Significance
Agricultural systems are drivers of global environmental degradation. Insecticides, in particular, are highly biologically active substances that can threaten the ecological integrity of aquatic and terrestrial ecosystems. Despite widespread insecticide application to croplands worldwide, no comprehensive field data-based evaluation of their risk to global surface waters exists. Our data show, for the first time to our knowledge at the global scale, that more than 50% of detected insecticide concentrations (n = 11,300) exceed regulatory threshold levels. This finding indicates that surface water pollution resulting from current agricultural insecticide use constitutes an excessive threat to aquatic biodiversity. Overall, our analysis suggests that fundamental revisions of current regulatory procedures and pesticide application practices are needed to reverse the global environmental impacts of agrochemical-based high-intensity agriculture.
Keywords: global surface waters, insecticide contamination, agriculture, regulatory risk assessment, biodiversity
Abstract
Compared with nutrient levels and habitat degradation, the importance of agricultural pesticides in surface water may have been underestimated due to a lack of comprehensive quantitative analysis. Increasing pesticide contamination results in decreasing regional aquatic biodiversity, i.e., macroinvertebrate family richness is reduced by ∼30% at pesticide concentrations equaling the legally accepted regulatory threshold levels (RTLs). This study provides a comprehensive metaanalysis of 838 peer-reviewed studies (>2,500 sites in 73 countries) that evaluates, for the first time to our knowledge on a global scale, the exposure of surface waters to particularly toxic agricultural insecticides. We tested whether measured insecticide concentrations (MICs; i.e., quantified insecticide concentrations) exceed their RTLs and how risks depend on insecticide development over time and stringency of environmental regulation. Our analysis reveals that MICs occur rarely (i.e., an estimated 97.4% of analyses conducted found no MICs) and there is a complete lack of scientific monitoring data for ∼90% of global cropland. Most importantly, of the 11,300 MICs, 52.4% (5,915 cases; 68.5% of the sites) exceeded the RTL for either surface water (RTLSW) or sediments. Thus, the biological integrity of global water resources is at a substantial risk. RTLSW exceedances depend on the catchment size, sampling regime, and sampling date; are significantly higher for newer-generation insecticides (i.e., pyrethroids); and are high even in countries with stringent environmental regulations. These results suggest the need for worldwide improvements to current pesticide regulations and agricultural pesticide application practices and for intensified research efforts on the presence and effects of pesticides under real-world conditions.
At present, 15.3 × 106 km2 of available croplands (Fig. 1) are cultivated worldwide; thus, agriculture (croplands and pasture) constitutes the world’s largest terrestrial biome (1). Agricultural expansion and intensification led to a >750% increase in pesticide production between 1955 and 2000 (2). Moreover, pesticides represent a US$50 billion market worldwide (3). However, agricultural pesticide use leads to the exposure of nontarget ecosystems such as surface waters (4, 5). In this study, we focused on insecticides because they exhibit a high potential toxicity to aquatic organisms (6) that are crucial for ecosystem functions (7), and we analyzed exposure data obtained for surface waters because these waters are likely to be exposed to agricultural insecticide inputs (4, 5, 8) while providing essential environmental and human health-related ecosystem services (9).
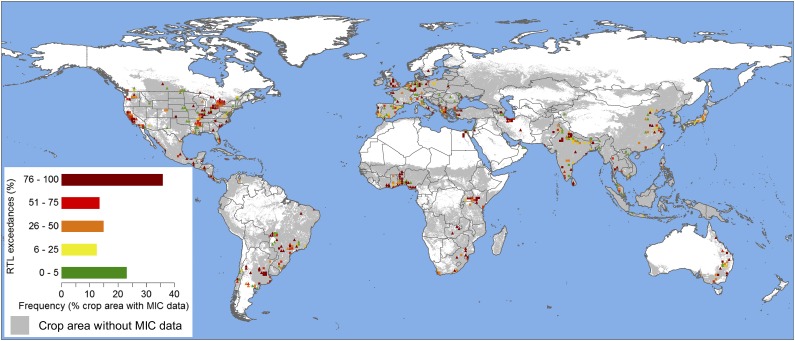
Fig. 1.
Global crop area and the distribution of regulatory threshold level (RTL) exceedance rates for reported measured insecticide concentrations (MICs, n = 10,659) aggregated in 1° grid cells. Information on insecticide surface water exposure was available for only 1.62 million km2 (10.6%) of the 15.3 million km2 of global croplands (1). Rectangles (n = 307) represent subclassified cropped areas with five or more MICs, and triangles (n = 290) display grid cells with fewer than 5 MICs. Please note that 641 MICs could not be allocated to a specific grid cell due to the provision of imprecise location information in the studies. The horizontal bars in the legend illustrate the relative distributions of the respective insecticide RTL exceedance classes among the global cropped area with information on insecticide exposure.
Although the importance of nutrient levels and habitat degradation for surface water impairment is well understood (9), the same cannot be said for insecticides or pesticides in general (5, 9) (Fig. 1). A recent study (10) showed that in Europe, organic chemicals and pesticides specifically threaten freshwater integrity. Based on model predictions, another study (8) identified river fragmentation and nutrient loading as greater threats to aquatic biodiversity than pesticides; however, this study did not consider differences in pesticide toxicities. In response to the inherent toxicity of pesticides and their intentional release into the environment, elaborate environmental risk assessment procedures (SI Appendix, SI Discussion) (11, 12) defining a legally accepted regulatory threshold level (RTL) for each compound (see SI Appendix, Table S1 for the RTLs of the 28 insecticides considered here) have been developed; thus, pesticides are among the most intensively tested and regulated chemicals (13) (SI Appendix, Table S2), possibly contributing to the general perception of their environmental safety.
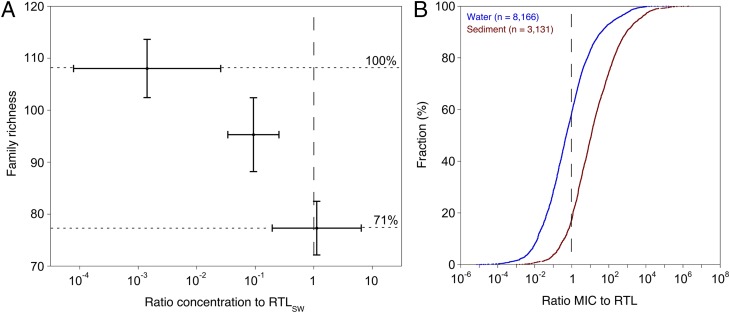
A recent study (14) using field data obtained from Germany, France, and Australia showed that elevated pesticide levels affect regional freshwater invertebrate biodiversity. This analysis ruled out confounding factors and used exposure data based on methods reflecting short-term pesticide concentrations. Transferring the standard toxicity values used in this study into RTLs clearly illustrates that species richness is reduced at the taxonomic family level by ∼30% at the RTL and by ∼12% at a factor of 10 below the RTL (Fig. 2A). Field studies (15, 16) reporting measured insecticide concentrations (MICs) up to 250 times RTL detected decreases in family richness of up to 63%. Any exceedance of the RTL thus indicates a risk of incurring clearly unacceptable effects on aquatic biodiversity. The overarching question now is how widespread and common this risk is, i.e., do MICs exceed their RTLs in the surface waters globally?
Fig. 2.
Observed ecological effects of pesticide exposure on regional surface water biodiversity and distribution curves for global reported measured insecticide concentrations (MICs) in water and sediment relative to regulatory threshold levels (RTLs). (A) Dependency of mean macroinvertebrate family richness at 60 agricultural stream sites on mean aqueous pesticide concentration to RTLSW ratios. Data on family richness, pesticide exposure levels, and categories were taken from ref. 14. The vertical dashed line indicates the RTLSW, and the error bars denote 95% confidence intervals. (B) Blue represents the concentrations in water relative to the substance-specific RTLSW (n = 8,166), and brown represents the concentrations in sediment relative to the substance-specific RTLSED (n = 3,131). The vertical dashed line indicates the RTL.
The few large-scale studies of insecticide exposure in surface waters have either examined sites in spatially restricted areas (10, 17, 18); lacked a quantitative data analysis (4); or followed other, rather specific objectives (18, 19) (SI Appendix, SI Discussion). However, the results obtained in these studies suggest that exceedances of threshold values occur, particularly for insecticides. These studies also showed that insecticides are only present for very short periods (i.e., less than 1% of the year) in agriculturally influenced surface waters. It follows that most traditionally operated, fixed interval-based sampling campaigns inherently miss insecticide exposure (20). To avoid bias resulting from an excessive number of samples without quantifiable insecticide levels, exposure assessments of insecticides using monitoring data must be based solely on quantifiable concentrations in aquatic environments, i.e., those above the limit of quantification (LOQ) (SI Appendix, SI Discussion) (20).
Based on prior investigations (4, 10, 17, 18), we hypothesized that MICs in surface waters exceed their RTLs at dimensions underestimated by regulators and the general public. We tested this first hypothesis using a metaanalysis of global insecticide monitoring data from international peer-reviewed publications (Methods).
The majority of the 28 insecticides included in our analysis (SI Appendix, Table S1) are currently approved in the United States and the European Union. They represent all major insecticide classes and those compounds that are important for global agriculture in terms of annual application rates (SI Appendix, SI Methods). A total of 11,300 MICs [representing an estimated 2.6% of the population of analyses conducted (SI Appendix, SI Discussion)] caused by agricultural nonpoint source pollution from 838 studies published between 1962 and 2012 were compared with their respective RTLs for surface water (RTLSW; n = 8,166) or sediment (RTLSED; n = 3,134). Specifically, we used the RTLSW derived from the official US Environmental Protection Agency’s regulatory risk assessment for the evaluation of MICSW detected in the United States and Canada, the official European RTLSW for the evaluation of MICSW detected in European Union member states, and the average of the two values for the evaluation of MICSW detected in other parts of the world (SI Appendix, Table S1 and Methods). Notably, the United States’ and European Union’s RTLSW values do not differ consistently, i.e., some individual RTLSW values are higher in the United States or the European Union. Our analysis is based on more than 2,500 surface water sites located in 73 countries worldwide (Fig. 1 and SI Appendix, Fig. S1 and SI Discussion) and includes freshwater (n = 9,910 concentrations) and estuarine (n = 1,390 concentrations) systems with catchment sizes between 0.002 and 3,400,000 km2 (SI Appendix, Table S3).
Results and Discussion
Our global analysis shows that no scientific investigations of insecticide surface water exposure exist for large portions (i.e., ∼90%) of high-intensity agricultural areas (Fig. 1). For example, no MICs were reported for Russia or several other post-Soviet states or from large parts of Africa or northwestern South America, although croplands dominate large areas in these regions. The most important outcome of our study is that among the 11,300 insecticide concentrations detected, 52.4% exceeded their specific threshold levels. Approximately 40.8% of the MICSW values (which are considered directly bioavailable due to their presence in the water phase) (21, 22) were above their respective RTLSW values (Fig. 2B). Thus, our results demonstrate that in at least 3,331 cases distributed globally (Fig. 1), the regional biodiversity of surface waters is at risk for impairment due to insecticide contamination (Fig. 2 A and B) (14). Importantly, these risks were defined only for individual compounds, without considering the potential effects of mixture toxicity (see below on this topic). The application of only the United States (54% RTLSW exceedances) or European Union (35.1% RTLSW exceedances) RTLSW to global MICSW did not alter the overall findings of our metaanalysis. When the dataset was rigorously restricted based on land use and entry routes to only those exposure incidents that were definitely linked to agricultural nonpoint entries (SI Appendix, SI Discussion), the results were even more striking (49.7% RTLSW exceedance; SI Appendix, Table S4).
The 82.5% RTLSED exceedances (2,584 cases) reported herein (Fig. 2B) also signify remarkable environmental risks. Sediment samples reflect exposure conditions over longer time spans compared with those of water samples, and the high exceedance levels (i) support the data reported for water, (ii) are likely due to the high hydrophobicity of many insecticides, (iii) imply long-term (chronic) risks to sediment-dwelling organisms (23), and (iv) indicate that both major aquatic ecosystem components are at risk.
Overall, the data regarding insecticide exposure (Fig. 2B) and their attributable ecological effects (Fig. 2A) reveal for the first time to our knowledge at the global scale that, in concert with nutrients and habitat degradation, agricultural insecticide use is likely a driver for biodiversity loss in agriculturally impacted aquatic ecosystems (8, 9, 24). This synthesis responds to a request to quantify the “concentrations of […] pollutants in the global environment” (25), made with regard to pollution as one of the two planetary boundaries that have not yet been quantified. Our approach is based on an extended version of the approach used in ref. 8 as it analyzes empirical monitoring data and employs for the first time to our knowledge a global risk-based evaluation that considers the fact that individual insecticide toxicities span several orders of magnitude. Applying the available insecticide monitoring results to areas that currently lack information on insecticide exposure (i.e., ∼90% of global cropland) reveals that the surface waters located in ∼65% of global cultivated areas are at risk for exposure to insecticide RTL exceedance rates of more than 25% (Fig. 1). However, future studies are needed to quantify the uncertainty related to extending the present risk predictions to all global cropland.
Please note that there are a number of aspects that require further consideration in the assessment of insecticide risks. First, the published insecticide monitoring results to which we refer in our analysis most likely underestimate the actual exposure levels because it is extremely difficult to capture transient insecticide peak concentrations; ∼84.4% of the reported water-phase concentrations were measured using sampling strategies likely to miss the short-term insecticide peaks (20). Highly transient exposures are, according to ref. 20, typical for insecticides in agricultural surface waters. Even considerably contaminated sites regularly exhibit detectable insecticide concentrations for only a few (i.e., 3–4) hours during ∼4–6 d/y coinciding with typical application patterns (e.g., in the spring/summer). Organisms present at such sites receive their entire annual insecticide exposure dose during these short time periods during which short-term peak exposure incidents occur, and these incidents may cause long-term ecological perturbations (4, 14) due to the high intrinsic toxicity of insecticides (6, 26). Therefore, environmental science is faced with the challenge of being able to detect very low absolute levels of insecticides occurring stochastically in time and space that lead to negative ecological impacts. It is thus likely that insecticides are regularly underestimated in their importance as a driver of aquatic biodiversity decline. Second, an in-depth evaluation of the field studies underlying this metaanalysis showed that the majority of sites received either repeated contamination peaks over short periods or concurrent exposure to a number of different pesticides. For example, 81.3% of the samples that were analyzed for the presence of additional compounds (n = 4,198) contained up to 31 additional pesticides; this finding indicates that although disregarded in the regulatory risk assessment (11, 27), overall pesticide effects in the field are driven by repetitive exposure peaks and mixture toxicity (the simultaneous exposure of organisms to a multitude of different compounds). Third, unacceptable ecological effects on aquatic organisms are likely to occur in the field at concentrations well below the RTL (Fig. 2A) (7, 14). Applied to the data compiled here, this consideration means that in virtually all cases where an insecticide had been detected (ratio MIC to RTL ≥10−3; Fig. 2B), the consequence is a negative impact on regional biodiversity (Fig. 2A).
Based on these three considerations, both the actual insecticide contamination of surface waters and the resulting ecological risks are, in reality, even greater than indicated in this study based on the assessed literature and current regulatory procedures for insecticide risk assessment. In this context, the comparison of MICSW to other established threshold levels such as science-based environmental quality standards (EQSs) [which, in contrast to RTLs, do not tolerate (transient) clear effects on aquatic organisms], leads to an even higher threshold level exceedance rate of 70.1% (n = 7,821; SI Appendix, SI Methods). However, a concentration exceeding the RTL measured at a given site does not necessarily indicate that large stretches of the associated surface water are exposed and therefore harbor risks to aquatic fauna. For example, aquatic vegetation can reduce the negative impacts of pesticides (26). Nonetheless, the fact that RTL exceedances are so widespread and lead to detectable biodiversity reductions clearly highlights the global problem we are facing as a result of insecticide use in agriculture.
In addition to improving the efficiency of insecticides and reducing insect/pest resistance, the research and development (R&D) of insecticide compounds have focused on being more environmentally friendly, with the intention of reducing risks to surface waters as nontarget ecosystems (28, 29). However, a recent study (18) showed that the FOCUS model, used for the regulatory exposure assessment in the European Union, underpredicts field concentrations of newer, increasingly used insecticides such as hydrophobic pyrethroids. Specifically, the ratio of the predicted insecticide surface water concentrations to the MICSW was significantly lower for pyrethroids than for organochlorines and organophosphorus insecticides. The authors partially attributed these results to the inadequacies of the runoff model termed “pesticide root zone model” (PRZM), which is also used for the authorization of pesticide compounds in other countries such as the United States (30). Therefore, our second hypothesis was that newer, more recently developed and registered insecticide classes (SI Appendix, Table S5) show higher RTL exceedances.
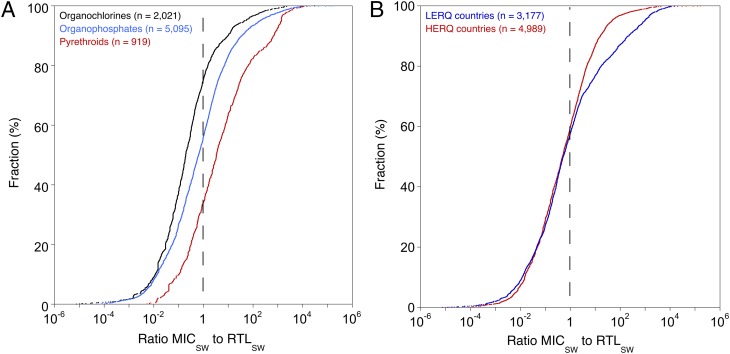
Contemporary insecticides, such as pyrethroids, showed a significantly higher percentage of RTLSW exceedance (65.8%) compared with both organophosphates (43.7%; P < 0.001) and organochlorines (24.4%; P < 0.001), and the latter two also differed significantly (P < 0.001; Fig. 3A and SI Appendix, Table S6). Although first introduced to the global crop protection market in 1973 (SI Appendix, Table S5), pyrethroids have gained prominence in part due to concerns over organophosphates and human health. In our comparison of insecticide classes, we specifically considered differences in bioavailability and the ratios between the RTLSW and the LOQ in additional linear model analyses; neither aspect altered the general picture of significant differences among the compound classes. In particular, considering only the freely dissolved [and therefore directly bioavailable (31)] fraction analyzed in water samples of the highly hydrophobic [organic carbon/water partitioning coefficients (KOC) of 105–107 (32)] pyrethroids did not reduce their concentration to RTLSW ratios (SI Appendix, Table S7 and SI Discussion). This finding indicates that the significantly higher RTLSW exceedance frequency for highly sorptive pyrethroids is not biased by potential bioavailability limitations. In addition, considering the lower RTLSW of pyrethroids associated with their comparably higher toxicity to aquatic organisms, and thus lower distances between RTLSW and LOQs (SI Appendix, Table S8), did not disprove our findings; however, the discrepancies among insecticide classes were reduced (SI Appendix, Table S9 and SI Discussion).
Fig. 3.
Effect of insecticide class and country environmental regulations on the distribution curves for reported measured insecticide concentrations in the water phase (MICSW) relative to substance-specific regulatory threshold levels (RTLSW). (A) Black represents data obtained for organochlorine insecticides (n = 2,021), blue represents data obtained for organophosphate insecticides (n = 5,095), and red represents data obtained for pyrethroid insecticides (n = 919); 6.1% of the MICSW of neonicotinoids (n = 131) exceeded the RTLSW (not displayed). (B) Distribution curves for MICSW relative to substance-specific RTLSW. Blue represents concentrations measured in countries with low environmental regulatory quality (LERQ; n = 3,177), and red represents data measured in countries with high environmental regulatory quality (HERQ; n = 4,989). The vertical dashed lines indicate the RTLSW.
Overall, we conclude that the environmental risk is even higher for newer-generation insecticides, such as pyrethroids, compared with older-generation insecticides. Further, these increased risks indicate a failure of R&D efforts to develop more environmentally friendly insecticides to improve surface water protection. Current risk management obligations and application practices for pyrethroids in agriculture obviously do not result in surface water exposure levels that adhere to the strict RTLs triggered by their extremely high invertebrate toxicities (6). However, in contrast to pyrethroids, a valid conclusion for neonicotinoid MICSW (RTLSW exceedances: 6.1%; n = 131) is hindered due to insufficient data. Nonetheless, recent studies (19, 33) on agricultural neonicotinoid use reveal environmental concerns for both aquatic and terrestrial ecosystems.
Our third hypothesis is that countries with a high environmental regulatory quality (HERQ) should exhibit markedly less frequent RTL exceedances than those with a low environmental regulatory quality (LERQ) (SI Appendix, Table S10). RTLSW exceedances were indeed significantly more frequent in the LERQ countries (P < 0.001; SI Appendix, Table S6). This pattern also holds true when accounting for differences in RTL/LOQ ratios (SI Appendix, Table S9). Although not unexpected, this finding is alarming considering that recent and anticipated future agricultural expansion and intensification have occurred and will occur in biodiversity-rich tropical LERQ countries (1). In these countries, pesticide regulations are insufficiently enforced (5, 34) (SI Appendix, SI Discussion) and surface waters are already exposed to numerous other stressors (9). The absolute percentage of the detected RTLSW exceedance (39.9%) in the HERQ countries (such as the United States, Canada, Germany, Japan, and Australia), is only slightly lower than that in the LERQ countries (42.2%; Figs. 1 and 3B). Therefore, our data show that the actual extent to which surface waters are contaminated with insecticides is not controlled effectively by increasingly stringent environmental regulations at present. However, in the LERQ countries, substantially larger surface water systems and longer sampling intervals were considered in the monitoring campaigns (SI Appendix, Table S11), decreasing the likelihood of determining insecticide peak exposure incidences (SI Appendix, Table S6) (20). The application of more targeted insecticide sampling strategies (20) is needed in the future to adequately reflect the risks to the surface waters of LERQ countries.
Overall, RTL exceedances depend on multiple factors, including insecticide classes, environmental regulatory standards, catchment size, sampling regime, and sampling date (SI Appendix, Table S6). We identified a significant interaction among insecticide class, the quality of countries’ regulatory standards, and sampling date (SI Appendix, Tables S6, S12, and S13, Fig. S2, and SI Discussion). Unlike in HERQ countries, the risks of organochlorine and organophosphorus insecticide exposure in LERQ countries have increased over the last three decades due to increased insecticide use and simultaneously weak or even nonexistent pesticide regulation schemes.
Taken together, our results seriously challenge the protectiveness of the current regulatory insecticide risk assessments and management procedures at the global scale. Although, for example, major EU and US pesticide legislations were already enforced at the beginning of the 1990s (SI Appendix, Table S2), 54.2% (n = 4,686; and 49.5%, n = 2,681 when considering HERQ countries only) of the MICs reported since 2000 have exceeded their respective RTLs (SI Appendix, Fig. S3 A and B). Targeted postregistration monitoring schemes and regulatory actions are needed, considering that 18 and 24 of the 28 insecticide compounds included in our metaanalysis are currently approved in EU countries and in the United States, respectively. The high numbers of threshold exceedances worldwide are caused by failures of either regulatory exposure assessment (18) or farmers’ adherence to prescribed risk management obligations (35).
Edge-of-field runoff was an important route of entry for insecticides in our dataset, comprising 72.4% of cases for which an entry route was specified (SI Appendix, Table S3). In addition to application patterns and geographical and meteorological conditions, the physicochemical properties of an insecticide (such as its hydrophobicity) are crucial components of its potential to enter a surface water via runoff (36, 37). Empirical studies (38, 39) suggest that lower runoff losses to surface waters occur for strongly sorbed compounds. This potential provides opportunities for the more efficient use of insecticides based on modeling of their runoff potential. However, the potential risks of insecticide surface water impairments are driven not only by the respective entry pathways and probabilities of exposure but also by the intrinsic toxicity, which varies considerably among different classes of insecticides (40). Thus, any risk mitigation attempt must consider both entry probability and toxicity.
To date, agriculture occupies ∼40% of the world’s land surface and agricultural production is forecast to undergo substantial intensification (1, 2). This situation leads to the projection that future agricultural activities may rival climate change in their environmental impacts (2). Reforming conventional agricultural systems and adopting promising approaches from organic farming (41), including the elimination of pesticides wherever applicable, in concert with the closing of yield gaps on underperforming lands (1, 42) and precision agricultural techniques (43), are possible ways to meet the twin challenges of providing sufficient food for a growing human population and reversing the global environmental impacts of agrochemical-based high-intensity agriculture.
Methods
We conducted a comprehensive literature search of multiple databases to identify scientific studies in eight different languages reporting on agricultural insecticide concentrations in global surface waters. We evaluated more than 200,000 database entries and examined ∼20,000 articles in greater detail. The studies had to meet the following selection criteria to be included in our metaanalysis: (i) only peer-reviewed studies were considered to ensure that minimum scientific standards were met; (ii) the studies had to be written in one of the following eight languages: Chinese, English, French, German, Japanese, Russian, Spanish, and Portuguese; and (iii) the MICs reported resulted from agricultural nonpoint source pollution (excluding urban, industrial, and public health activities; aquaculture; atmospheric deposition; forest application; sheep dipping; golf course applications; accidental spills; intentional water contamination; and in-crop use) and were detected in perennial freshwater or estuarine surface water bodies (SI Appendix, SI Methods).
Regulatory threshold levels were applied as follows to assess the ecological importance of reported insecticide exposure data (SI Appendix, SI Methods, and Table S1): aqueous concentrations measured in the United States, Canada, or the European Union were compared with the respective regulatory threshold levels (RTLSW), which are defined as part of the US (differentiated further into freshwater and estuarine RTLSW) or EU pesticide legal registration procedures; and aqueous concentrations measured in other parts of the world were compared with the average values of the US and EU RTLSW (SI Appendix, Table S1), as both regulatory risk assessments are considered highly elaborated and science based. Sediment or suspended-particle exposure was evaluated using the respective RTLSED. The concentration of each insecticide was compared with its respective RTL, irrespective of how many compounds were measured in a given sample. To focus on the potential ecological risks of the highly relevant short-term exposure peaks of insecticides in surface waters, and considering that insecticide exposure occurs less than 1% of the time per year, we used only insecticide concentrations above the LOQ, as suggested by ref. 20 (see also SI Appendix, SI Discussion for further details). The aggregate exceedance frequencies for all studies considered were computed across multiple sites and plotted as distribution curves.
In addition to information on insecticide concentrations, we collected information on several covariates (i.e., sampling location, catchment size, sampling interval, and sampling date) that might influence insecticide exposure and used these data in a linear model analysis (SI Appendix, SI Methods) with the logarithm of the MICSW to RTLSW ratio as the dependent variable to test for differences among specific insecticide classes (organochlorines, organophosphates/carbamates, and pyrethroids) and between countries’ environmental regulatory standards (HERQ vs. LERQ countries, classified based on environmental, regulatory, and economic indices) (SI Appendix, SI Methods). We also evaluated the effects of the organic carbon/water partitioning coefficient (KOC), the bioavailability of highly sorptive pyrethroids, and the differences in the RTLSW/LOQ ratios on the concentration to RTLSW ratios using two additional linear model analyses (SI Appendix, SI Discussion).
Supplementary Material
Acknowledgments
We thank Walter H. Schreiber and Ralf B. Schäfer for statistical advice; Jörg Rapp and Caroline Nägele for support with Fig. 1; Ralf B. Schäfer and Mikhail A. Beketov for support with Fig. 2A; Sascha Bub for support with database generation; Navin Ramankutty, Chris Metcalfe, Martin Scheringer, Ralf B. Schäfer, Carsten A. Brühl, James M. Dabrowski, Erin R. Bennett, and two anonymous reviewers for their valuable comments on earlier drafts of the manuscript; and David Imo, Niklas Keck, Bonny Krell, Koffi Tassou, and Sie Yung for translating the foreign-language studies. This study was funded by the German Society for the Advancement of Sciences (DFG SCHU 2271/6-1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.M.B. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited at math.uni-landau.de/ecotox/publications/stehleandschulz2015.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500232112/-/DCSupplemental.
References
- 1.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 2.Tilman D, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292(5515):281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 3. Specialists in Business Information (November 24, 2008) Record increase in global pesticides market to accelerate into 2013. Available at www.sbireports.com/about/release.asp?id=1249. Accessed February 12, 2013.
- 4.Schulz R. Field studies on exposure, effects, and risk mitigation of aquatic nonpoint-source insecticide pollution: A review. J Environ Qual. 2004;33(2):419–448. doi: 10.2134/jeq2004.4190. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313(5790):1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 6.US Environmental Protection Agency 2014 ECOTOXicology Database System, Version 4.0. Available at http:/cfpub.epa.gov/ecotox/. Accessed March 18, 2013. [PubMed]
- 7.Schäfer RB, et al. Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ Sci Technol. 2012;46(9):5134–5142. doi: 10.1021/es2039882. [DOI] [PubMed] [Google Scholar]
- 8.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 9.Finlayson CM, et al. In: Inland Water Systems. Ecosystems and Human Well-Being: Current State and Trends. Hassan R, Scholes R, Ash N, editors. Vol 1. Island Press; Washington, DC: 2005. pp. 551–583. [Google Scholar]
- 10.Malaj E, et al. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci USA. 2014;111(26):9549–9554. doi: 10.1073/pnas.1321082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Commission Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off J Eur Union L. 2009;309(1):1–50. [Google Scholar]
- 12.FIFRA 1947. Federal Insecticide, Fungicide and Rodenticide Act, U.S. Federal Law amended in 1972 and 1988.
- 13.European Crop Protection Association . The ABCs of Crop Protection: Fast Facts for European Policy-Makers. ECPA; Brussels: 2003. [Google Scholar]
- 14.Beketov MA, Kefford BJ, Schäfer RB, Liess M. Pesticides reduce regional biodiversity of stream invertebrates. Proc Natl Acad Sci USA. 2013;110(27):11039–11043. doi: 10.1073/pnas.1305618110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz R, Liess M. A field study of the effects of agriculturally derived insecticide input on stream macroinvertebrate dynamics. Aquat Toxicol. 1999;46(3-4):155–176. [Google Scholar]
- 16.Bollmohr S, Schulz R. Seasonal changes of macroinvertebrate communities in a Western cape river, South Africa, receiving nonpoint-source insecticide pollution. Environ Toxicol Chem. 2009;28(4):809–817. doi: 10.1897/08-228R.1. [DOI] [PubMed] [Google Scholar]
- 17.Gilliom RJ. Pesticides in U.S. streams and groundwater. Environ Sci Technol. 2007;41(10):3408–3414. doi: 10.1021/es072531u. [DOI] [PubMed] [Google Scholar]
- 18.Knäbel A, Stehle S, Schäfer RB, Schulz R. Regulatory FOCUS surface water models fail to predict insecticide concentrations in the field. Environ Sci Technol. 2012;46(15):8397–8404. doi: 10.1021/es301649w. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey CA, et al. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ Int. 2015;74:291–303. doi: 10.1016/j.envint.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Stehle S, Knäbel A, Schulz R. Probabilistic risk assessment of insecticide concentrations in agricultural surface waters: A critical appraisal. Environ Monit Assess. 2013;185(8):6295–6310. doi: 10.1007/s10661-012-3026-x. [DOI] [PubMed] [Google Scholar]
- 21.Weston DP, Lydy MJ. Urban and agricultural sources of pyrethroid insecticides to the Sacramento-San Joaquin Delta of California. Environ Sci Technol. 2010;44(5):1833–1840. doi: 10.1021/es9035573. [DOI] [PubMed] [Google Scholar]
- 22.Spurlock F, Bacey J, Starner K, Gill S. A probabilistic screening model for evaluating pyrethroid surface water monitoring data. Environ Monit Assess. 2005;109(1-3):161–179. doi: 10.1007/s10661-005-5847-3. [DOI] [PubMed] [Google Scholar]
- 23.Du J, Pang J, You J. Bioavailability-based chronic toxicity measurements of permethrin to Chironomus dilutus. Environ Toxicol Chem. 2013;32(6):1403–1411. doi: 10.1002/etc.2192. [DOI] [PubMed] [Google Scholar]
- 24.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344(6187):1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 25.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 26.Stehle S, et al. Pesticide risk mitigation by vegetated treatment systems: a meta-analysis. J Environ Qual. 2011;40(4):1068–1080. doi: 10.2134/jeq2010.0510. [DOI] [PubMed] [Google Scholar]
- 27.US Environmental Protection Agency 2015 Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization. Available at www.epa.gov/oppefed1/ecorisk_ders/toera_analysis_eco.htm. Accessed February 12, 2015. [PubMed]
- 28.Lamberth C, Jeanmart S, Luksch T, Plant A. Current challenges and trends in the discovery of agrochemicals. Science. 2013;341(6147):742–746. doi: 10.1126/science.1237227. [DOI] [PubMed] [Google Scholar]
- 29.Devine GJ, Furlong MJ. Insecticide use: Contexts and ecological consequences. Agric Human Values. 2007;24(3):281–306. [Google Scholar]
- 30.US Environmental Protection Agency 2015 Technical Overview of Ecological Risk Assessment—Analysis Phase: Exposure Characterization. Available at www.epa.gov/oppefed1/ecorisk_ders/toera_analysis_exp.htm. Accessed February 13, 2015. [PubMed]
- 31.Yang W, Spurlock F, Liu W, Gan J. Inhibition of aquatic toxicity of pyrethroid insecticides by suspended sediment. Environ Toxicol Chem. 2006;25(7):1913–1919. doi: 10.1897/05-616r.1. [DOI] [PubMed] [Google Scholar]
- 32.Laskowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol. 2002;174:49–170. doi: 10.1007/978-1-4757-4260-2_3. [DOI] [PubMed] [Google Scholar]
- 33.Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon H, Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511(7509):341–343. doi: 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- 34.Ecobichon DJ. Pesticide use in developing countries. Toxicology. 2001;160(1-3):27–33. doi: 10.1016/s0300-483x(00)00452-2. [DOI] [PubMed] [Google Scholar]
- 35.De Snoo GR. Variations in agricultural practice and environmental care. In: Den Hond F, Groenewegen P, van Straalen NM, editors. Pesticides: Problems, Improvements, Alternatives. Blackwell; Oxford: 2003. pp. 100–112. [Google Scholar]
- 36.Capel PD, Larson SJ, Winterstein TA. The behaviour of 39 pesticides in surface waters as a function of scale. Hydrol Processes. 2001;15(7):1251–1269. [Google Scholar]
- 37.Reichenberger S, Bach M, Skitschak A, Frede H-G. Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness: A review. Sci Total Environ. 2007;384(1-3):1–35. doi: 10.1016/j.scitotenv.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Riise G, Lundekvam H, Wu QL, Haugen LE, Mulder J. Loss of pesticides from agricultural fields in SE Norway—runoff through surface and drainage water. Environ Geochem Health. 2004;26(2-3):269–276. doi: 10.1023/b:egah.0000039590.84335.d6. [DOI] [PubMed] [Google Scholar]
- 39.Patakioutas GI, Karras G, Hela D, Albanis TA. Pirimiphos-methyl and benalaxyl losses in surface runoff from plots cultivated with potatoes. Pest Manag Sci. 2002;58(12):1194–1204. doi: 10.1002/ps.589. [DOI] [PubMed] [Google Scholar]
- 40.Spurlock F, Lee M. Synthetic pyrethroid use patterns, properties, and environmental effects. In: Gan J, Spurlock F, Hendley P, Weston D, editors. Synthetic Pyrethroids, Occurrence and Behavior in Aquatic Environments. American Chemical Society; Washington, DC: 2008. pp. 3–25. [Google Scholar]
- 41.Seufert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. 2012;485(7397):229–232. doi: 10.1038/nature11069. [DOI] [PubMed] [Google Scholar]
- 42.Mueller ND, et al. Closing yield gaps through nutrient and water management. Nature. 2012;490(7419):254–257. doi: 10.1038/nature11420. [DOI] [PubMed] [Google Scholar]
- 43.Gebbers R, Adamchuk VI. Precision agriculture and food security. Science. 2010;327(5967):828–831. doi: 10.1126/science.1183899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.