Significance
About one third of patients with type 1 diabetes mellitus develop nephropathy, which often progresses to end-stage renal diseases. The present study demonstrates that below normal transforming growth factor (TGF) β1 expression ameliorates the nephropathy and decreased glomerular filtration rate resulting from long-standing type 1 diabetes, while above normal TGFβ1 expression makes both worse. Reducing TGFβ1 expression in the glomerulus is more important in avoiding the decrease in glomerular filtration rate than altering expression in the tubule, while expression in the tubule is more important in controlling interstitial fibrosis and albuminuria. Suppressing TGFβ1 action in the kidney as a whole, or specifically in podocytes, could be a promising option for treating/preventing the progressive deterioration of renal function in diabetes.
Keywords: aldosterone, glomerular filtration rate, glomerulosclerosis, megalin, nephrin
Abstract
Nephropathy develops in many but not all patients with long-standing type 1 diabetes. Substantial efforts to identify genotypic differences explaining this differential susceptibility have been made, with limited success. Here, we show that the expression of the transforming growth factor β1 gene (Tgfb1) affects the development of diabetic nephropathy in mice. To do this we genetically varied Tgfb1 expression in five steps, 10%, 60%, 100%, 150%, and 300% of normal, in mice with type 1 diabetes caused by the Akita mutation in the insulin gene (Ins2Akita). Although plasma glucose levels were not affected by Tgfb1 genotype, many features of diabetic nephropathy (mesangial expansion, elevated plasma creatinine and urea, decreased creatinine clearance and albuminuria) were progressively ameliorated as Tgfb1 expression decreased and were progressively exacerbated when expression was increased. The diabetic 10% hypomorphs had comparable creatinine clearance and albumin excretion to wild-type mice and no harmful changes in renal morphology. The diabetic 300% hypermorphs had ∼1/3 the creatinine clearance of wild-type mice, >20× their albumin excretion, ∼3× thicker glomerular basement membranes and severe podocyte effacement, matching human diabetic nephropathy. Switching Tgfb1 expression from low to high in the tubules of the hypomorphs increased their albumin excretion more than 10-fold but creatinine clearance remained high. Switching Tgfb1 expression from low to high in the podocytes markedly decreased creatinine clearance, but minimally increased albumin excretion. Decreasing expression of Tgfb1 could be a promising option for preventing loss of renal function in diabetes.
Diabetes is the number one cause of end-stage renal disease in the United States and many other developed countries. However, despite having similar levels of blood glucose only 20–40% of all diabetic patients develop diabetic nephropathy. In diabetic nephropathy, increased expression of transforming growth factor β1 (TGFβ1) has been demonstrated to promote accumulation of extracellular matrix components (1), apoptosis (2), dedifferentiation of podocytes (3), and epithelial–mesenchymal transition of proximal tubules (4), all of which are thought to facilitate a decline in nephron number and renal function.
Tgfb1-null mice on a mixed genetic background show severe multiorgan inflammation with massive infiltration of lymphocytes and macrophages that culminates in death by 3–4 wk of age (5, 6). Their death effectively prevents determining whether absence of TGFβ1 influences the development of nephropathy. To overcome this problem and also to allow the study of the effects of above-normal TGFβ1, we have generated mice with five genetically graded levels of TGFβ1, and have made them diabetic with the Ins2Akita mutation, which causes pancreatic beta-cell dysfunction and type 1 diabetes.
Here we show that the features characteristic of diabetic nephropathy are progressively minimized as Tgfb1 expression is decreased below normal and are progressively exacerbated when expression is increased above normal.
Generation of Akita Diabetic Mice Having Five Genetically Different Levels of Tgfb1 Expression
We recently described the generation of C57BL/6 mice having a low-expressing Tgfb1 allele (Tgfb1L), which can be switched to high expressing form (Tgfb1H) by exposure to Cre recombinase, and the combination of these low and high expressing alleles with the wild-type allele (Tgfb1+) to produce mice having Tgfb1 mRNA expression graded in five steps from 10% to 300% normal (7). We have now crossbred these mice with mice having the Akita mutation in the Ins2 gene (8) to generate type 1 diabetic mice with different TGFβ1 levels. Male C57BL/6 Akita diabetic mice with the following five genotypes were studied: Tgfb1L/L: Ins2Akita/+ (hereafter called L/L:A/+), Tgfb1L/+:Ins2Akita/+ (L/+:A/+), Tgfb1+/+:Ins2Akita/+ (WT:A/+), Tgfb1H/+:Ins2Akita/+ (H/+:A/+), and Tgfb1H/H:Ins2Akita/+ (H/H:A/+).
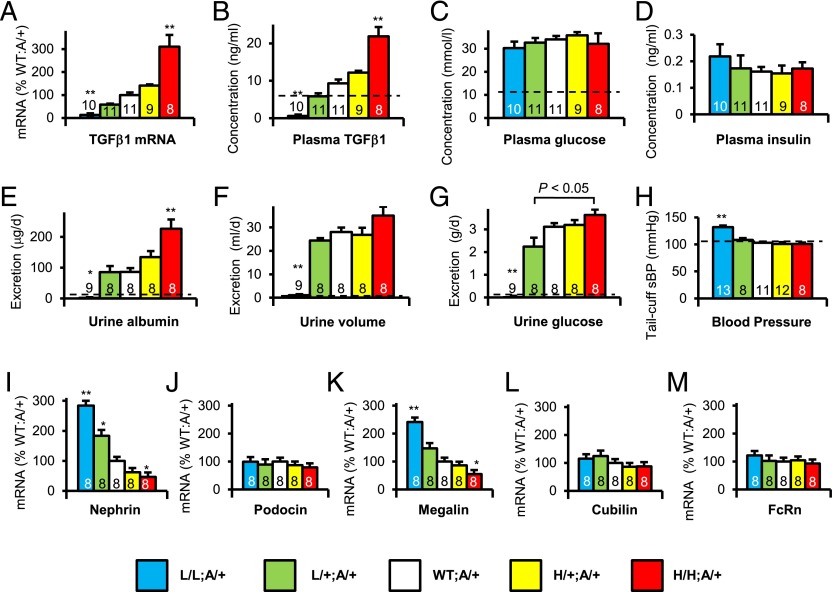
Fig. 1A shows that Akita diabetic mice with the five Tgfb1 genotypes have a graded expression of Tgfb1 mRNA in their kidneys, and that their plasma TGFβ1 levels have a similar gradation (Fig. 1B). They all have about three times the plasma concentration of glucose and about one third the plasma insulin concentration of wild-type nondiabetic C57BL/6 mice, indicative of type 1 diabetes. These plasma glucose and plasma insulin concentrations were not significantly affected by the Tgfb1 genotype (Fig. 1 C and D and SI Appendix, Fig. S8).
Fig. 1.
Characterization at age 40 wk of Akita diabetic mice having five genetically determined levels of Tgfb1 expression. (A) Tgfb1 mRNA in the kidney. (B) Plasma concentration of TGFβ1. (C) Plasma glucose concentration. (D) Plasma insulin concentration. (E) Urinary albumin excretion. (F) Urine Volume. (G) Urine glucose excretion. (H) Systolic blood pressure. (I) Renal mRNA expression of Nephrin. (J) Podocin. (K) Megalin. (L) Cubilin. (M) Neonatal Fc receptor (FcRn). All of the mice were Akita diabetic. Bars are color coded to indicate Tgfb1 and Ins2 genotypes: blue (L/L:A/+), green (L/+:A/+), white (WT:A/+), yellow (H/+:A/+), red (H/H:A/+). *P < 0.05, **P < 0.01 vs. WT:A/+.
General Characteristics of Akita Diabetic Mice with Five Graded Expressions of Tgfb1
The body weights of the L/L:A/+ Akita diabetic mice were about 15% less than those of the Akita mice with the other Tgfb1 genotypes (SI Appendix, Table S1), a result similar to our previous finding with nondiabetic L/L mice (7). The heart weights of the L/L:A/+ and H/H:A/+ mice were, respectively, ∼10% and 20% lower than that of the mice with the other Tgfb1 genotypes, but heart weight/body weight ratios did not differ significantly among all five genotypes (SI Appendix, Table S1). Heart rates were not significantly different (SI Appendix, Fig. S1). The kidney weight/body weight ratio was ∼15% lower in the L/L:A/+ mice and ∼30% higher in the H/H:A/+ mice in comparison with Akita diabetic mice with wild-type Tgfb1 expression (SI Appendix, Table S1). Plasma cholesterol and plasma triglyceride concentrations were not affected by the Tgfb1 genotypes.
Effects of Tgfb1 on Urinary Excretion of Albumin, Water, and Glucose
Because nephropathy/renal failure in human patients is associated with long-term diabetes, the effects of graded expression of Tgfb1 were studied in mature adult 40-wk-old C57BL/6 Akita diabetic mice. Using metabolic cages, we found that the L/L:A/+ diabetic mice, like the nondiabetic L/L mice, excreted very little amount of albumin (Fig. 1E and SI Appendix, Fig. S2). However, higher levels of TGFβ1 led to progressive increases in urinary albumin excretion (Fig. 1E), ranging from microalbuminuria in the L/+:A/+ diabetic mice (∼80 µg/day) to macroalbuminuria in the H/H:A/+ diabetic mice (∼200 µg/day). The urine volumes of the L/L:A/+ Akita diabetic mice (Fig. 1F) were much reduced compared with the polyuric urine volumes of the other Akita diabetic mice (∼1 mL/day versus ∼30 mL/day). In addition to not having polyuria, the L/L Akita mice did not have glucosuria (Fig. 1G), even though they had about three times normal plasma glucose concentration and about one third normal plasma insulin concentration (Fig. 1 C and D). Nondiabetic L/L mice also excreted very little amount of glucose (SI Appendix, Fig. S11). The L/L:A/+ mice had systolic blood pressures ∼20 mmHg above normal (Fig. 1H). These unusual features are seen in nondiabetic L/L mice, caused by their having ∼2× normal plasma aldosterone concentrations (7). Our L/L:A/+ diabetic hypomorphs also have plasma aldosterone concentrations about twice that of diabetic mice that are wild type at the Tgfb1 locus (SI Appendix, Fig. S6). Thus, although the 10% hypomorphs developed additional features associated with their hyperaldosteronism, we conclude that higher-than-normal expression of Tgfb1 in the Akita diabetic mice caused increased albumin excretion, whereas lower than normal expression decreased the albuminuria.
Effects of Tgfb1 on Expression of Genes Affecting Renal Function
To uncover factors affecting the nephropathy in our diabetic mice with graded expression of Tgfb1, we determined the expression in the kidney of mRNAs coding for proteins involved in renal function and albumin excretion (Fig. 1 I–M). Nephrin (mouse gene: Nphs1) and podocin (Nphs2) were chosen because they are expressed in renal podocytes and have mutations that cause congenital nephrotic syndromes in humans (9–12). Megalin (Lrp2) and cubilin (Cubn), both expressed in the brush border of renal proximal tubules, were chosen because of their known contribution to the endocytosis of low molecular weight proteins and albumin (13–17). Neonatal Fc receptor (Fcgrt) was included because it is also expressed in the brush border but its primary effects are on the urinary excretion of immunoglobulins rather than of albumin (18, 19). The results show that genetic increases in the expression of Tgfb1 in the Akita diabetic mice caused progressive decreases in the renal expression of nephrin and of megalin, ranging from ∼250% normal in the L/L:A/+ mice to ∼50% normal in the H/H:A/+ mice. Expression of cubilin and of the neonatal Fc receptor was unaffected. We conclude that progressive increases in Tgfb1 expression from 10% to 300% normal are accompanied by progressive decreases in nephrin and megalin expression from ∼250% to ∼50% normal but without changes in the expression of podocin, cubulin, and the neonatal Fc receptor.
Excretory Function of Nondiabetic and Akita Diabetic Mice with Graded Tgfb1 Expression
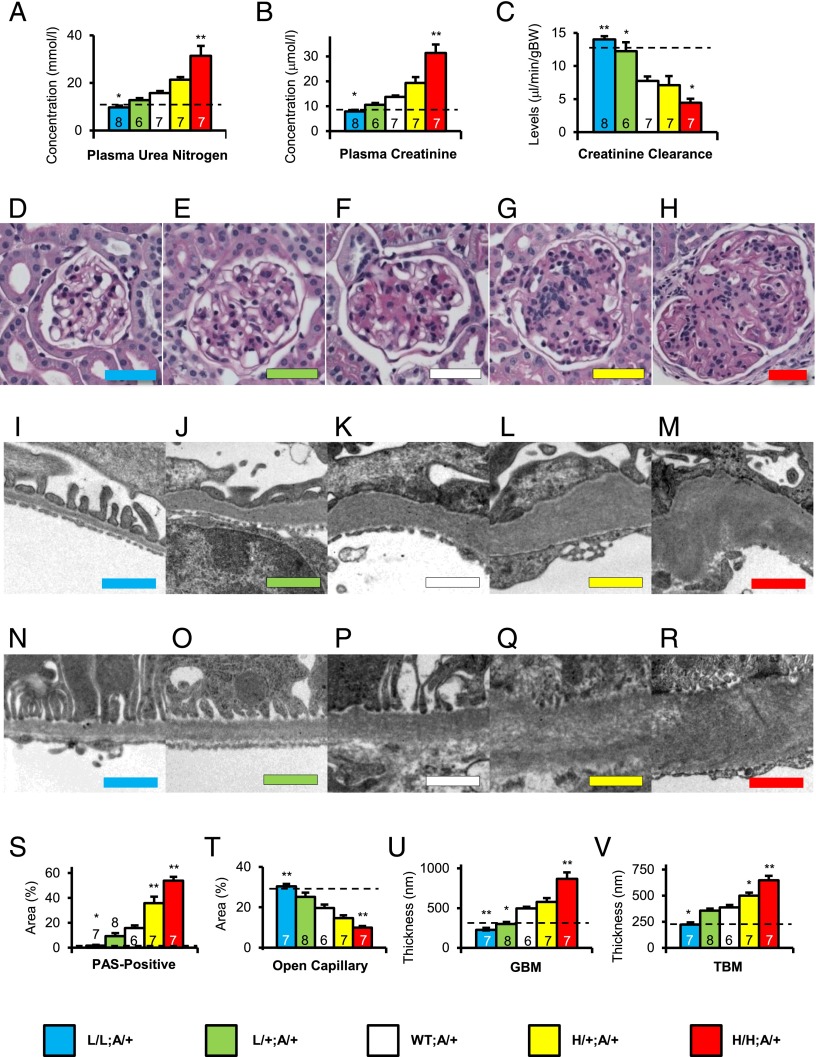
The effects of graded expression of Tgfb1 on renal function were studied in mature adult 40-wk-old Akita diabetic mice and their nondiabetic counterparts. The results show that in the nondiabetic mice changes in Tgfb1 expression had no significant effects on glomerular filtration rate (GFR) as judged by plasma levels of urea nitrogen and creatinine, and creatinine clearance (SI Appendix, Figs. S3–S5). However, in the Akita diabetic mice we found a highly significant inverse gradation in GFR as Tgfb1 expression varied (Fig. 2 A–C). Thus, GFR was greater than in the 10% hypomorphs (L/L:A/+) than in diabetic mice wild type at the Tgfb1 locus (WT:A/+), but was less in the hypermorphs (H/+:A/+ and H/H:A/+) than in the WT:A/+ mice. We conclude that GFR decreases in Akita diabetic mice as the expression of Tgfb1 increases. In statistical confirmation of this inverse relationship, nonparametric regression analyses showed that Tgfb1 mRNA expression, plasma urea nitrogen, plasma creatinine, and creatinine clearance (Figs. 1A and 2 A–C) were all strongly related to the Tgfb1 genotypes (R2 ≥ 0.57 for all four variables). Two-way ANOVA showed that the interaction of Tgfb1 and Akita genotype on renal function was highly significant (P < 0.0001). We found one factor that probably contributes to this interaction; namely, the plasma level of TGFβ1 in the five genotypes (Fig. 1B) proved to be generally about 50% higher in the 40-wk-old diabetic mice than in their nondiabetic counterparts at 12 wk age (7), as it is in human patients (1). We conclude that the GFR of the diabetic mice is highly dependent on the level of expression of Tgfb1, ranging from approximately twice normal in the L/L:A/+ mice to approximately half normal in the H/H:A/+ mice.
Fig. 2.
Renal excretory function, glomerular histology and ultrastructure at 40 wk of age in Akita mice with five levels of Tgfb1 expression. (A) Plasma urea nitrogen concentration. (B) Plasma creatinine concentration. (C) Creatinine clearance. (D–H) Glomerular histology; periodic acid-Schiff (PAS) staining with hematoxylin. (Color-coded scale bar: 50 μm) (D) L/L:A/+. (E) L/+:A/+. (F) WT:A/+. (G) H/+:A/+. (H) H/H:A/+. (I–M) Glomerular basement membrane ultra-structure; color-coded scale bar = 1 μm. (I) L/L:A/+. (J) L/+:A/+. (K) WT:A/+. (L) H/+:A/+. (M) H/H:A/+. (N–R) Peri-tubular basement membrane ultra-structure; color-coded scale bar = 1 μm. (N) L/L:A/+. (O) L/+:A/+. (P) WT:A/+. (Q) H/+:A/+. (R) H/H:A/+. (S–V) Renal phenotypes in nondiabetic and diabetic mice of the five Tgfb1 genotypes. (S) Fraction of PAS-positive mesangial material per total glomerular tuft cross-sectional area. (T) Fraction of open capillary area per total glomerular tuft cross-sectional area. (U) Thickness of glomerular basement membrane (GBM). (V) Thickness of tubular basement membrane (TBM) in the proximal tubule. Bars and images are color coded as indicated. Dotted lines indicate nondiabetic WT levels. *P < 0.05, **P < 0.01 vs. WT:A/+.
Renal Morphology in Nondiabetic and Akita Diabetic Mice with Graded Tgfb1 Expression
To uncover the causes of the progressive changes in GFR in our diabetic mice, we evaluated the microscopic and ultramicroscopic status of their glomeruli (Fig. 2 D–R). The microscopic studies showed that the Akita mice with wild-type Tgfb1 alleles (WT:A/+) at age 40 wk had pathological changes in their glomeruli typical of diabetic nephropathy, including mesangial cell expansion, less open capillaries and accumulation of periodic acid-Schiff (PAS)-positive materials (Fig. 2F). These pathological changes were completely absent in the Akita mice with the lowest expression of Tgfb1 (L/L:A/+; Fig. 2D), were still reduced in the L/+:A/+ mice (Fig. 2E) relative to those in the diabetic mice with wild-type Tgfb1 (Fig. 2F), but were progressively exacerbated as expression of Tgfb1 increased above normal in the H/+:A/+ and H/H:A/+ Akita diabetic mice (Fig. 2 G and H). The glomeruli of the H/H:A/+ diabetic mice showed essentially all of the pathological features that are observed in humans with advanced diabetic nephropathy, including glomerulosclerosis as indicated by glomerular morphology, accumulation of extracellular matrix, mesangial expansion, and nodular lesions (SI Appendix, Fig. S7P).
The pathology revealed by light microscopy was further evaluated by electron microscopy (Figs. 2 I–M and SI Appendix, Fig. S7 A–J). The results confirmed that the Akita mice with wild-type Tgfb1 alleles (WT:A/+) at age 40 wk had ultrastructural changes typical of advanced diabetic nephropathy, including a several-fold increase in the thickness of the glomerular basement membrane (GBM) together with marked podocyte effacement (Fig. 2K). Both were progressively exacerbated in the H/+:A/+ and H/H:A/+ mice with above normal expression of Tgfb1 (Fig. 2 L, M, and U and SI Appendix, Fig. S7O). The glomerular ultrastructural pathology was substantially corrected when Tgfb1 expression was about half normal in the L/+:A/+ mice (Fig. 2 I and U and SI Appendix, Fig. S7O), and the ultrastructure of the glomeruli in the L/L:A/+ mice was indistinguishable from that in wild-type nondiabetic C57BL/6 mouse except that the thickness of the GBM was about half normal (Fig. 2 I and U and SI Appendix, Fig. S7O). Podocyte effacement was present in the glomeruli of all of the diabetic mice except the L/L:A/+ mice with one tenth normal Tgfb1 expression. We did not observe any obvious lesions in the renal vasculature other than in the glomeruli of the Akita mice. We conclude that the nephropathy observed in the mature Akita mice with type 1 diabetes is strongly affected by the expression of Tgfb1, ranging from almost the same as in nondiabetic Tgfb1 wild-type mice (in the 10% hypomorphs) to severe diabetic nephropathy (in the 300% hypermorphs).
The thickness of the tubular basement membrane (TBM) changed with Tgfb1 expression in the same manner as the thickness of the GBM, although somewhat less dramatically (Fig. 2 N–R).
We quantitated the renal pathology in the five Tgfb1 genotypes by measuring the fraction of PAS-positive area per glomerular tuft area, an indicator of mesangial expansion, and found that PAS-positive material increased more than 10-fold in the Akita diabetic animals as Tgfb1 expression increased (Fig. 2S), but increased only slightly in the nondiabetic mice (SI Appendix, Fig. S7K). The open capillary area in the L/L:A/+ hypomorphs decreased from almost twice that in the WT:A/+ diabetic mice to less than one third in the H/H:A/+ diabetic mice (Fig. 2T), but was not significantly affected by Tgfb1 expression in the nondiabetic mice (SI Appendix, Fig. S7L). The thicknesses of the GBM and TBM in the diabetic mice were affected in remarkably similar ways by the changes in Tgfb1 expression (Fig. 2 U and V), but were not significantly affected in the nondiabetic mice (SI Appendix, Fig. S7 M and N). Thus, the quantitative evaluation of the light and electron microscope images of the glomeruli confirm the conclusions drawn from their visual inspection, namely that below normal Tgfb1 expression ameliorates the nephropathy caused by diabetes, whereas above-normal expression exacerbates it.
Effects of Podocyte-Specific or Proximal Tubule-Specific Switching of Tgfb1 from Low to High in the L/L:A/+ Diabetic Mice
To determine whether the increased urinary albumin and decreased renal function occurring in the L/L Akita mice is due to changes in the podocytes, we generated L/L:A/+ P mice having a Cre transgene driven by the promoter of the podocin gene (Nphs2), which is active only in podocytes. Likewise, we generated L/L:A/+ T mice having a Cre transgene driven by the promoter of the gamma-glutamyl transferase 1 gene (Ggt1), which is active only in the brush border of Tubule cells. The podocin-Cre switches Tgfb1 gene expression from low to high in podocytes during development and life-long thereafter. The tubule-specific Ggt1-Cre is inducible by tamoxifen and switches Tgfb1 expression from low to high in proximal tubule cells after mice are treated with tamoxifen.
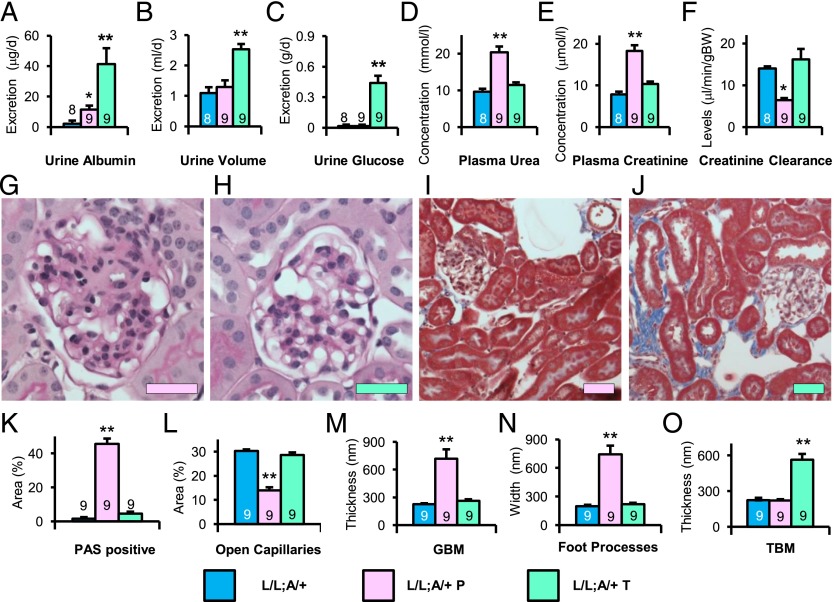
Switching Tgfb1 expression from low to high in either the tubules or the glomeruli of the L/L:A/+ mice had no effect on the hyperglycemia caused by the Akita mutation (SI Appendix, Fig. S8). However, the blood pressure of the L/L:A/+ T diabetic mice in which Cre recombination had been induced in the tubules was decreased by ∼10 mmHg (from ∼130 mmHg to ∼120 mmHg; SI Appendix, Fig. S9), and the decreased urine volume of the L/L:A/+ mice was increased (from ∼1.1 mL/day to ∼2.5 mL/day; Fig. 3B), indicating that the abnormally high blood pressure and abnormally low urine volumes of the L/L:A/+ mice are at last partly due to low expression of Tgfb1 in the tubules.
Fig. 3.
Renal function and histology at age 40 wk in L/L Akita diabetic mice with Tgfb1 switched from low to high by podocyte-specific or proximal tubule-specific induction of Cre recombinase. (A) Urinary albumin excretion. (B) Urine volume. (C) Urine glucose excretion. (D) Plasma urea nitrogen concentration. (E) Plasma creatinine concentration. (F) Creatinine clearance. (G and H) Periodic acid-Schiff (PAS) staining with hematoxylin of the glomerulus. (Scale bar: 50 μm.) (G) L/L:A/+ mice with podocyte-specific switching (L/L:A/+ P). (H) L/L:A/+ mice with proximal tubule-specific switching (L/L:A/+ T). (I and J) Masson’s trichrome staining of renal cortex. (I) L/L:A/+ P. (J) L/L:A/+ T. (K) Percentage area of PAS-positive mesangial material per glomerular tuft. (L) Percentage of open capillary area per glomerular tuft. (M) Thickness of glomerular basement membrane (GBM). (N) Width of podocyte foot processes. (O) Thickness of tubular basement membrane (TBM) in proximal tubules. Bars are color coded to indicate switching: blue (L/L:A/+; no switching), pink (L/L:A/+ P, in podocytes), and turquoise (L/L:A/+ T, in tubules). *P < 0.05, **P < 0.01 vs. L/L:A/+ mice.
Switching Tgfb1 expression from low to high in the glomeruli prevented the development in the L/L:A/+ mice of the lower than normal plasma urea nitrogen and plasma creatinine and higher creatinine clearance indicative of increased GFR (pink bars in Fig. 3 D–F). Switching in tubules had no effect on the GFR (turquoise bars in Fig. 3 D–F). Urinary excretion of albumin and glucose was markedly affected by switching Tgfb1 expression from low to high in the tubules (turquoise bar in Fig. 3 A and C), but only slightly by glomerulus-specific switching (pink bar in Fig. 3 A and C). We conclude that GFR is affected by Tgfb1 expression in the glomerulus, whereas albumin and glucose excretion is affected by Tgfb1 expression in the tubule.
Changes in the renal pathology of the L/L:A/+ Akita mice after switching Tgfb1 expression from low to high in podocytes or tubules were in line with the changes in GFR and albumin excretion. Thus, the glomeruli of the L/L:A/+ diabetic mice appeared normal except for a slightly above normal open capillary area, but switching Tgfb1 expression from low to high in their podocytes damaged them and they became the same as in the WT:A/+ mice (Fig. 3 G, I, and K–L). In contrast, switching expression of Tgfb1 in the tubules of the L/L:A/+ T mice left the glomeruli unchanged, but fibrosis in the tubulointerstitium was markedly enhanced (Fig. 3 H, J, and K–L). We conclude that Tgfb1 expression in the glomerulus controls the glomerulosclerosis, whereas Tgfb1 expression in the tubule controls tubulointerstitial fibrosis.
Transmission electron microscopy showed that the podocyte-specific switching of Tgfb1 expression in the L/L:A/+ P mice prevented the development of the abnormally thin GBM that occurs in the L/L:A/+ mice, but had little effects on the thickness of the TBM (Fig. 3 M–O and SI Appendix, Fig. S10 A and C). In contrast, tubule-specific switching from low to high had no effects on the thickness of the GBM, but the thickness of TBM was much increased (Fig. 3 M–O and SI Appendix, Fig. S10 B and D). We conclude that Tgfb1 expression in the glomerulus controls the thickness of the GBM, whereas Tgfb1 expression in the tubule controls the thickness of the TBM.
Discussion
The most life-threatening component of diabetic nephropathy is the decrease in GFR, which develops in many type 1 diabetic patients as a result of mesangial expansion and the accumulation of extracellular matrix in the glomerulus (glomerulosclerosis) together with increased GBM thickness, podocyte foot process retraction/effacement, and tubulointerstitial fibrosis. We have shown here that all these features are present in mature Akita type 1 diabetic mice, which have increased expression of Tgfb1 as a result of genetic changes. Thus, their GFR is decreased to about half normal and the pathognomonic features of diabetic nephropathy are present in their kidneys.
The H/H:A/+ mice with three times normal expression of Tgfb1 exhibit better than any previous mouse model the features typically seen in patients with long-term type 1 diabetes. However, none of these features is present in L/L:A/+ Akita diabetic mice with one tenth normal expression of Tgfb1. Indeed, the GFR in the L/L:A/+ mice is greater than normal, the GBM is thinner than normal, and they have less than normal PAS-positive material in their glomeruli. When Tgfb1 expression was changed less dramatically from 150% normal in the H/+:A/+ mice to 60% normal in the L/+:A/+ mice, we found that the GFR still increased and renal morphological abnormalities still decreased in a graded fashion. Thus, above normal Tgfb1 expression exacerbates and below normal expression ameliorates the diabetic nephropathy that develops in Akita diabetic mice.
The progressive inverse changes in the renal expression of the mRNAs of nephrin and megalin, but not of podocin, cubilin, and neonatal Fc receptor, that occurred as Tgfb1 expression was altered are striking, but of uncertain importance. The 3/4 and 1/2 normal expression of nephrin and megalin in the H/+:A/+ and H/H:A/+ diabetic mice are likely to be phenotypically unimportant, because heterozygous null mice for these genes have 1/2 normal expression and no overt phenotypes. However, whether the Tgfb1-dependent two- to threefold increases of nephrin and megalin expression in the L/+:A/+ and L/L:A/+ mice contribute to the diabetic phenotypes is uncertain, and must await further studies, as must deciphering why only two of these five genes are affected even though all are known to be involved in albumin excretion.
It is important to note that the L/L:A/+ Akita diabetic mice did not exhibit the polyuria characteristic of diabetes. Nor did they have glucosuria, even though they had about three times normal plasma glucose concentration and about 1/3 normal plasma insulin concentration. Additionally, they had systolic blood pressures ∼20 mmHg above normal. The absence of polyuria and the increased blood pressure were expected because comparable changes occur in their nondiabetic counterparts (7). We did not anticipate the absence of glucosuria, but the joint absence of polyuria and glucosuria is understandable in light of the demonstration (20) that more than 200 water molecules are coupled to each sugar molecule cotransported by Na+/glucose cotransporters, which are highly expressed in the proximal tubule. Support for this inference is provided by our finding that the absence of polyuria and glucosuria and the increased blood pressure were all partly corrected in the diabetic mice with Tgfb1 expression switched from low to high specifically in tubules.
By switching Tgfb1 expression in the 10% hypomorphs from low to high specifically in podocytes, we showed that the GFR is affected by Tgfb1 expression in the glomerulus, as would be expected given the changes that increased Tgfb1 expression causes in the morphology of the glomerulus. Switching in tubules did not alter the GFR.
Our experiments show that albumin excretion in the Akita diabetic mice is greatly affected by Tgfb1 expression. Thus, it is comparable to nondiabetic wild type in the 10% hypomorphs, but increases from microalbuminuria to macroalbuminuria as Tgfb1 expression increases from 1/2 to ∼3 times normal. Whether the proteinuria in diabetic nephropathy is due to podocyte/glomerular dysfunction and/or to tubule dysfunction is a subject of debate (21–24). We found that the albuminuria of the 10% hypomorphs was increased 20 fold by switching Tgfb1 expression from low to high in the tubules, but only fourfold by switching in the podocytes. Consequently, our results favor the view that the albuminuria that develops in type 1 diabetic mice is mainly due to decreased tubular reabsorption/degradation rather than to increased glomerular filtration.
The absence of nephropathy in the diabetic 10% hypomorphs indicates that substantially decreasing Tgfb1 expression in diabetics could have therapeutic benefits. Unfortunately, the 10% hypomorphs develop aldosteronism as a result of the very low Tgfb1 expression in the adrenal gland (7). Consequently, lowering expression to this degree throughout the body is contraindicated. Nevertheless, our results indicate that this problem might be avoided, while still retaining efficacy, if the reduction of Tgfb1 expression was only in the kidney.
In summary, we have studied the renal phenotype of mature Akita diabetic male mice having five genetically controlled levels of TGFβ1 and have demonstrated that below normal Tgfb1 expression ameliorates the decreased GFR and nephropathy that result from long-standing type 1 diabetes, whereas above normal Tgfb1 expression exacerbates these abnormalities. We have also shown that reducing Tgfb1 expression in the glomerulus is more important in avoiding the decrease in GFR than altering expression in the tubule, whereas expression in the tubule is more important in controlling interstitial fibrosis and albuminuria. Suppressing TGFβ1 action in the kidney as a whole, or specifically in podocytes, could be a promising option for treating/preventing the progressive deterioration of renal function that leads to end-stage renal disease in many diabetic patients.
Materials and Methods
Animals.
To study the effects of TGFβ1 on the phenotype in diabetes, we crossbred heterozygous and homozygous mice having hypomorphic (L) or hypermorphic (H) alleles for TGFβ1 on a C57BL/6 genetic background (7) with mice having heterozygous Akita mutation in the insulin 2 gene, which is an animal model of type 1 diabetes mellitus (Akita mice), on a C57BL/6 genetic background (The Jackson Laboratory) (8). Because the L allele can be converted into the H allele by Cre-loxP recombination, we used this property to generate mice with tissue-specific overexpression of TGFβ1 in the hypomorphs. To study the effects of proximal tubule-specific overexpression on the phenotype in the L/L Akita mice, we used the Ggt1 promoter-driven Cre transgene (Ggt1-cre/ERT2; European Mouse Mutant Archive) (25), which is induced by tamoxifen injection (50 mg/kg/day IP in sesame oil for 5 d) at age 4 wk. To study the effects of podocyte-specific overexpression on the phenotype in the L/L Akita mice, we used the podocin (Nphs2) promoter-driven Cre transgene (Nphs2-cre; The Jackson Laboratory) (26). All mice were kept under the husbandry conditions in conformance with guidelines of University of North Carolina Institutional Animal Care and Use Committee.
Measurement of Biological Parameters.
Plasma glucose levels were determined with the glucose oxidase method (Wako Chemical). Plasma insulin levels were determined with ELISA (Crystal Chem). Plasma urea nitrogen concentrations and plasma and urine electrolytes were determined with the Vitros 250 Chemistry system (Ortho-Clinical Diagnostics). Plasma total cholesterol (Wako) and triglyceride (Stanbio Laboratory) were measured with enzymatic colorimetric methods. Plasma creatinine levels were studied with liquid chromatography tandem mass spectrometry (LC-MS/MS) as described (27). Plasma TGFβ1 and aldosterone were studied with ELISA (Quantikine Mouse/Rat/Porcine/Canine TGFβ1 Immunoassay, R&D Systems; Aldosterone EIA kit, Enzo Life Sciences). Metabolic balance studies were performed using metabolic cages (Solo Mouse Metabolic Cage; Tecniplast).
Histology.
After the inferior vena cava is cut, the left ventricle was punctured by a 23-gauge needle and perfused with PBS for 3 min and with 4% paraformaldehyde for 5 min. Thereafter, the tissues were dissected out and put in 4% paraformaldehyde at least 3 d. These were then paraffin embedded and sectioned. The stained sections were prepared by Center for Gastrointestinal Biology and Diseases Histology Core and imaged on an Olympus BX61 microscope. For electron microscopy, grids were prepared by Microscopy Services Laboratory and imaged on a Zeiss TEM 910 transmission electron microscope.
Blood Pressure and Pulse Rate Measurement.
We measured blood pressure and pulse rate with the tail-cuff method (28).
Quantitative Reverse Transcription-PCR.
Total RNA was extracted from different tissues and the mRNAs were assayed by quantitative reverse transcription-PCR as described (29). The primers and the probes used to measure the mRNAs are shown in SI Appendix, Table S2.
Statistical Analysis.
Data are expressed as means ± SEs. To compare groups, we used one-factor or two-factor ANOVA. Post hoc pairwise comparisons were performed by Tukey–Kramer Honestly Significance Differences test (JMP 9.0; SAS Institute).
Supplementary Material
Acknowledgments
This work was supported by NIH Grants HL49277, HL70523, HL71266, and DK34987 and by Career Development Award 2006-102 from Juvenile Diabetes Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504777112/-/DCSupplemental.
References
- 1.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 3.Herman-Edelstein M, et al. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: A model for diabetic podocytopathy. Diabetes. 2011;60(6):1779–1788. doi: 10.2337/db10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisberg M, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9(7):964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 5.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni AB, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakoki M, et al. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1. Proc Natl Acad Sci USA. 2013;110(14):5600–5605. doi: 10.1073/pnas.1302641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46(5):887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 9.Kestilä M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 10.Holzman LB, et al. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56(4):1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Boute N, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24(4):349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 12.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10(1):1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Christensen EI, Birn H. Megalin and cubilin: Synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280(4):F562–F573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- 14.Yammani RR, et al. Loss of albumin and megalin binding to renal cubilin in rats results in albuminuria after total body irradiation. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R339–R346. doi: 10.1152/ajpregu.00752.2001. [DOI] [PubMed] [Google Scholar]
- 15.Gekle M, et al. Transforming growth factor-beta1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol. 2003;552(Pt 2):471–481. doi: 10.1113/jphysiol.2003.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moestrup SK, et al. Binding and endocytosis of proteins mediated by epithelial gp330. Ann N Y Acad Sci. 1994;737:124–137. doi: 10.1111/j.1749-6632.1994.tb44306.x. [DOI] [PubMed] [Google Scholar]
- 17.Leheste JR, et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155(4):1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haymann JP, et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11(4):632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- 19.Sarav M, et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol. 2009;20(9):1941–1952. doi: 10.1681/ASN.2008090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loo DD, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1996;93(23):13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker BJ, Rasch R, Blantz RC. Glomerular filtration and tubular reabsorption of albumin in preproteinuric and proteinuric diabetic rats. J Clin Invest. 1993;92(2):686–694. doi: 10.1172/JCI116638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagtalunan ME, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tojo A, et al. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol. 2001;116(3):269–276. doi: 10.1007/s004180100317. [DOI] [PubMed] [Google Scholar]
- 24.Russo LM, et al. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20(3):489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dworniczak B, et al. Inducible Cre/loxP recombination in the mouse proximal tubule. Nephron, Exp Nephrol. 2007;106(1):e11–e20. doi: 10.1159/000100554. [DOI] [PubMed] [Google Scholar]
- 26.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35(1):39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71(3):266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 28.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25(5):1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci USA. 2002;99(7):4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.