Significance
Ecological-niche differentiation in diploid sexual–polyploid asexual complexes has been observed within and among many taxa, yet the relative contributions of reproductive system and ploidy are not fully understood. Here, we assess niche characteristics of sexual diploid, apomictic (asexual) diploid, and triploid Boechera (Brassicaceae) lineages. We find strong evidence for widespread hybridization and, to a lesser degree, ploidy variation as factors that together overcome the adaptive disadvantages of apomictic (i.e., asexual) reproduction. When controlling for ploidy, we find only modest evidence for putatively asexually driven ecological-niche divergence among reproductive systems, a finding that contradicts the well-supported patterns of geographic parthenogenesis.
Keywords: Boechera, UPGRADE2, APOLLO, geographic parthenogenesis, niche conservation
Abstract
Asexual reproduction is expected to reduce the adaptive potential to novel or changing environmental conditions, restricting or altering the ecological niche of asexual lineages. Asexual lineages of plants and animals are typically polyploid, an attribute that may influence their genetic variation, plasticity, adaptive potential, and niche breadth. The genus Boechera (Brassicaceae) represents an ideal model to test the relative ecological and biogeographic impacts of reproductive mode and ploidy because it is composed of diploid sexual and both diploid and polyploid asexual (i.e., apomictic) lineages. Here, we demonstrate a strong association between a transcriptionally conserved allele and apomictic seed formation. We then use this allele as a proxy apomixis marker in 1,649 accessions to demonstrate that apomixis is likely to be a common feature across the Boechera phylogeny. Phylogeographic analyses of these data demonstrate (i) species-specific niche differentiation in sexuals, (ii) extensive niche conservation between differing reproductive modes of the same species, (iii) ploidy-specific niche differentiation within and among species, and (iv) occasional niche drift between apomicts and their sexual ancestors. We conclude that ploidy is a substantially stronger and more common driver of niche divergence within and across Boechera species although variation in both traits may not necessarily lead to niche evolution on the species scale.
Sexual reproduction offers several evolutionary advantages over asexuality, including accelerated adaptation to variation in environments (1), competitors (2), and parasites (3). As such, evolutionary transitions from sexual to asexual reproduction or outcrossing to selfing may have a strong impact on an organism’s ecological distribution and adaptive potential (4, 5). Because reproductive-mode divergence can occur on short temporal scales (6), comparisons between closely related taxa that differ in reproductive mode offer unique opportunities to study the adaptive significance of sexuality at micro- (i.e., population) (7) and macroevolutionary (i.e., species) (8) levels.
Apomixis, the asexual formation of seeds via meiotically unreduced gametes, is rare among angiosperm genera (∼1.1%) (9). It is nonetheless an evolutionarily important trait capable of fixing the entire genome as one linkage group across generations, conferring potential fitness advantages associated with the now-fixed genotype, such as yield, in ecological (10) and agricultural settings (11). Apomicts seem to have evolved from sexual ancestors independently in several distantly related taxa (12) and can experience advantages, such as reduced or no allocation to male function (in hermaphroditic taxa) (13) and reproductive assurance (sensu ref. 14), which together enhance their colonizing abilities (15). These advantages may be tempered by disadvantages imparted by the absence of recombination, such as increased deleterious mutation accumulation (16) and poor responses to selection imposed by changing environments (17).
Comparisons between asexuals and their sexual ancestors shed light upon the processes contributing to the evolution and maintenance of apomixis. Both novel mutations (i.e., gain-of-function mutation; sensu ref. 18), and/or hybridization (19) have been proposed to induce apomixis although recurrent hybridization may obscure origin and age estimations of natural apomictic lineages [e.g., Boechera (20) and Taraxacum (21); but see ref. 22]. One explanation for the success of apomicts in mixed reproductive systems follows from the fact that many of them display strong evidence for niche differentiation from their sexual progenitors, a pattern termed “geographic parthenogenesis” (GP) (23, 24). The ubiquity of GP has led to the hypothesis that niche differentiation, rather than niche conservation, governs the ecology of apomictic lineages (ref. 25; but see ref. 26). GP could be explained by (i) an escape from competition between sexuals and apomicts occupying similar niches (27–29), (ii) selection for asexual genotypes with wider ecological tolerance compared with sexuals (“general purpose” genotype model) (30), and (iii) niche partitioning between sexual parents and their hybrid apomictic progeny, the latter of which have a fixed subset of genetic variation from the sexuals (“frozen-niche variation” model) (31). Despite substantial evidence for GP, the factors responsible for this pattern are poorly understood. For example, because GP is commonly observed in diploid sexual–polyploid asexual complexes (25, 32), it is speculated that ploidy could be the primary source of GP rather than reproductive mode (14, 25, 33).
The North-American genus Boechera (Brassicaceae) is an ideal system to study the ecological and evolutionary dynamics of reproductive-mode divergence. The genus is monoecious, and, in addition to broad ecological ranges and high intra- and interspecies diversity (34), Boechera possesses three reproductive-mode classes: diploid sexuals versus triploid and diploid pseudogamous apomicts (35). A number of lines of evidence demonstrate that the switch from sex to apomixis has occurred multiple times during the evolution of Boechera (36–38). As such, Boechera offers replicated events of sexual-apomictic transitions within a ploidy level and enables comparisons between reproductive modes without the confounding effects of variable ploidy.
Here, we document the associations between reproductive-mode variation and the extensive ecological and physiological diversity of Boechera to test two hypotheses: (i) Apomixis is a recent evolutionary development arising only in a related subset of haplotypes, and (ii) niche evolution is an intrinsic factor of reproductive-mode divergence (i.e., geographic parthenogenesis) and not a covariate of ploidy variation. To assess these hypotheses, we examine the phylogeographic distribution of APOLLO (apomixis-linked locus) (39) and UPGRADE2 (unreduced pollen grain development) (40), two alleles whose expression is highly correlated with apomeiotic egg and pollen formation in Boechera, respectively, to infer the ecological niches of 1,649 single samples from different populations per species of diploid sexual and apomictic Boechera. Our data provide phylogeographic evidence for multiple origins of apomictic cytotypes in Boechera and support a frozen-niche variation model for diploid apomixis niche evolution. Importantly, we provide statistical evidence that ploidy variation, both within and among species, is a stronger driver of niche evolution than reproductive mode.
Results
A Molecular Marker Predicts Apomixis in Boechera.
We used the flow cytometric seed screen (FCSS) (41) to functionally infer reproductive mode in 275 Boechera accessions from 22 species (Dataset S1). Each plant was additionally genotyped for the presence or absence of apomixis-specific alleles (hereafter “allele class”) of two genes associated with apomeiotic pollen (UPGRADE2) (40) and egg cell formation (APOLLO) (39) (Fig. S1). FCSS revealed that apomixis-specific alleles were nearly fixed among plants determined to be apomictic (UPGRADE2, 96.06%; APOLLO, 98.39%, respectively). Sexuals were virtually free from the apomictic APOLLO allele (frequency, 2.27%) whereas 34.48% of sexuals had the apomictic UPGRADE2 allele. The tighter association of the apomictic APOLLO allele with apomixis (logistic regression model; predictor variable = APOLLO, covariates = FCSS and taxon data, n = 256, eB = 2835.48, χ2 = 306.49, P < 0.0001) (Dataset S1), relative to the apomictic UPGRADE2 allele (logistic regression model; predictor variable = UPGRADE2, covariates = FCSS and taxon data, n = 272, eB= 60.48, χ2 = 139.59, P < 0.0001) (Dataset S1), led us to use the APOLLO polymorphism as a proxy marker for apomixis.
Broad Phylogenetic Distribution of Apomixis.
We genotyped the APOLLO and UPGRADE2 allele classes in 1,374 additional herbaria accessions, representing 84 of the 111 accepted Boechera species and nine species of four closely related genera (Dataset S1 and Table S1). A subset of 1,010 accessions were previously genotyped for several chloroplast markers (20). The chloroplast DNA (cpDNA) haplotypes were used to determine the phylogenetic distribution of APOLLO and UPGRADE2 allele classes on a genus-wide scale (n = 1,649) because true species-specific cpDNA-haplotype lineages are rare (i.e., in total, seven maternal phylogenetic lineages) due to haplotype sharing among species (20).
On a genus-wide scale, apomixis, as defined by the presence of the apomictic APOLLO allele, was found in all cpDNA-haplotype lineages and in 49.31% of all Boechera cpDNA haplotypes (Dataset S1). Apomixis frequencies did not vary between the major cpDNA-haplotype lineages (two-tailed Fisher’s exact tests between cpDNA-haplotype lineages I, II, and III; VI and VII were excluded because n ≤ 5 accessions; P ≥ 0.071) (Dataset S1), except for lineages IV and V, where apomixis frequencies were strongly reduced compared with 49.31% average apomixis frequency (two-tailed Fisher’s exact test; lineage IV, 1 of 13 accessions, P = 0.0033; lineage V, 2 of 21 accessions, P = 0.0002) (Dataset S1).
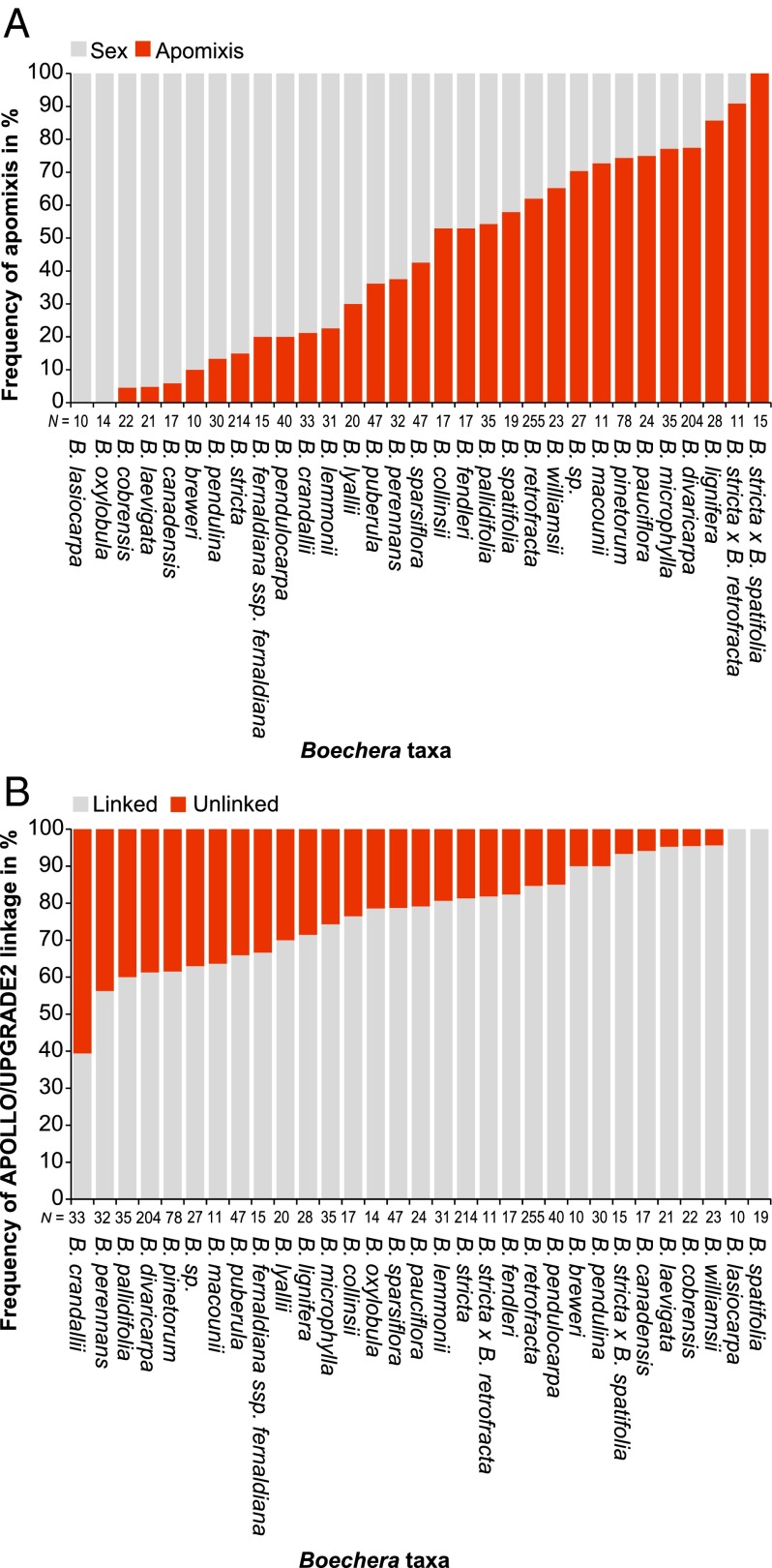
Of the 31 Boechera species characterized by more than 10 accessions, 29 species contained both apomictic and sexual individuals (Table S1). There was wide variation in the frequency of apomictic individuals across species (range, 0–100%; median, 42.55%) (Fig. 1A). For example, Boechera retrofracta and Boechera divaricarpa, both large groups with wide geographic distributions, were characterized by both reproductive modes (61.96%, and 77.45% apomixis, respectively) (Fig. 1A and Table S1). By contrast, Boechera stricta, with the widest distribution of any species (42), was predominantly sexual with a few apomicts (n = 214, 83.71% sexual) (Fig. 1A and Table S1). As seen previously (43), our data also show that hybrids, such as B. stricta × B. retrofracta and B. stricta × Boechera spatifolia, have the highest frequencies of apomixis (n = 11, 93.33% and n = 15, 100%, respectively) (Fig. 1A and Table S1).
Fig. 1.
Frequency of (A) apomixis inferred from the presence of the female-apomeiosis marker allele of APOLLO and (B) linkage of male- and female-apomeiosis marker alleles across Boechera species. Here, the term “linkage” refers to the cooccurrence or coabsence of both apomictic alleles from the UPGRADE2 and APOLLO genes per individual accession.
UPGRADE2 and APOLLO Are Linked and Geographically Dispersed.
The apomixis-specific alleles of UPGRADE2 and APOLLO were detected in 41.73% and 46.15% of the tested accessions, respectively (Dataset S1). For the purpose of this paper, we use the term “linkage” to describe cooccurrence or coabsence of the apomictic UPGRADE2 and APOLLO alleles in single individuals, as determined through PCR. In that light, 77.08% of all accessions (n = 1,584) demonstrated linkage of both allele classes (i.e., both apo-alleles cooccurred or were coabsent in an individual). The number of accessions demonstrating linkage between both allele classes varied from 39.39% to 100% among species (Fig. 1B and Dataset S1).
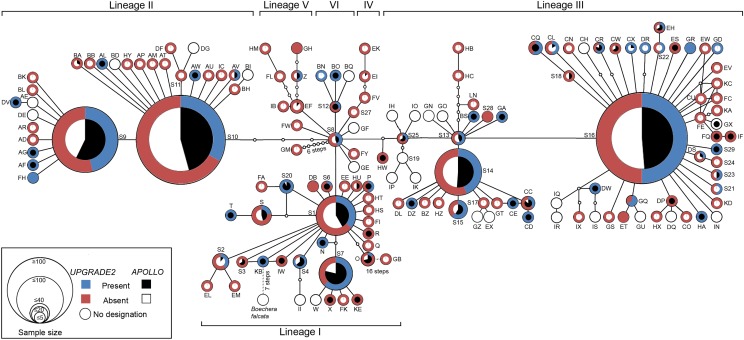
Individuals carrying the oldest cpDNA haplotypes AA, AB, and AC, which are represented by suprahaplotype S8 (∼0.7–2 million y) (44) (Fig. 2), had either none, both, or one of the apomixis-related APOLLO and UPGRADE2 alleles. There was no evidence for overrepresentation of either allele class in ancient or recent cpDNA-haplotype carriers (APOLLO, r2 = 0.499; UPGRADE2, r2 = 0.281) (Table S2). We also found that all cpDNA haplotypes associated with the apomixis-specific alleles of one or both loci are interconnected in the phylogenetic network (Fig. 2).
Fig. 2.
Genus-wide phylogenetic distribution of apomixis in Boechera. The phylogenetic distributions of apomictic APOLLO and UPGRADE2 alleles reflect the range of chloroplast haplotype diversity of sexual Boechera accessions. Haplotype node sectors indicate the frequency of accessions carrying the male and the female apomeiosis alleles versus those lacking one or both alleles.
The apomictic alleles of APOLLO and UPGRADE2 were each observed only in a single accession of Boechera sister genera (APOLLO, Cusickiella quadricostata; UPGRADE2, Polyctenium fremontii) (Dataset S1 and Table S1). In contrast to the apomictic APOLLO allele, the apomictic UPGRADE2 allele was not detected in any of the genera in neighboring clades (Table S1 and GenBank nucleotide collection search, www.ncbi.nlm.nih.gov/genbank/, release 205.0). However, the two apomictic alleles were never linked in outgroups of the Boechera phylogeny.
Sexual and Apomictic Boechera Do Not Differ in Genus-Wide Geographic Range.
We used a constrained correspondence analysis (CCA) to compare the geographic distribution of both allele classes of the proxy apomixis marker APOLLO within and across species. Statistical differences among groups were determined by 10,000 permutations. First, tests of geographic divergence conducted by partitioning ecological-niche differentiation among accessions showed no significant differences between sexual and apomictic Boechera on a genus-wide scale (n = 1,595, Pspat = 0.549) (Fig. 3 and Table 1). Both allele classes of APOLLO spanned nearly the entire geographic distribution of the total sample, which has a latitude range from 31°34'N to 72°46'N and a longitude range from −50°28'W to −151°22'W. Thereby, sexuals ranged from 31°34'N, −91°12'W to 72°46'N, −56°10'W, and apomicts ranged from 32°2'N, −115°54'W to 69°40'N, −50°28'W (Fig. 3, Fig. S2, and Table S3).
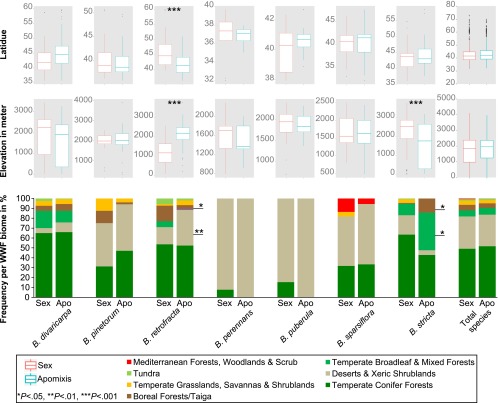
Fig. 3.
Species-specific variation of niche occupation and niche partitioning between sexual and apomictic Boechera in a subset of species where statistical comparisons could be made. In some species, apomixis is constrained to a subset of climates (e.g., B. retrofracta) whereas, in others, apomixis is found across the entire ecological niche (e.g., B. divaricarpa). Asterisks denote significant differences between distribution of sexual and apomictic Boechera based on two-tailed Fisher’s exact tests (α = 0.05; *P < 0.05; **P < 0.01, ***P < 0.001).
Table 1.
Widespread niche conservation between reproductive modes, and isolation-by-ploidy niche differentiation within and among species of Boechera
| Subset | Sex | Apo | Variables selected* | Fspat† | Pspat† | Feco‡ | Peco‡ | PPeco‡ | α§ |
| Subset “reproduction” | |||||||||
| All accessions¶ | 869 | 726 | Bio10, elevation | 0.32 | 0.5498 | 0.59 | 0.1615 | 0.1593 | 0.0500 |
| All 2׶ | 269 | 125 | Bio2, bio6, bio8, bio9, bio11, bio12, bio19, elevation | 2.86 | 0.0789 | 2.94 | 0.0145 | 0.0129 | 0.0250 |
| All 3׶ | 22 | 101 | Bio14, bio16 | 0.02 | 0.8647 | 2.00 | 0.2398 | 0.2405 | 0.0250 |
| Boechera collinsii | 8 | 9 | Bio3, elevation | 1.94 | 0.1613 | 0.51 | 0.3015 | 0.3086 | 0.0026 |
| Boechera crandallii | 26 | 7 | Bio3, bio16 | 17.74 | 0.0002 | 6.34 | 0.0177 | 0.0200 | 0.0026 |
| Boechera divaricarpa¶ | 46 | 158 | Bio4, bio14, bio18 | 0.39 | 0.5236 | 1.79 | 0.1579 | 0.1554 | 0.0026 |
| Boechera fendleri | 8 | 8 | Bio3, bio9 | 0.27 | 0.5884 | 2.45 | 0.1330 | 0.1324 | 0.0026 |
| Boechera lemmonii | 23 | 7 | Bio2, bio5, bio7, elevation | 2.04 | 0.1618 | 3.50 | 0.0080 | 0.0093 | 0.0026 |
| Boechera lyallii | 14 | 5 | Bio8, bio18 | 0.67 | 0.4204 | 0.05 | 0.8187 | 0.8164 | 0.0026 |
| Boechera microphylla | 8 | 27 | Bio6, bio11 | 1.08 | 0.3161 | 5.82 | 0.0108 | 0.0105 | 0.0026 |
| Boechera pallidifolia¶ | 16 | 19 | Bio5, bio8 | 0.10 | 0.7508 | 1.94 | 0.1662 | 0.1647 | 0.0026 |
| Boechera pauciflora | 6 | 18 | Bio4, bio9, bio10 | 1.32 | 0.2665 | 1.42 | 0.2516 | 0.2503 | 0.0026 |
| Boechera pendulocarpa | 32 | 8 | Bio16, elevation | 8.57 | 0.0074 | 2.92 | 0.0799 | 0.0719 | 0.0026 |
| Boechera perennans¶ | 20 | 11 | Bio3, bio18 | 0.11 | 0.7428 | 0.53 | 0.5492 | 0.5456 | 0.0026 |
| Boechera pinetorum¶ | 20 | 57 | Bio3, bio14, bio17 | 0.38 | 0.5571 | 1.27 | 0.2539 | 0.2582 | 0.0026 |
| Boechera puberula¶ | 30 | 17 | Bio5, bio9 | 0.49 | 0.4937 | 1.82 | 0.1619 | 0.1627 | 0.0026 |
| Boechera retrofracta (all)¶ | 97 | 158 | Bio2, bio3, elevation | 34.22 | 0.0001 | 24.35 | 0.0001 | 0.0001 | 0.0026 |
| Boechera retrofracta (2×)¶ | 30 | 27 | Bio2, bio3, elevation | 7.25 | 0.0074 | 14.30 | 0.0001 | 0.0002 | 0.0026 |
| Boechera sparsiflora¶ | 25 | 20 | Bio5, bio8, bio12, bio18, bio19 | 0.67 | 0.4087 | 0.68 | 0.5215 | 0.5189 | 0.0026 |
| Boechera spatifolia | 8 | 11 | Bio15, bio19 | 1.97 | 0.1453 | 8.14 | 0.0131 | 0.0113 | 0.0026 |
| Boechera stricta¶ | 182 | 32 | Bio16, bio18 | 14.05 | 0.0003 | 0.16 | 0.8313 | 0.8291 | 0.0026 |
| Boechera williamsii | 8 | 15 | Bio2, elevation | 5.18 | 0.0199 | 14.67 | 0.0010 | 0.0008 | 0.0026 |
| Subset “ploidy” | 2x | 3x | |||||||
| All¶ | 414 | 123 | Bio3, bio4, bio5, bio11, bio15, bio18 | 0.15 | 0.6877 | 0.30 | 0.5885 | 0.0144 | 0.0500 |
| Sex¶ | 269 | 22 | Bio1, bio11, bio14 | 0.49 | 0.4668 | 6.97 | 0.0052 | 0.0177 | 0.0250 |
| Apo¶ | 125 | 101 | Bio4, bio18 | 0.08 | 0.7725 | 0.06 | 0.8132 | 0.0379 | 0.0250 |
| Boechera collinsii | 3 | 3 | Bio4, bio6 | 7.84 | 0.0974 | 31.10 | 0.0129 | 0.0108 | 0.0063 |
| Boechera divaricarpa | 3 | 35 | Bio4, bio9, bio19 | 3.10 | 0.0748 | 2.87 | 0.0968 | 0.0063 | 0.0063 |
| Boechera lignifera | 4 | 3 | Bio7, bio8 | 0.07 | 0.8595 | 12.18 | 0.0285 | 0.0293 | 0.0063 |
| Boechera pallidifolia | 22 | 3 | Bio2, bio17 | 0.43 | 0.4872 | 11.58 | 0.0008 | 0.0009 | 0.0063 |
| Boechera retrofracta | 57 | 35 | Bio3, elevation | 16.82 | 0.0001 | 17.85 | 0.0001 | 0.0001 | 0.0063 |
| Boechera retrofracta (apo)¶ | 27 | 35 | Bio3, elevation | 5.71 | 0.0217 | 5.13 | 0.0043 | 0.0049 | 0.0063 |
| Boechera spatifolia | 16 | 3 | Bio4, bio18 | 1.21 | 0.3187 | 0.63 | 0.3567 | 0.3462 | 0.0063 |
| Boechera stricta × spatifolia (apo) | 10 | 5 | Bio4, bio5 | 0.62 | 0.6798 | 51.04 | 0.0001 | 0.0001 | 0.0063 |
Bio1, annual mean temperature; bio2, mean monthly temperature range; bio3, isothermality; bio4, temperature seasonality; bio5, maximum temperature of warmest month; bio6, minimum temperature of coldest month; bio7, temperature annual range; bio8, mean temperature of wettest quarter; bio9, mean temperature of driest quarter; bio10, mean temperature of warmest quarter; bio11, mean temperature of coldest quarter; bio12, annual precipitation; bio13, precipitation of wettest month; bio14, precipitation of driest month; bio15, precipitation seasonality; bio16, precipitation of wettest quarter; bio17, precipitation of driest quarter; bio18, precipitation of warmest quarter; bio19, precipitation of coldest quarter.
Post hoc permutation test for CCA on geographic distances to detect differences in spatial patterns between each sample group. Significant differences are shown in bold.
Post hoc permutation test for CCA on ecological variables (bio1 to 19, elevation) without (Peco) and with spatial covariate (partial Peco). Significant values are shown in bold.
Bonferroni corrected threshold for partial P values assuming α* ≈ α/M with M = number of independent tests.
To determine the ecological niche of each APOLLO allele class, we assessed the values of 19 bioclimatic variables (www.worldclim.org/) and elevation for each accession. Random forest classification was used to select variables based on their importance for each model (Table 1). On the genus-wide scale, there was no signature of ecological-niche differentiation between sexuals and apomicts (PPeco = 0.159) (Figs. 3 and 4 A and C and Table 1), with both reproductive modes typically found in temperate conifer forests and desert/xeric shrublands (82.96% and 84.87% respectively) (Fig. 3, Dataset S1, and Table S4). Sexuals and apomicts were found in similar mean annual temperatures (apomicts, lower quartile = 2 °C, upper quartile = 6 °C; sexuals, lower quartile = 2 °C, upper quartile = 7 °C), annual precipitation (apomicts, 356–603 mm, sexuals, 336–643 mm), and elevation (apomicts, 1,402–2,520 m; sexuals, 1,126–2,469 m) (Fig. 3 and Table S3). No geographic differentiation was found between ploidy classes across the entire sample (Pspat = 0.687) (Table 1). However, diploid genotypes had climatic distributions that were different from those of triploids (PPeco = 0.014) (Table 1).
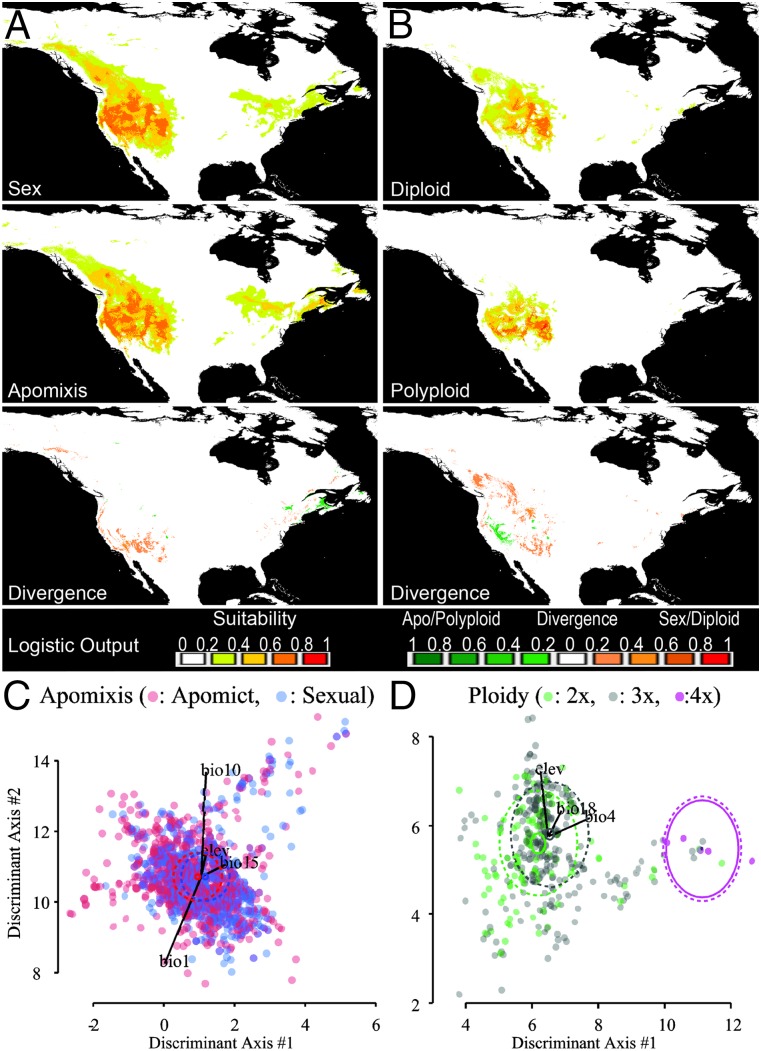
Fig. 4.
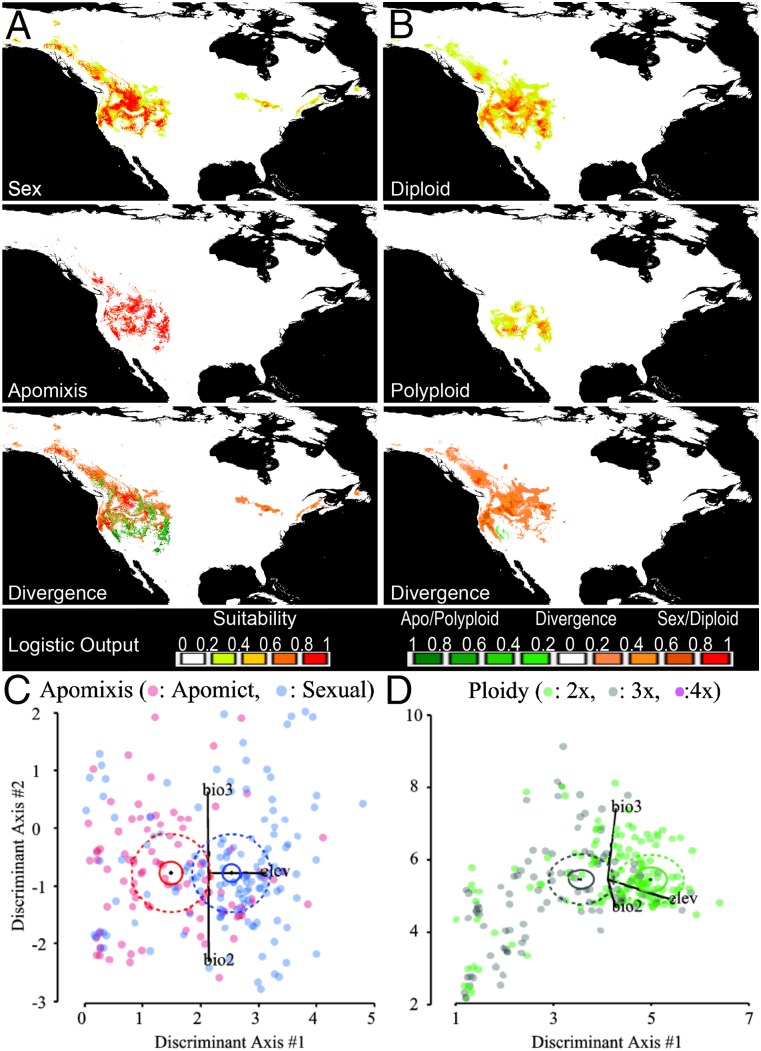
Lack of niche differentiation on genus-wide level. Across all Boechera species, there is no evidence of an association between niche differentiation and reproductive-mode divergence (A and C), but significant evidence between diploids and triploids (B and D). Strong differentiation between tetraploidy and all other ploidy levels is observed although this result is based on only six observations. The Upper panels show Maxent predictive ecological models of Boechera accessions. Habitat suitability is represented using different colors from low (green) to high (red). Strength of distribution differences is displayed for the surplus of apomicts or polyploids (shades of green) and the surplus of sexuals or diploids (shades of red). The Lower panels depict constrained discriminant function score distributions, where dashed ellipses represent 50% normal intervals and solid black vectors represent the scaled direction and effect of the labeled explanatory variables.
Comparisons between reproductive mode and ploidy class independently revealed genus-wide evidence of ecological-niche differentiation between sexual and apomictic genotypes, independent of ploidy (PPeco = 0.013) (Table 1). Within apomicts, diploids and triploids displayed different ecological distributions (PPeco = 0.037) (Fig. 4 B and D and Table 1). Combined, these results point to a weak, but significant, pattern of GP for both ploidy and reproductive mode across Boechera.
Ploidy and Reproduction Independently Influence Niche Partitioning in Boechera.
Lack of niche differentiation at the genus level could be an artifact of among-species apomixis-independent divergence (Fig. 3). Alternatively, niche conservation may reflect extended periods of sympatry between sexuals and apomicts, as observed for other agamic complexes (e.g., Taraxacum officinale) (45). We thus tested the effect of both reproductive mode and ploidy separately on within-species niche variation. Across the 84 Boechera species, we had sufficient replication within each of the three ploidy classes and two reproductive modes to conduct within-species tests for 7 and 18 species, respectively.
There was no evidence for significant geographic divergence for 6 of 7 species at the ploidy level, and for 15 of 18 species at the reproductive-mode level (Table 1). This pattern was bolstered by a paired Student’s t test demonstrating that the geographic range areas of sexuals and apomicts within species were similar (r2 = 0.83, P < 0.0001; paired t test, df = 18, P = 0.342) (Table S3). We did detect geographic divergence between apomicts and sexuals in three species (B. crandallii, Pspat = 0.0002; B. retrofracta, Pspat = 0.0001; and B. stricta, Pspat = 0.0003) (Fig. 3, Table 1, and Fig. S3). Differences between ploidy levels were observed only in B. retrofracta (Pspat = 0.0001) (Table 1).
A combined CCA using niche models for each ploidy class or each reproductive-mode class per species in addition to spatial distribution as a covariate revealed significant local niche differentiation between sexuals and apomicts in 2 of 18 species (e.g., B. retrofracta) (Fig. 5 A and C and Table 1). A within-species test of the independent effects of allele and ploidy classes in 57 diploid sexual and 27 diploid apomictic B. retrofracta accessions confirmed niche differentiation between reproductive modes in diploids (PPeco = 0.0002) (Fig. 5 A and C and Table 1). Additionally, comparisons between apomictic accessions also revealed significant niche differentiation between ploidy levels (PPeco = 0.0049) (Fig. 5 B and D, Fig. S4, and Table 1). On the species level (i.e., for B. retrofracta), apomictic diploids had a wider ecological-niche distribution compared with apomictic polyploids whereas, at the genus-wide scale, the trend was opposite (Fig. S4). This observation points to varying directions of niche differentiation among species. Overall, local niche differentiation with ploidy as cofactor (4 of 7 species) (Table 1) occurred significantly more frequently than with reproductive-mode divergence (2 of 18 species, Fisher’s exact test, P = 0.032) (Table 1).
Fig. 5.
Additive effects of ploidy and reproductive mode for niche partitioning in B. retrofracta. Within B. retrofracta, significant differentiation among reproductive mode (A and C) and, to a much stronger degree, ploidy (B and D) is observed. The Upper panels show Maxent predictive ecological models of different accessions. Habitat suitability is represented by different colors from low (green) to high (red). Strength of distribution differences is displayed for the surplus of apomicts or polyploids (shades of green) and the surplus of sexuals or diploids (shades of red). The Lower panels depict constrained discriminant function score distributions, where dashed ellipses represent 50% normal intervals and solid black vectors represent the scaled direction and effect of the labeled explanatory variables.
Discussion
APOLLO and UPGRADE2 Are Linked and Conserved in Apomicts.
We used quantitative analyses of the penetrance of apomictic seed formation and a large-scale screening of apomictic seed formation in a variety of Boechera taxa (Dataset S1) to demonstrate that presence of the female apomeiosis-linked allele of the APOLLO gene (39) can be used as a proxy marker for apomixis. A parallel analysis of the same samples for the presence of the male-apomeiosis allele UPGRADE2 (40) demonstrated a weaker positive correlation with apomictic seed production (Dataset S1). These results imply segregation between the UPGRADE2 and APOLLO alleles and are consistent with the fact that not all apomictic genotypes produce unreduced pollen (40, 46). For example, APOLLO and UPGRADE2 are unlinked in some accessions of Boechera microphylla (31.82%) (Fig. 1B), which could explain the absence of unreduced pollen in some apomictic accessions (47).
We used the apomeiosis-linked APOLLO allele to screen for the potential for apomictic seed production in a large number of herbarium accessions (n = 1,373; taken from ref. 20) for which no seed material existed. These results demonstrated that (i) some taxa that were previously classified as purely sexual [e.g., B. stricta (48) and B. crandallii (49)] are likely to contain apomicts (Fig. 1A and Table S1) and (ii) taxa formerly considered as purely apomictic [e.g., B. divaricarpa (37, 48)] are likely to be characterized by both sexual and apomictic members (Fig. 1A and Table S1). Our demonstration that the majority of tested taxa (93.55% of the 31 Boechera species with n ≥ 10 accessions) (Table S1) contain both sexual and apomictic members is consistent with recent taxonomic reassessments of Boechera, whereby morphological differences between sexual and hybrid apomictic members of a species are considered as significant characters for taxon subdivision (see the Flora of North America (Vol. 7) website, floranorthamerica.org/). Nonetheless, our data imply that morphological divergence has not yet been accompanied by niche differentiation (as measured here) between apomicts and sexuals in the majority of tested species (88.89%) (Table 1). Considering the already established complex influences of adaptation, hybridization, and polyploidy on the morphological evolution of Boechera, for example with previously observed variability in relative levels of meiotically reduced and unreduced pollen even among obligate apomicts (40), it is not surprising that our ability to resolve ploidy and/or reproduction-associated effects relied upon species-level rather than genus-level comparisons.
The high frequency of phylogenetically and geographically distant taxa in which the apomixis-specific alleles of APOLLO or UPGRADE2 were linked in a subset of their individuals (96.43%) (Dataset S1), in conjunction with their conserved polymorphisms and complex DNA sequences (39, 40) and the fact that each allele was discovered in an independent experiment (39, 40), implies that each allele is part of, or is tightly linked to, the genetic networks leading to apomeiotic egg and pollen formation.
The cooccurrence of apomixis-specific alleles of both the APOLLO and UPGRADE2 genes across the majority of Boechera taxa (Table S1) is indicative of either their independent origins, followed by complementation through hybridization, or common ancestry with regard to their origin. Our data more strongly support the former by the fact that the 5′ UTR polymorphism that defines the apomictic APOLLO allele predates the origin of the genus Boechera (39). In contrast to the apomictic APOLLO allele, the apomictic UPGRADE2 allele was only found in two single accessions among 2 of 14 species tested, belonging to a more broadly defined genus Boechera (e.g., Boechera laevigata) (50) or closely related genera (i.e., Cusickiella) while not being detected in distant plant taxa (e.g., Arabidopsis and Brassica) (Dataset S1, Table S1, and GenBank nucleotide collection, www.ncbi.nlm.nih.gov/genbank/, release 205.0).
Similar Haplotype Diversity in Sexuals and Apomicts Mirrors Reticulate Spread of Apomixis Alleles.
Hybridization can be considered as a potential inducer of apomixis (19). Intra- and interspecific gene flow from apomicts to sexuals via apomictic pollen is possible (48) and likely facilitated the horizontal transfer of apomixis across Boechera (43, 51). Nevertheless, in Boechera, hybridization and apomixis are closely (47) but not exclusively (43) associated. Thus, if hybridization per se is not the induction mechanism of apomixis in Boechera, our data together imply that (i) APOLLO and UPGRADE2 arose independently of one another in different Boechera species/populations and (ii) these apomixis alleles were brought together via hybridization between plants carrying APOLLO and/or UPGRADE2, which facilitated the transfer of both alleles into different sexual genetic backgrounds (i.e., species), leading to the reticulate phylogeographic pattern shown here (Figs. 2–4, Table 1, Fig. S3, and Tables S3 and S4).
Widespread hybridization (44, 48) reflects similar apomixis frequencies across the major cpDNA-haplotype lineages (lineage I = 54.26%, lineage II = 47.74%, and lineage III = 50.64% apomixis) (Figs. 1B and 2 and Tables S2 and S4), corroborates previous findings of high genotypic diversity in other agamic complexes (e.g., T. officinale) (52), and is furthermore supported by a computational study (53). It remains unclear whether the observed phylogenetic and geographic cooccurrence of apomixis-specific alleles is due to multiple independent introgressions of both alleles, or a single introgression event involving both alleles followed by dispersion of the linked alleles via hybridization throughout the genus.
Niche Conservation Between Reproductive Modes, Isolation-By-Ploidy, and Occasional Niche Drift Between Apomicts and Their Sexual Ancestors.
Seen from a genus-wide level, apomicts and sexuals share similar habitats and climatic limits (Figs. 3 and 4 A and C and Table 1), an observation that could be explained by the spread of apomixis into different sexual backgrounds in an “infectious” fashion (sensu ref. 54). A dynamic equilibrium between generation and neutral loss of asexual lineages (55) could thus have led to the broad niche conservation and lack of GP in Boechera, in contrast to other agamic complexes (e.g., Ranunculus auricomus complex) (56).
Despite the decreased statistical power of species-level analyses due to our inability to infer and test multiple populations per species from the herbarium dataset, these analyses were still able to resolve divergent patterns. Species-specific niche occupation (Fig. 3, Fig. S3, and Table S3) reflects the divergence and adaptation processes that characterize the evolutionary success of Boechera (34). However, there is also significant local adaptation within Boechera species [e.g., B. stricta (57) and B. spatifolia (58)] that seems to be associated with reproductive mode, which covaried with niche occupation in 2 of 18 species with n ≥ 5 accessions per reproductive mode (Fig. 5 A and C and Table 1). Importantly, these niche differences were present despite identical ploidy levels (see B. retrofracta and Boechera williamsii) (Table 1).
Niche conservation between reproductive modes in the majority of the tested species (88.89%) (Table 1) could have a number of explanations. First, an ancestral and independently derived apomictic lineage may have evolved to occupy a similar niche as a particular sexual species (i.e., evolutionary convergence). This scenario is unlikely considering (i) multiple lines of evidence for repeated separate transitions from sex to apomixis in Boechera (36–38), (ii) that cpDNA haplotypes are distributed across multiple habitats, (iii) that cpDNA haplotypes are partially shared by sexual and apomictic accessions (24.63%, n = 203) (Tables S3 and S4), and (iv) that sexuals and apomicts display a similar range of genetic diversity as a reflection of their phylogenetic relationships (i.e., cpDNA haplotypes per individual) (Table S4) (59). Therefore, a more parsimonious scenario is favored whereby introgression of apomixis factors into different sexual backgrounds is accompanied by the establishment of independent apomixis lineages. Considering this, the observed niche conservation between sexual and apomictic conspecifics could be explained by the apomictic lineages being too young to have diverged (i.e., recently induced) (60) or that niche differentiation is not possible due to genetic constraints: for example, a genetic bottleneck having stronger effects on sexuals versus apomicts due to inbreeding (61).
Stronger patterns of GP in other agamic complexes could reflect the fact that apomicts in most other plant species are polyploids (reviewed in ref. 56; but see ref. 62) whereas diploid apomixis is relatively frequent in Boechera (i.e., apomixis frequency in diploids, 31.33%, n = 399; and in polyploids, 81.40%, n = 129) (Dataset S1). Ploidy variation, rather than reproductive-mode divergence, seems to be the common driver of niche differentiation (Table 1) although we cannot yet infer which specific aspect of polyploidy (e.g., genetic composition, genome size, or deleterious allele masking, etc.) accounts for the observed differences in niche occupation between diploids and polyploids. Reproductive isolation-by-ploidy between mostly diploid sexuals and polyploid apomicts in other agamic complexes is enhanced by reduced fertility in their hybrid progeny (48), reduced levels of backcrossing (e.g., in the R. auricomus complex) (63), and enhanced colonizing abilities for disturbed areas and species-range edges due to altered ecological tolerances (60, 64), processes that would equally explain the more common pattern of ploidy-driven niche divergence within and across Boechera species. Our inability to identify geographic range size variation between reproductive modes (Table S3), or through divergence between geographic distance (i.e., latitude levels) and niche specificity (Table 1), underlines the importance of multiparametric analyses for tests of GP between reproductive groups.
Conclusions
Here, we present, to our knowledge, the first evidence of isolation-by-ploidy and reproductive mode as independent forces of species-specific niche differentiation in an agamic complex. Together with species-specific habitat variation (Fig. 3 and Table S3), isolation-by-ploidy has a more ubiquitous effect than reproductive mode on niche differentiation across Boechera (Table 1). Niche conservation between reproductive modes within species is the most distinctive pattern observed (Table 1), and therefore we reject our initial hypothesis of niche evolution as an intrinsic factor of reproductive-mode divergence.
Our data alternatively support an extended frozen niche variation model to explain habitat differences between sexuals and apomicts. This model implies that niche occupation by apomicts reflects a subset of that of their parental sexual taxa, whereby apomictic progeny adopt the adaptive peak of sexuals to their ecological niche (31).
This extended model hinges upon multiple origins of the apomictic phenotype from different sexual backgrounds in Boechera. Introgression of apomixis alleles from apomictic diploids into different diploid sexual genotypes (43, 48) may have facilitated the enormous genetic diversity characteristic of apomictic Boechera (Table S4) (see also ref. 43). Our data demonstrate that apomictic Boechera for the most part drift into new niches by virtue of ploidy variation although evidence for occasional ploidy-independent niche drift was also found (e.g., Fig. 5 A and C, Table 1, and Fig. S3).
Therefore, the evolutionary success of apomictic Boechera seems to have been driven by a number of processes. First, we hypothesize that the ongoing hybridization-driven spread of apomixis alleles in an infectious manner into different sexual genetic backgrounds has led to the establishment of apomixis in different niches. In a second step, we believe that recurrent polyploidy mediated by the production of meiotically unreduced gametes has, through a yet-unknown mechanism, enabled polyploid apomicts to diverge into novel niches. In addition, ploidy-independent niche differentiation arising after reproductive-mode transition further complicates the signature of natural selection in wild populations. In this regard, analyses of comprehensive data matrices have enabled us to disentangle at least some determinants of niche differentiation in a mixed reproductive mode and have led us to question whether geographic parthenogenesis in plants is an exception rather than the rule.
Materials and Methods
Detailed methods can be found in SI Materials and Methods. Briefly, 1,649 accessions of Boechera and closely related genera were screened for apomixis by a PCR-based detection method of apomixis-specific polymorphisms in two independent genetic factors (39, 40). Phylogenetic distribution of apomixis in Boechera was plotted onto a haplotype network using the TCS 1.21 software (65) with a connection limit of 95% and the trnLF dataset from ref. 20. Maxent ecological-niche variation models (66) for constraining variables such as reproductive modes and ploidy were plotted with DIVA GIS version 7.5 (www.diva-gis.org/) using ecological parameters from the WorldClim database (67). Geographic and ecological distances between ploidy levels or between reproductive modes were evaluated with a stepwise constrained correspondence analysis (CCA) (68) using the R programming environment version 3.1.1 (69) and the vegan package version 2.0-10 (70), followed by permutations to test for significance (70).
Supplementary Material
Acknowledgments
We thank the reviewers for valuable comments on the manuscript. We also thank Heidi Block, Robert Jerchel, and Sheila Milker for technical support. We thank M. Neiman and D. Paczesniak for providing valuable comments on the manuscript. This study was supported by funding from the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) to the Apomixis Research Group at IPK.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423447112/-/DCSupplemental.
References
- 1.Candolin U, Heuschele J. Is sexual selection beneficial during adaptation to environmental change? Trends Ecol Evol. 2008;23(8):446–452. doi: 10.1016/j.tree.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Begon M, Wall R. Individual variation and competitor coexistence: A model. Funct Ecol. 1987;1(3):237–241. [Google Scholar]
- 3.Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc Natl Acad Sci USA. 1990;87(9):3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuellig MP, Kenney AM, Sweigart AL. Evolutionary genetics of plant adaptation: Insights from new model systems. Curr Opin Plant Biol. 2014;18:44–50. doi: 10.1016/j.pbi.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paquin CE, Adams J. Relative fitness can decrease in evolving asexual populations of S. cerevisiae. Nature. 1983;306(5941):368–370. doi: 10.1038/306368a0. [DOI] [PubMed] [Google Scholar]
- 6.Stebbins GL. Flowering Plants: Evolution Above the Species Level. Harvard Univ Press; Cambridge, MA: 1974. [Google Scholar]
- 7.Richardson JL, Urban MC, Bolnick DI, Skelly DK. Microgeographic adaptation and the spatial scale of evolution. Trends Ecol Evol. 2014;29(3):165–176. doi: 10.1016/j.tree.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Mayr E. Animal Species and Evolution. Harvard Univ Press; Cambridge, MA: 1963. [Google Scholar]
- 9.Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc Lond. 1997;61(1):51–94. [Google Scholar]
- 10.Harper AB. The selective significance of partial apomixis. Heredity. 1982;48(1):107–116. [Google Scholar]
- 11.Hanna WW, Bashaw EC. Apomixis: Its identification and use in plant breeding. Crop Sci. 1987;27(6):1136–1139. [Google Scholar]
- 12.Van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms: A reappraisal. In: Bakker F, Chatrou L, Gravendeel B, Pelser PB, editors. Plant Species-Level Systematics: New Perspectives on Pattern and Process. Gantner; Ruggell, Liechtenstein: 2005. pp. 101–116. [Google Scholar]
- 13.Noirot M, Couvet D, Hamon S. Main role of self-polination rate on reproductive allocations in pseudogamous apomicts. Theor Appl Genet. 1997;95(3):479–483. [Google Scholar]
- 14.Stebbins GL. Variation and Evolution in Plants. Columbia Univ Press; New York: 1950. [Google Scholar]
- 15.Van Dijk PJ. 2007. Potential and realized costs of sex in dandelions (Taraxacum officinale s.l.). Apomixis: Evolution, Mechanisms and Perspectives, eds Hörandl E, Grossniklaus U, Van Dijk P, Sharbel TF (Gantner, Ruggell, Liechtenstein)
- 16.Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6(11):836–846. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- 17.Muller HJ. The relation of recombination to mutational advance. Mutat Res. 1964;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- 18.Vielle-Calzada J-P, Crane CF, Stelly DM. Apomixis: The asexual revolution. Science. 1996;274(5291):1322–1323. [Google Scholar]
- 19.Carman JG. 1997. The gene effect: Genome collisions and apomixis. The Flowering of Apomixis: From Mechanisms to Genetic Engineering, eds Savidan Y, Carman JG, Dresselhaus T (International Maize and Wheat Improvement Center, IRD, European Commission DC VI (FAIR), Mexico City), Vol 15.
- 20.Kiefer C, Dobeš C, Sharbel TF, Koch MA. Phylogeographic structure of the chloroplast DNA gene pool in North American Boechera: A genus and continental-wide perspective. Mol Phylogenet Evol. 2009;52(2):303–311. doi: 10.1016/j.ympev.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Menken SBJ, Smit E, Den Nijs HJCM. Genetical population structure in plants: Gene flow between diploid sexual and triploid asexual dandelions (Taraxacum section Ruderalia) Evolution. 1995;49(6):1108–1118. doi: 10.1111/j.1558-5646.1995.tb04437.x. [DOI] [PubMed] [Google Scholar]
- 22.Pellino M, et al. Asexual genome evolution in the apomictic Ranunculus auricomus complex: Examining the effects of hybridization and mutation accumulation. Mol Ecol. 2013;22(23):5908–5921. doi: 10.1111/mec.12533. [DOI] [PubMed] [Google Scholar]
- 23.Vandel A. La parthénogenese geographique: Contribution a l’étude biologique et cytologique de la parthénogenese naturelle. Bull Biol Fr Belg. 1928;62:164–182. [Google Scholar]
- 24.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Univ of California Press; Berkeley, CA: 1982. [Google Scholar]
- 25.Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41(10):1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Fukuhara T, Huziwara Y. 1982. Studies on the Asian Eupatorias. I. Eupatorium chinense var. simplicifolium from the Rokko Mountains. Bot Mag (Tokyo) 95:261–280.
- 27.Vrijenhoek RC. Factors affecting clonal diversity and coexistence. Am Zool. 1979;19:787–797. [Google Scholar]
- 28.Baker HG. 1965. Characteristics and modes of origin of weeds. The Genetics of Colonizing Species, eds Baker HG, Stebbins GL (Academic, New York), pp 147–168.
- 29.Levin DA. Pest pressure and recombination systems in plants. Am Nat. 1975;109(968):437–451. [Google Scholar]
- 30.Lynch M. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q Rev Biol. 1984;59(3):257–290. [Google Scholar]
- 31.Vrijenhoek RC. 1984. Ecological differentiation among clones: The frozen niche variation model. Population Biology and Evolution, Proceedings in Life Sciences, eds Wöhrmann K, Loeschcke V (Springer, Heidelberg), pp 217–231.
- 32.Hörandl E. The complex causality of geographical parthenogenesis. New Phytol. 2006;171(3):525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- 33.Lundmark M, Saura A. Asexuality alone does not explain the success of clonal forms in insects with geographical parthenogenesis. Hereditas. 2006;143:23–32. doi: 10.1111/j.2006.0018-0661.01935.x. [DOI] [PubMed] [Google Scholar]
- 34.Rushworth CA, Song B-H, Lee C-R, Mitchell-Olds T. Boechera, a model system for ecological genomics. Mol Ecol. 2011;20(23):4843–4857. doi: 10.1111/j.1365-294X.2011.05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böcher TW. 1951. Cytological and embryologal studies in the amphiapomictic Arabis holboellii complex. Biologiske Skrifter/Kongelige Danske Videnskabernes Selskab 6(7):1–59.
- 36.Sharbel TF, Mitchell-Olds T. Recurrent polyploid origins and chloroplast phylogeography in the Arabis holboellii complex (Brassicaceae) Heredity (Edinb) 2001;87(Pt 1):59–68. doi: 10.1046/j.1365-2540.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 37.Kiefer C, Koch MA. A continental-wide perspective: The genepool of nuclear encoded ribosomal DNA and single-copy gene sequences in North American Boechera (Brassicaceae) PLoS ONE. 2012;7(5):e36491. doi: 10.1371/journal.pone.0036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aliyu OM, Seifert M, Corral JM, Fuchs J, Sharbel TF. Copy number variation in transcriptionally active regions of sexual and apomictic Boechera demonstrates independently derived apomictic lineages. Plant Cell. 2013;25(10):3808–3823. doi: 10.1105/tpc.113.113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corral JM, et al. A conserved apomixis-specific polymorphism is correlated with exclusive exonuclease expression in premeiotic ovules of apomictic boechera species. Plant Physiol. 2013;163(4):1660–1672. doi: 10.1104/pp.113.222430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mau M, et al. The conserved chimeric transcript UPGRADE2 is associated with unreduced pollen formation and is exclusively found in apomictic Boechera species. Plant Physiol. 2013;163(4):1640–1659. doi: 10.1104/pp.113.222448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000;21(1):97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- 42.Windham MD, Al-Shehbaz IA. New and noteworthy species of Boechera (Brassicaceae) III: Additional sexual diploids and apomictic hybrids. Harv Pap Bot. 2007;12(1):235–257. [Google Scholar]
- 43.Lovell JT, et al. On the origin and evolution of apomixis in Boechera. Plant Reprod. 2013;26(4):309–315. doi: 10.1007/s00497-013-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobeš CH, Mitchell-Olds T, Koch MA. Extensive chloroplast haplotype variation indicates Pleistocene hybridization and radiation of North American Arabis drummondii, A. x divaricarpa, and A. holboellii (Brassicaceae) Mol Ecol. 2004a;13(2):349–370. doi: 10.1046/j.1365-294x.2003.02064.x. [DOI] [PubMed] [Google Scholar]
- 45.Verduijn MH, Van Dijk PJ, Van Damme JMM. Distribution, phenology and demography of sympatric sexual and asexual dandelions (Taraxacum officinale s.l.): Geographic parthenogenesis on a small scale. Biol J Linn Soc Lond. 2004;82(2):205–218. [Google Scholar]
- 46.Aliyu OM, Schranz ME, Sharbel TF. Quantitative variation for apomictic reproduction in the genus Boechera (Brassicaceae) Am J Bot. 2010;97(10):1719–1731. doi: 10.3732/ajb.1000188. [DOI] [PubMed] [Google Scholar]
- 47.Beck JB, et al. Does hybridization drive the transition to asexuality in diploid Boechera? Evolution. 2012;66(4):985–995. doi: 10.1111/j.1558-5646.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 48.Schranz ME, Dobeš C, Koch MA, Mitchell-Olds T. Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae) Am J Bot. 2005;92(11):1797–1810. doi: 10.3732/ajb.92.11.1797. [DOI] [PubMed] [Google Scholar]
- 49.Roy BA. The breeding systems of six species of Arabis (Brassicaceae) Am J Bot. 1995;82(7):869–877. [Google Scholar]
- 50.Kiefer C, Dobeš C, Koch MA. Boechera or not? Phylogeny and phylogeography of eastern North American Boechera species (Brassicaceae) Taxon. 2009;58(4):1109–1121. [Google Scholar]
- 51.Dobeš C, Sharbel TF, Koch M. Towards understanding the dynamics of hybridization and apomixis in the evolution of the genus Boechera (Brassicaceae) Syst Biodivers. 2007;5(3):321–331. [Google Scholar]
- 52.Lyman JC, Ellstrand NC. Clonal diversity in Taraxacum officinale (compositae), an apomict. Heredity. 1984;53(1):1–10. [Google Scholar]
- 53.Adolfsson S, Bengtsson BO. The spread of apomixis and its effect on resident genetic variation. J Evol Biol. 2007;20(5):1933–1940. doi: 10.1111/j.1420-9101.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- 54.Spillane C, Curtis MD, Grossniklaus U. Apomixis technology development: Virgin births in farmers’ fields? Nat Biotechnol. 2004;22(6):687–691. doi: 10.1038/nbt976. [DOI] [PubMed] [Google Scholar]
- 55.Janko K, Drozd P, Flegr J, Pannell JR. Clonal turnover versus clonal decay: A null model for observed patterns of asexual longevity, diversity and distribution. Evolution. 2008;62(5):1264–1270. doi: 10.1111/j.1558-5646.2008.00359.x. [DOI] [PubMed] [Google Scholar]
- 56.Hörandl E, Paun O. 2007. Patterns and sources of genetic diversity in apomictic plants: Implications for evolutionary potentials. Apomixis: Evolution, Mechanisms and Perspectives, eds Hörandl E, Grossniklaus U, Van Dijk P, Sharbel TF (Gantner, Ruggell, Liechtenstein), pp 169–194.
- 57.Anderson JT, Lee C-R, Mitchell-Olds T. Life-history QTLS and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution. 2011;65(3):771–787. doi: 10.1111/j.1558-5646.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovell JT, Grogan K, Sharbel TF, McKay JK. Mating system and environmental variation drive patterns of adaptation in Boechera spatifolia (Brassicaceae) Mol Ecol. 2014;23(18):4486–4497. doi: 10.1111/mec.12879. [DOI] [PubMed] [Google Scholar]
- 59.Mogie M. The Evolution of Asexual Reproduction in Plants. Chapman & Hall; London: 1992. [Google Scholar]
- 60.Stebbins GL. Chromosomal Evolution in Higher Plants. Edward Arnold; London: 1971. [Google Scholar]
- 61.Haag CR, Ebert D. A new hypothesis to explain geographic parthenogenesis. Ann Zool Fenn. 2004;41(4):539–544. [Google Scholar]
- 62.Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol Evol. 2005;20(9):495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Paun O, Greilhuber J, Temsch EM, Hörandl E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae) Mol Ecol. 2006;15(4):897–910. doi: 10.1111/j.1365-294X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- 64.Mogie M. A model for the evolution and control of generative apomixis. Biol J Linn Soc Lond. 1988;35(2):127–153. [Google Scholar]
- 65.Clement M, Posada D, Crandall KA. TCS: A computer program to estimate gene genealogies. Mol Ecol. 2000;9(10):1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 66.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190(3–4):231–259. [Google Scholar]
- 67.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 68.Legendre P, Legendre L. 1998. Numerical Ecology (Elsevier, Amsterdam), 2nd English Ed.
- 69.R Development Core Team 2008. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing Vienna)
- 70.Oksanen J, et al. 2008 Vegan: Community Ecology Package. R package version 2.0-10. Available at vegan.r-forge.r-project.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.