and attachment security (41, 42), both of which might contribute to the intervention effects on stress response system functioning. Maternal presence has a strong regulatory influence on the ANS and HPA axis in rodents and primates (11, 43), and insecure attachment is associated with elevated cortisol reactivity and vagal withdrawal in young children (44, 45). Moreover, placement before 24 months in BEIP is also associated with substantially greater odds of developing a secure attachment (42), indicating a similar sensitive period for attachment security as we observe for cortisol reactivity and vagal regulation. Caregiving quality and downstream effects on attachment security might therefore be mechanisms underlying these effects.
Severe early-life psychosocial deprivation is associated with a persistent and pervasive pattern of blunted ANS and HPA axis response to both social and nonsocial stressors, particularly for children in the CAUG who had more prolonged institutional care. Children exposed to institutional rearing exhibited reduced SNS activation to social stressors, blunted vagal withdrawal to a nonsocial stressor, and blunted cortisol reactivity, indicating a consistent pattern of reduced engagement of stress response systems to environmental challenges following early psychosocial deprivation. This pervasive pattern of stress response system hyporesponsivity is inconsistent with patterns observed in the rodent literature following maternal deprivation (1–3, 46) and challenges some prevailing conceptual models of early-life adversity and stress response system development, which argue that adverse environments should lead to elevated physiological reactivity (47, 48). The observed pattern of cortisol hyporesponsivity is consistent with several prior studies in humans documenting an association between early-life deprivation and low levels of morning cortisol and blunted cortisol responses to social challenge (20, 28, 32), although other work has found elevated basal cortisol and heightened cortisol reactivity in children who have experienced social deprivation (17, 27). The experimental design of the current study clarifies these inconsistencies in prior observational studies. The complex nature of human attachment and social interaction with caregivers might be one domain in which direct parallels with the animal literature are limited, potentially related to the fact that the attachment relationship between children and caregivers is a necessary scaffold for development of numerous uniquely human capacities, including emotion regulation and language (49, 50). Methodological differences that are difficult to reconcile could also contribute, including different methods used to elicit physiological responses in animals (e.g., restraint or shock) and humans (e.g., social or cognitive challenges).
Maternal presence reduces activation of the HPA axis and ANS in young rodents exposed to a stressor (51), and in humans, the presence of a supportive caregiver is associated with reduced HPA axis reactivity in young children (52). Together, these findings suggest that the absence of a caregiver might lead to chronic elevations in activity of the HPA axis and ANS in early childhood, which may ultimately lead to reduced responsiveness of these systems to the environment later in life. Indeed, studies of nonhuman primates have observed that maternal deprivation is associated with a pattern of heightened cortisol production in the first year of life but low morning levels and a flattened diurnal rhythm later in childhood (53, 54), with childhood hypocortisolism most extreme among infants with the highest cortisol levels in infancy (53). One mechanism that could explain this pattern is down-regulation of corticotropin-releasing hormone (CRH) receptors in the pituitary due to chronic CRH hypersecretion from the hypothalamus (55); chronic CRH hypersecretion would be associated with elevated cortisol levels initially and reduced levels later in development once receptors have been down-regulated. Indeed, reductions in CRH receptors in the pituitary have been observed in adult rodents exposed to maternal deprivation (3). An alternative mechanism involves heightened negative feedback sensitivity to glucocorticoids in the hippocampus following exposure to elevated cortisol levels early in development, which would inhibit CRH production in the hypothalamus and, ultimately, lead to lower cortisol levels over time (56).
Regardless of the mechanism, the pattern of hyporesponsivity of the ANS and HPA axis observed among previously institutionalized children is likely to have downstream consequences for their physical and mental health. Cortisol has regulatory influences on inflammatory responses and other immune processes, glucose metabolism, and numerous aspects of central nervous system functioning (56, 57). Patterns of hypocortisolism similar to those observed among CAUG children are thought to contribute to heightened risk for health problems including chronic fatigue, pain syndromes, and autoimmune conditions (58). Moreover, the pattern of blunted ANS reactivity among the CAUG children is similar to patterns identified among children with disruptive behavior disorders, including oppositional defiant disorder and conduct disorder (59, 60). Together, the patterns of blunted stress reactivity among children who remained in institutional care might lead to heightened risk for multiple physical and mental health problems.
Future research is needed to determine the specific aspects of improved caregiving quality that remediate the effects of adverse early environments on stress response system development. These advances will inform caregiving practices for the millions of abandoned and neglected children worldwide and inform the nature and timing of interventions for children exposed to a range of adverse early environments.
Materials and Methods
Sample.
The BEIP is a longitudinal study of a sample of children raised from early infancy in institutions in Bucharest, Romania, and the only randomized controlled trial of foster care as an alternative to institutional rearing for abandoned children (61). A sample of 136 children (aged 6–30 months) was recruited from each of the six institutions for young children in Bucharest. An age-matched sample of 72 community-reared children was recruited from pediatric clinics in Bucharest and comprised the NIG. Half of children in the institutionalized group were randomized to a foster care intervention, resulting in two groups: the foster care group (FCG) and the group who received care as usual [prolonged institutional care (CAUG)]. The study design and methods have been described in detail (61). Physiological reactivity was first assessed at age 12 y, 8 y after the formal randomized controlled trial was completed and the local authorities began to manage foster care.
No differences were found between the CAUG and FCG in sex distribution, age, birth weight, or percentage of life spent in the institution. The mean age at foster care placement was 22.97 months. A total of 138 children participated in the 12-y physiological reactivity assessment (CAUG, n = 43; FCG, n = 48; NIG, n = 47; see SI Materials and Methods for CONSORT diagram).
Ethical Issues.
The BEIP was initiated in collaboration with the Romanian government. Study procedures were approved by local commissions on child protection in Bucharest, the Romanian ministry of health, and later an ethics committee including appointees from government and Bucharest University academic departments. In addition, the institutional review boards of the institutions of the three principal investigators reviewed and approved the study. A detailed accounting of efforts to ensure ethical integrity has been published (62–64).
Procedures.
Approximately 30 min after arriving for the laboratory session, participants provided a baseline saliva sample and were hooked up to the physiological monitoring equipment, described in greater detail below. Next, they completed a 5-min baseline resting period where they were asked to sit quietly without moving. Participants then completed three laboratory-based procedures designed to elicit a physiological response: (i) a peer-evaluation task (65) that was passive in nature (i.e., did not require active responses by the participant); (ii) an evaluated social performance task requiring instrumental cognitive responses—the TSST (66), a widely used stress induction procedure that has been used with children and adolescents (67, 68); and (iii) a nonsocial task designed to elicit frustration that required active responses. Each task was followed by a 5-min recovery period during which children were asked to sit quietly, to eliminate carryover effects from one task to another. See SI Materials and Methods for greater details about each of these tasks.
Physiological Measures.
Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier by using a modified Lead II configuration (right clavicle, left lower torso, and right leg ground). Cardiac impedance recordings were obtained with a Bio-Impedance Technology model HIC-2500 impedance cardiograph. One pair of mylar tapes encircled the neck and another pair encircled the torso. A continuous 500-µA AC 95 kHz current was passed through the two outer electrodes, and basal thoracic impedance (z0) and the first derivative of basal impedance (dz/dt) was measured from the inner electrodes. A Biopac MP150 integrated the ECG and impedance cardiography (ICG) signals, sampled at 1.0 kHz, using Acqknowledge software. A Colin Prodigy II oscillometric blood pressure machine (Colin Medical Instruments) was used to obtain blood pressure recordings at predetermined times during the study (after the first minute of the two negative feedback portions of the peer evaluation task and after the first and fourth minute of each component of the TSST and the frustration task). See SI Materials and Methods for information on ECG and ICG scoring.
We examined two neuroendocrine markers that reflect HPA axis functioning: cortisol and DHEA-S. Cortisol is the most widely used marker of HPA axis activity in human studies, and DHEA-S appears to have protective effects against some of the negative downstream effects of glucocorticoids, including in the hippocampus (69). Saliva samples were obtained with cryovial tubes [Immuno-Biological Laboratories (IBL)] by using the drool method. Participants expectorated ∼1.5 mL of saliva into a cryovial with a plastic straw. Saliva samples were stored immediately at −20 °C until they were shipped on dry ice to a laboratory in Boston. Samples were assayed for cortisol and DHEA-S by using commercially available luminescence immunoassay kits (CLIA; IBL). Intraassay and interassay coefficients of variance were acceptable (cortisol: 5.11% and 5.37%; DHEA-S: 6.50% and 5.79%, respectively). See SI Materials and Methods for information on sensitivity of immunoassays and range of values for our sample.
Analysis Methods.
We examined group differences in ANS reactivity by using univariate ANCOVAs with group (CAUG, FCG, NIG) as a between-subject factor, controlling for sex. Group differences were followed up with a series of contrasts to first assess intervention effects (CAUG vs. FCG) and then to examine persistent effects of maternal deprivation (FCG vs. NIG; and CAUG vs. NIG). Age was not included as a covariate because no group differences were observed in age at testing, F(1,135) = 0.27, P = 0.76, and inclusion of age worsened model fit in HPA axis models (described below). Reactivity scores were created by subtracting the baseline value of each physiological parameter from the value during task administration. For the peer evaluation task, we subtracted the mean baseline value of each physiological parameter from the mean value across the two evaluation periods of the task. For the TSST, mean baseline values were subtracted from the first minute of each portion of the task (preparation, speech, math), which is standard practice given rapid habituation during the task. For the frustration task, we subtracted mean values in the training phase from the test phase.
Group differences in cortisol and DHEA-S were examined by using multilevel modeling. Both cortisol and DHEA-S were skewed and were log-transformed before analysis. A series of two-level models (observations over time nested within persons) were estimated. This approach allowed us to simultaneously estimate the variance in cortisol and DHEA-S both within and between children over time. All variables were centered before analysis. We first estimated an unconditional growth model that predicted each neuroendocrine marker by Time, with baseline coded as zero. We added quadratic and cubic terms for Time to the model to determine the functional form of the growth trajectory and tested the difference in model fit between the linear, quadratic, and cubic models. We next examined group differences in intercepts (value at baseline) and slopes (linear change over time) of each marker by examining the interaction of time variables (i.e., linear, quadratic, cubic) with group status, controlling for sex. The best-fitting model for cortisol reactivity included linear, quadratic, and cubic terms for Time, and modeled Time as a random effect (i.e., allowed it to vary across children). The best-fitting model for DHEA-S included linear and quadratic terms for Time, and modeled Time as a random effect. Group differences were examined with the same contrasts described above for ANS markers.
Supplementary Material
Acknowledgments
We thank the caregivers and children who participated in this project and the Bucharest Early Intervention Project staff for their tireless work on our behalf. This work was supported by the John D. and Catherine T. MacArthur Foundation, the Binder Family Foundation, the Help the Children of Romania, Inc. Foundation, and National Institute of Mental Health Grants MH091363 (to C.A.N.), MH092526 (to K.A.M.), and MH092555 (to M.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423363112/-/DCSupplemental.
References
- 1.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 2.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 3.Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137(4):1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 5.Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 6.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196(1):199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Levine S. The influence of social factors on the response to stress. Psychother Psychosom. 1993;60(1):33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- 8.Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. Hormonal effects of early rearing conditions in the infant rhesus monkey. Am J Primatol. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- 9.Sackett GP, Bowman RE, Meyer JS, Tripp RL, Grady SS. Adrenocortical and behavioral reactions by differentially raised rhesus monkeys. Physiol Psychol. 1973;1:209–212. [Google Scholar]
- 10.Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. J Neuroendocrinol. 2000;12(8):723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- 11.Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta) Behav Neurosci. 1990;104(1):98–107. [PubMed] [Google Scholar]
- 12.Fahlke C, et al. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24(5):644–650. [PubMed] [Google Scholar]
- 13.Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JS, Novak MA, Bowman RE, Harlow HF. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev Psychobiol. 1975;8(5):425–435. doi: 10.1002/dev.420080507. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman J, et al. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42(8):669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- 17.Fries ABW, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Dev Psychobiol. 2008;50(6):588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bellis MD, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78(2):249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 19.Oosterman M, De Schipper JC, Fisher P, Dozier M, Schuengel C. Autonomic reactivity in relation to attachment and early adversity among foster children. Dev Psychopathol. 2010;22(1):109–118. doi: 10.1017/S0954579409990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMillan HL, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biol Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36(2):173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Lupie SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 25.Evans GW, Exner-Cortens D, Kim P, Bartholomew D. Childhood poverty and blood pressure reactivity to and recovery from an acute stressor in late adolescence: The mediating role of family conflict. Psychosom Med. 2013;75(7):691–700. doi: 10.1097/PSY.0b013e31829f9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 27.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 28.van der Vegt EJM, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults A study of international adoptees. Psychoneuroendocrinology. 2009;34(5):660–669. doi: 10.1016/j.psyneuen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 30.Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- 31.Elzinga BM, et al. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22(18):7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: A systematic review. Pediatrics. 2014;133(2):312–326. doi: 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brotman LM, et al. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64(10):1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- 36.Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36(4):531–539. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard K, Dozier M, Bick J, Gordon MK. Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Dev Psychopathol. 2014:1–13. doi: 10.1017/S095457941400073X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 39.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14(2):333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- 41.Smyke AT, Zeanah CH, Jr, Fox NA, Nelson CA., 3rd A new model of foster care for young children: The Bucharest early intervention project. Child Adolesc Psychiatr Clin N Am. 2009;18(3):721–734. doi: 10.1016/j.chc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Smyke AT, Zeanah CH, Fox NA, Nelson CA, Guthrie D. Placement in foster care enhances quality of attachment among young institutionalized children. Child Dev. 2010;81(1):212–223. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldji C, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Dev Psychobiol. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Hill-Soderlund AL, et al. Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Dev Psychobiol. 2008;50(4):361–376. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- 46.Lyons DM, et al. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinology. 1999;24(2):131–142. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- 47.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 48.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Ijzendoorn MH, Dijkstra J, Bus AG. Attachment, intelligence, and language: A metaanalysis. Soc Dev. 1995;4:115–128. [Google Scholar]
- 50.Cassidy J. Emotion regulation: Influences of attachment relationships. Monogr Soc Res Child Dev. 1994;59(2-3):228–249. [PubMed] [Google Scholar]
- 51.Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biol Psychiatry. 2002;52(11):1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- 55.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 56.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 57.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 59.Crowell SE, et al. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. J Abnorm Psychol. 2006;115(1):174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- 60.Boyce WT, et al. MacArthur Assessment Battery Working Group of the MacArthur Foundation Research Netwrok on Psychopathology and Development Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- 61.Zeanah CH, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopathol. 2003;15(4):885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 62.Miller FG. The randomized controlled trial as a demonstration project: An ethical perspective. Am J Psychiatry. 2009;166(7):743–745. doi: 10.1176/appi.ajp.2009.09040538. [DOI] [PubMed] [Google Scholar]
- 63.Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science. 2007;318(5858):1874–1875. doi: 10.1126/science.1153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeanah CH, et al. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Infant Ment Health J. 2006;27:559–576. doi: 10.1002/imhj.20107. [DOI] [PubMed] [Google Scholar]
- 65.Howarth GZ, Guyer AE, Pérez-Edgar K. Young children's affective responses to acceptance and rejection by peers: A computer-based task sensitive to variation in temperamental shyness and gender. Soc Dev. 2013;22(1):146–162. doi: 10.1111/sode.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 67.Buske-Kirschbaum A, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Stroud LR, et al. Stress response and the adolescent transition: Performance versus peer rejection stressors. Dev Psychopathol. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaminska M, Harris J, Gijsbers K, Dubrovsky B. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP. Implications for depression. Brain Res Bull. 2000;52(3):229–234. doi: 10.1016/s0361-9230(00)00251-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.