Significance
All mammals, from platypuses to humans, produce relatively immature offspring that are wholly dependent on their mother’s milk for their postnatal growth and development. However, the dynamic signaling and molecular mechanisms responsible for the transport of key constituents (e.g., calcium) into milk and for alveolar unit contraction and milk ejection are not fully understood. Using genetically modified mouse models, we demonstrate that the store-operated Ca2+ channel Orai1 delivers over 50% of the calcium ions present in milk. We also reveal an unanticipated role of Orai1 as a master regulator of oxytocin-mediated alveolar unit contractility, milk ejection, and pup survival. These results provide a unique mechanistic insight into the fundamentally mammalian process of lactation.
Keywords: calcium signaling, calcium channels, lactation, mammary gland, store-operated calcium entry
Abstract
The nourishment of neonates by nursing is the defining characteristic of mammals. However, despite considerable research into the neural control of lactation, an understanding of the signaling mechanisms underlying the production and expulsion of milk by mammary epithelial cells during lactation remains largely unknown. Here we demonstrate that a store-operated Ca2+ channel subunit, Orai1, is required for both optimal Ca2+ transport into milk and for milk ejection. Using a novel, 3D imaging strategy, we visualized live oxytocin-induced alveolar unit contractions in the mammary gland, and we demonstrated that in this model milk is ejected by way of pulsatile contractions of these alveolar units. In mammary glands of Orai1 knockout mice, these contractions are infrequent and poorly coordinated. We reveal that oxytocin also induces a large transient release of stored Ca2+ in mammary myoepithelial cells followed by slow, irregular Ca2+ oscillations. These oscillations, and not the initial Ca2+ transient, are mediated exclusively by Orai1 and are absolutely required for milk ejection and pup survival, an observation that redefines the signaling processes responsible for milk ejection. These findings clearly demonstrate that Ca2+ is not just a substrate for nutritional enrichment in mammals but is also a master regulator of the spatiotemporal signaling events underpinning mammary alveolar unit contraction. Orai1-dependent Ca2+ oscillations may represent a conserved language in myoepithelial cells of other secretory epithelia, such as sweat glands, potentially shedding light on other Orai1 channelopathies, including anhidrosis (an inability to sweat).
Mammary alveoli are comprised of two distinct epithelial cell types—an inner layer of alveolar luminal cells, which selectively extract nutrients from the maternal circulation for secretion into milk, and a meshwork of myoepithelial cells on the basal surface that are responsible for generating the contractile force necessary for milk ejection (1–3). The highly regulated passage of Ca2+ into milk by luminal epithelial cells during lactation implies the coordinated involvement of various Ca2+ channels, pumps, and calcium-sensing proteins (4, 5). A role for the plasma membrane Ca2+ ATPase 2 (PMCA2) isoform in the direct pumping of Ca2+ across the apical membrane of mammary luminal cells has been unambiguously demonstrated in transgenic mice (6–10); however, other key elements in milk Ca2+ transport are not well defined, in particular the mechanism of Ca2+ entry into luminal cells from the maternal circulation. Orai1 is a store-operated Ca2+ channel whose expression in the mammary gland is increased during lactation (11). Here we have used two Orai1 deletion mouse models to examine the role of Orai1 channels in lactation. Our findings reveal critical roles for this channel, in both the transport of Ca2+ into milk and the ejection of milk during nursing.
Results and Discussion
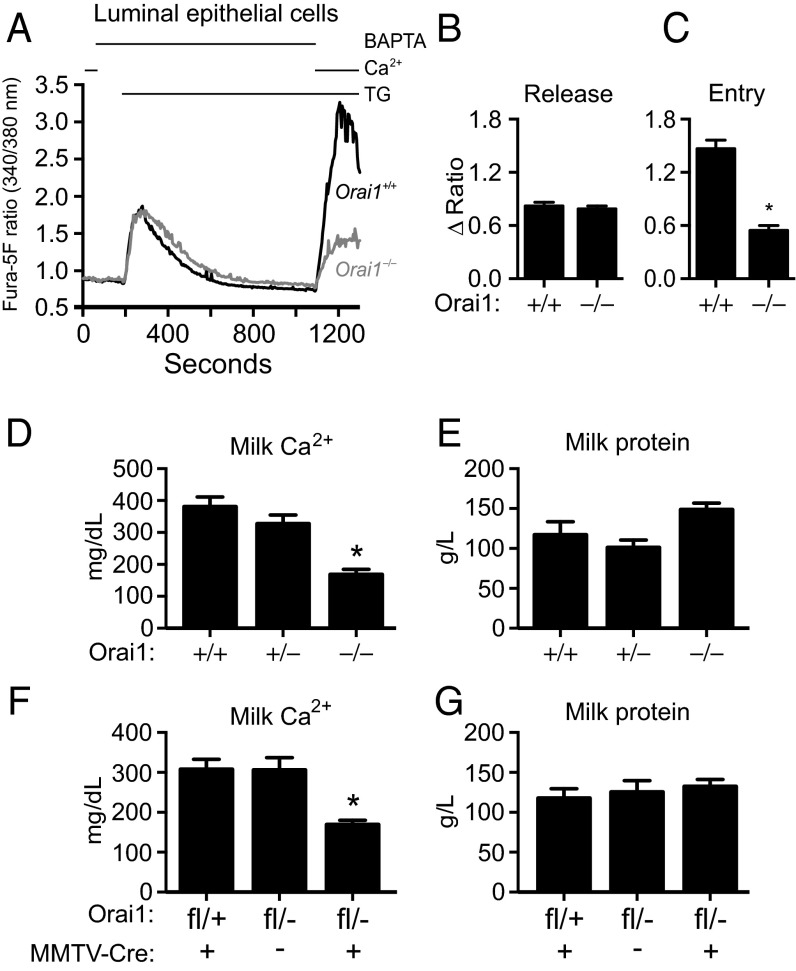
Gene expression of the store-operated calcium channel Orai1 increases in the mammary gland during lactation (11), and other expression studies and in vitro mammary models indirectly implicate a role for Orai1 in milk Ca2+ enrichment (12, 13). To directly determine if Orai1 is required for Ca2+ transport into milk during lactation, milk was collected from mice lacking Orai1 (Orai1−/−). These mice, generated by gene trap mutagenesis (14), showed more than 99% inhibition of Orai1 gene expression in the mammary gland (Fig. S1A), with no compensatory increase in the transcription of Orai2, Orai3, Stim1, or Stim2 (Fig. S1 B–E). Orai1 mRNA levels were significantly attenuated in both the milk-producing (luminal) epithelial cells (Fig. S1F) and contractile (myoepithelial) cells (Fig. S1G) of the mammary gland in Orai1−/− mice. To assess sites of Orai1 expression in the mammary gland, we exploited the β-galactosidase activity of the mutant fusion protein obtained by gene-trap (14). Orai1 expression was detected in both ducts and alveoli of the mammary gland (Fig. S1H). In addition to having significantly reduced Orai1 gene expression, thapsigargin (TG)-mediated store-operated Ca2+ entry (SOCE) was significantly attenuated in fura-5F–loaded luminal mammary epithelial cells isolated from Orai1−/− mice (Fig. 1 A–C). Residual Ca2+ entry in luminal Orai1−/− cells may be due to Ca2+ influx through Orai3 Ca2+ channels, which are regulated by the estrogen receptor-α (ERα) in breast cancer cell lines (15).
Fig. 1.
Orai1 KO mice have reduced milk Ca2+. (A) TG-induced Ca2+ entry in mammary luminal cells isolated from Orai1+/+ (n = 82 cells) and Orai1−/− (n = 86 cells) mice. Cells were bathed in nominally Ca2+-free HBSS supplemented with 1,2-bis(o-aminophenoxy)ethane-N,N,N′, N′-tetraacetic acid (BAPTA, 500 μM) for 2 min and treated with TG (2 μM) to deplete ER Ca2+ stores before Ca2+ readdition (2 mM). Peak ratio responses to (B) TG (150–270 s) and (C) Ca2+ readdition (1,000–1,180 s). Total Ca2+ and protein concentrations in milk collected from (D and E) lactating (day 3) Orai1−/− mice versus control genotypes and (F and G) mice with conditional deletion of Orai1 in the mammary epithelia (Orai1fl/−;MMTV-Cre) versus control genotypes (n = 3–4 mice). Data represent mean ± SEM; *P < 0.05, Student’s t test (B and C) or one-way ANOVA with Bonferroni posttests (D–G).
Total milk Ca2+ levels were measured on days 2 and 3 of lactation (Fig. 1D and Fig. S2A). The average milk Ca2+ concentration in Orai1+/+ dams on lactation day 3 was 380 mg/dL (∼95 mM). Milk Ca2+ was reduced by 55% in Orai1−/− mice (170 mg/dL, P < 0.05). Total milk protein and maternal serum Ca2+ levels were not reduced in Orai1−/− mice (Fig. 1E and Fig. S2 B and C), indicating that the reduction in milk Ca2+ in these mice was not merely a consequence of changes in the overall milk composition or the amount of Ca2+ available for transport into milk.
To confirm that reduced milk Ca2+ was not simply a consequence of altered Ca2+ handling in other tissues (e.g., intestinal Ca2+ absorption), we measured milk Ca2+ concentrations in mice with a conditional deletion of the Orai1 gene in the mammary gland (conditional Orai1 knockout, cKO). We confirmed that Orai1 was significantly decreased in the cKO mammary epithelial cells (Fig. S1 I–K). Total milk Ca2+ concentrations were significantly lower in cKO mice, whereas total milk protein levels were not reduced (Fig. 1 F and G and Fig. S2 D and E). Collectively, these data demonstrate that Orai1 has an important role in the transport of Ca2+ into milk during lactation across the basolateral membrane of luminal epithelial cells (13). Future studies could assess the role of sustained Orai1-mediated basolateral Ca2+ influx in the mammary gland during postlactational regression (involution), such as the implicated role of PMCA2 down-regulation during mammary gland involution (16).
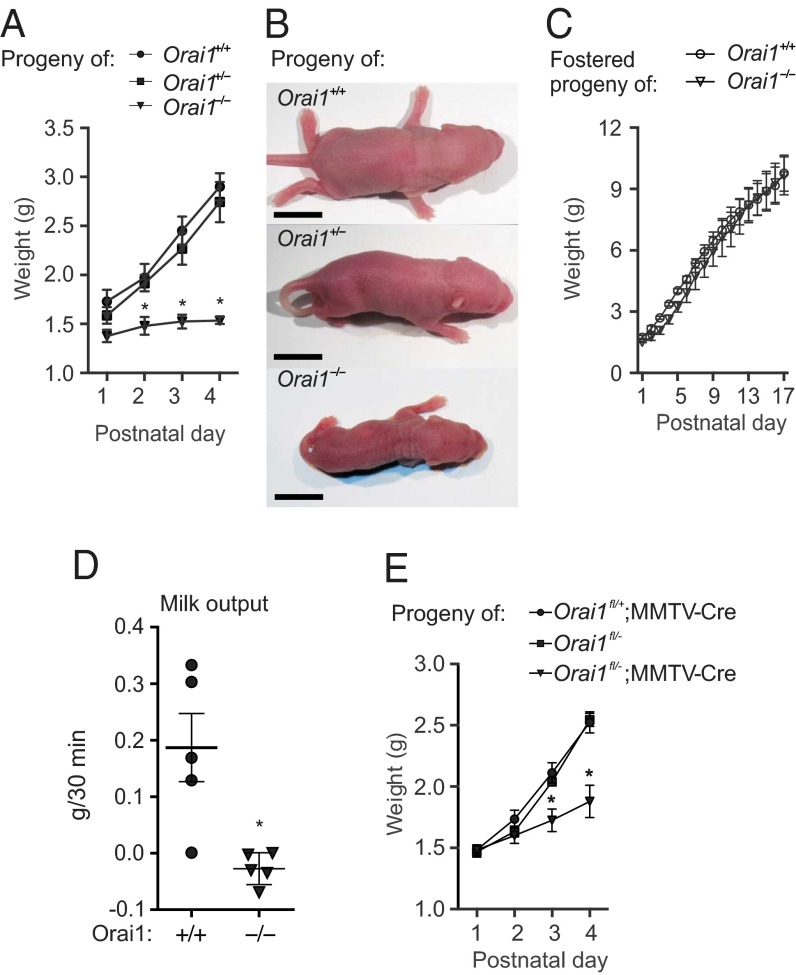
Orai1-deficient female mice were fertile and did not possess any overt gestational or parturition defects when mated to wild-type sires. Maternal nurturing behaviors were not altered in these mice, which built nests, readily retrieved pups that were removed from the nest, and were regularly observed allowing their pups to suckle. However, pups nursed by Orai1−/− mothers failed to thrive (Fig. 2 A and B). This is in sharp contrast to pups nursed by Orai1+/+ and Orai1+/− mothers, which exhibited a large (∼170%) increase in body weight between birth and postnatal day (PND) 4. Although Orai1−/− mice have a markedly reduced body size (14), the pups born to and nursed by Orai1−/− mothers are heterozygous for Orai1 and therefore were not predicted to have a growth-restricted phenotype. Moreover, failure to thrive in Orai1−/− litters was rescued by fostering newborn pups to lactating CD-1 foster mothers (Fig. 2C), indicating that this defect was not intrinsic to the pups.
Fig. 2.
Pups nursed by Orai1 KO mice fail to thrive. (A) Average weight of pups born to and nursed by Orai1−/− or control mothers (n = 3 litters), and (B) representative images of PND 4 pups from each litter. (Scale bar, 10 mm.) (C) Average weight of pups from Orai1+/+ and Orai1−/− litters fostered on PND 1 to lactating CD-1 foster mothers (n = 2 litters). (D) Milk output in Orai1+/+ and Orai1−/− dams (n = 5 mice). (E) Average weight of pups nursed by control mice and mice with conditional deletion of Orai1 in the mammary epithelium (Orai1fl/−;MMTV-Cre, n = 3–4 litters). Data represent mean ± SEM; *P < 0.05, two-way ANOVA with Bonferroni posttests (A and E) or Student’s t test (D).
These observations suggest that Orai1−/− mice have a further defect in lactation, beyond compromised enrichment of milk with Ca2+. Consistent with this theory was the observation that pups nursed by Orai1−/− mothers lacked visible milk spots in their stomachs (Fig. S3). To further verify the lactation defect in Orai1−/− mice, we obtained a timed milk volume estimate (Materials and Methods) in Orai1+/+ and Orai1−/− dams. Milk output was significantly higher in Orai1+/+ versus Orai1−/− dams (Fig. 2D). Pups nursed by cKO mice also showed significant runting on PND 3 and 4 (Fig. 2E). Collectively, our results reveal that mice lacking Orai1 in the mammary gland have a major defect in lactation, not only at the transport level for milk Ca2+ enrichment but also at the signaling level via a strong defect in milk expulsion and/or production.
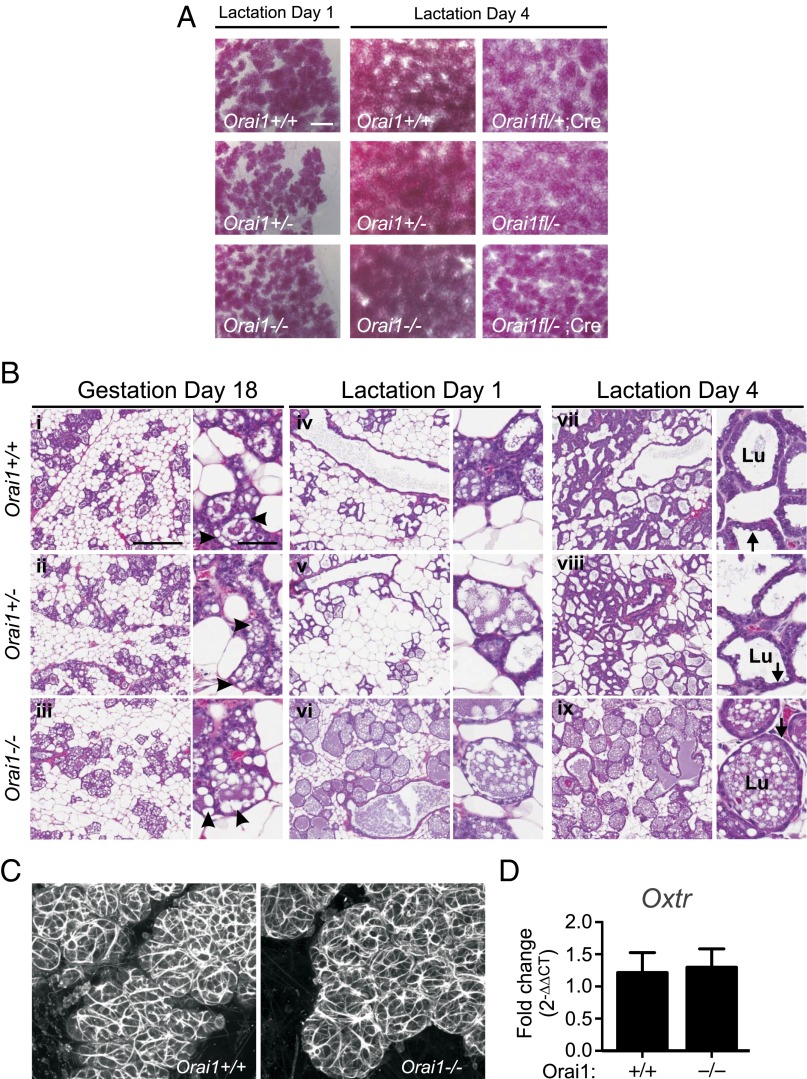
To identify the underlying cause for the lactation defect in Orai1 KO mice, we assessed mammary gland morphology with whole mounts and histological sections. In all genotypes, the ductal tree extended to the limits of the mammary fat pad and formed alveoli, which continued to proliferate in the days following parturition (Fig. 3A). These results indicate that expression of Orai1 is not essential for normal mammary gland development. In addition, the decrease in large cytoplasmic lipid droplets in luminal epithelial cells between late gestation (Fig. 3 B, i–iii, arrowheads) and early parturition (Fig. 3 B, iv–vi and vii–ix) is consistent with their secretion into milk (17) and suggests that secretory activation in the mammary gland also occurs independently of Orai1. Although the structural development and secretory capacity of the mammary gland were not grossly affected in Orai1-deficient mice, we observed clear differences in the appearance of alveoli with hematoxylin and eosin (H&E) staining during lactation (Fig. 3 B, vii–ix). Specifically, mammary glands from Orai1−/− mice exhibited alveolar dilation (Fig. 3 B, vii and viii vs. ix) (Lu, alveolar lumen), thinning of the secretory epithelium (Fig. 3 B, vii and viii vs. ix, arrows), and intense staining of milk proteins that remained trapped in ducts and alveolar lumens (Fig. 3 B, vii and viii vs. ix). This phenotype is consistent with the histological profile of milk stasis (18–20). Mammary glands of lactating Orai1 cKO mice were also engorged with milk (Fig. S4). Collectively, these data demonstrate that mammary glands of Orai1-deficient mice are able to develop normally and produce milk but that there is a severe defect in the milk ejection response, leading to the unproductive accumulation of milk in the mammary gland.
Fig. 3.
Orai1 KO mice demonstrate normal mammary gland development and secretory activation but impaired milk let down. (A) Mammary whole mounts from lactating Orai1−/− mice versus control genotypes (days 1 and 4 of lactation) and cKO mice (Orai1fl/−;MMTV-Cre) versus control genotypes (day 4 lactation, n = 3). (Scale bar, 400 µm.) (B) H&E staining of mammary glands from Orai1−/− or control mice on gestation day 18 (i–iii), lactation day 1 (iv–vi), and lactation day 4 (vii–ix) (n = 2–3). [Scale bars, 300 µm (60 µm, higher magnification image).] Lu, alveolar lumen. Arrows, secretory epithelium; arrowheads, cytoplasmic lipid droplets. (C) Actin staining of myoepithelial structures with phalloidin and (D) Oxtr mRNA levels in lactating mammary glands (n = 3). Data represent mean ± SEM; P > 0.05, Student’s t test.
Myoepithelial cells are responsible for generating the requisite contractile force for milk ejection. These cells form a basket-like network around mammary alveoli and contract in response to elevations in maternal serum oxytocin levels with suckling (18, 19). Impaired myoepithelial contractility could be caused by abnormal development or differentiation or by defects in cell signaling and function. No differences in myoepithelial structure or organization were observed in mammary tissue from Orai1−/− mice (Fig. 3C). Gene expression of the oxytocin receptor (Oxtr) was also not influenced by the absence of Orai1 (Fig. 3D), suggesting that this channel may instead regulate late-stage processes in mammary myoepithelial cells (e.g., oxytocin-mediated cell signaling).
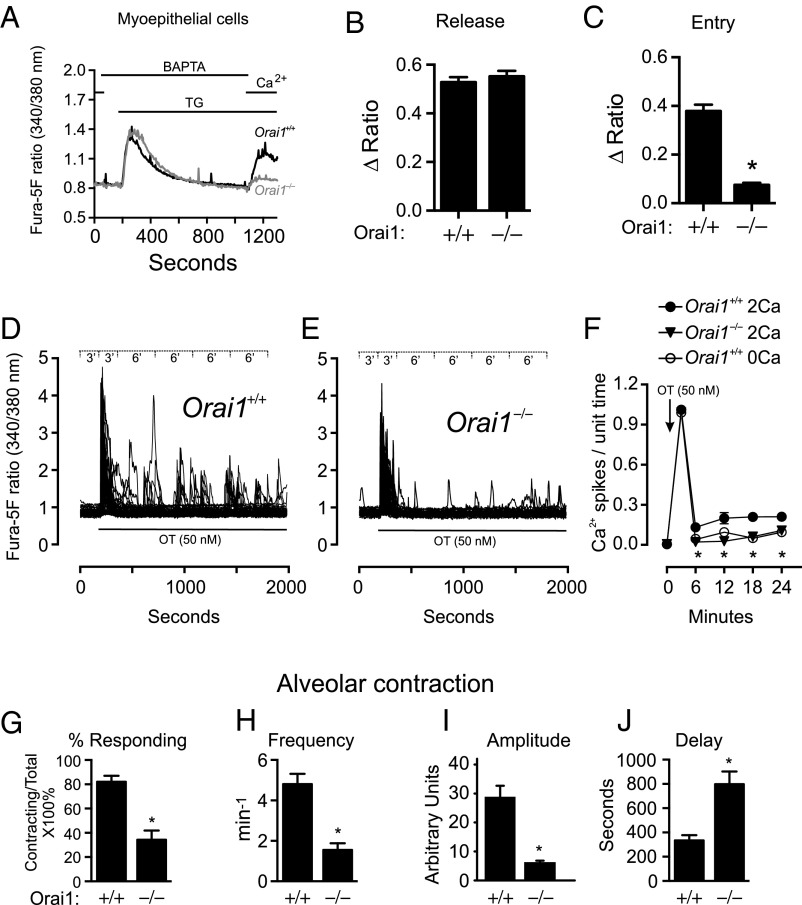
SOCE was significantly reduced in myoepithelial cells isolated from Orai1−/− mice (Fig. 4 A–C). The size of the ionomycin Ca2+-release transient was similar in both Orai1+/+ and Orai1−/− genotypes, indicating that the total Ca2+ content of internal stores was not altered in myoepithelial cells lacking Orai1 (Fig. S5 A and B).
Fig. 4.
Reduced oxytocin-mediated Ca2+ oscillations and alveolar unit contractility in Orai1−/− mice. (A) TG-induced Ca2+ entry in mammary myoepithelial cells isolated from Orai1+/+ (n = 159 cells) and Orai1−/− (n = 128 cells) mice. Cells were bathed in nominally Ca2+-free HBSS supplemented with BAPTA (500 μM) for 2 min and treated with TG (2 μM) to deplete ER Ca2+ stores before readdition of Ca2+ (2 mM). Peak ratio responses to (B) TG (150–270 s) and (C) Ca2+ readdition (1,000–1,180 s). Single-cell ratio responses to oxytocin (50 nM) in (D) Orai1+/+ and (E) Orai1−/− myoepithelial cells loaded with fura-5F; data binning periods are shown in Inset (n = 57 cells). (F) Average number of oxytocin-induced Ca2+ oscillations per data bin in Orai1+/+ myoepithelial cells with 2 mM Ca2+ (filled circle), Orai1−/− myoepithelial cells with 2 mM Ca2+ (filled triangle), and Orai1+/+ myoepithelial cells in the absence of extracellular Ca2+ (open circle) (n = 3 coverslips). Analyses of alveolar unit contractions in live tissue, showing (G) percentage of alveoli responding to oxytocin (50 nM) and (H) frequency, (I) amplitude, and (J) latency of alveolar unit contractions (n = 3 mice). Data represent mean ± SEM; *P < 0.05, Student’s t test (B, C, and G–J) or two-way ANOVA with Bonferroni posttests (F).
Oxytocin signaling is initiated by its binding to the Oxtr, a G protein-coupled receptor that signals through activation of phospholipase C (PLC) (21). PLC-inositol trisphosphate (IP3) signaling typically produces a transient elevation in intracellular Ca2+ levels ([Ca2+]i) due to the release of Ca2+ from IP3-sensitive stores (22). This initial release-phase Ca2+ response may be followed by a sustained elevation in global cytosolic Ca2+ due to Ca2+ entry across the plasma membrane (23). To assess the contribution of Ca2+ entry pathways in oxytocin signaling, we stimulated wild-type myoepithelial cells with oxytocin in the presence or absence of extracellular Ca2+. Under extracellular Ca2+ conditions, oxytocin produced a robust increase in intracellular Ca2+ that rapidly returned to baseline levels and was followed by an oscillatory phase, characterized by slow, irregular Ca2+ oscillations (Fig. 4D and Fig. S5C). Although no significant changes in the initial release-phase Ca2+ response or overall percentage of responding cells were observed in Orai1−/− cells (Fig. 4 E and F and Fig. S5D), the frequency of subsequent Ca2+ oscillations was significantly attenuated (Fig. 4 E and F). Notably, oscillations were reduced to levels comparable to that observed in wild-type myoepithelial cells when extracellular Ca2+ was absent (Fig. 4F), indicating that these Ca2+ oscillations are driven almost exclusively by Ca2+ entry through Orai1 channels.
These data led us to predict that myoepithelial contractility and milk ejection in lactating mammals is inextricably linked to Ca2+ entry through Orai1 channels. To test this hypothesis, we developed a strategy for visualizing oxytocin-induced alveolar unit contraction in live, excised mammary tissue. This technique afforded insight into the basic nature of milk ejection in mammals, demonstrating that mammary alveolar units are capable of rhythmic contractions (Movie S1). Alveolar unit contractions in mammary tissue removed from lactating wild-type mice appeared well coordinated, producing peristaltic waves to aid in milk expulsion, whereas contractions in tissue from Orai1−/− mice appeared poorly coordinated (Movie S2). The percentage of alveoli responding to oxytocin was significantly diminished (Fig. 4G), and responding glands did so with diminished frequency (Fig. 4H) and diminished amplitude (Fig. 4I and Fig. S5E). Of particular interest was the finding that the latency to the onset of alveolar unit contractions was substantially greater than the duration of the initial [Ca2+]i transient and was significantly greater for Orai1−/− mice than for wild-type mice (Fig. 4J and Fig. S5E).
Using this novel, live imaging strategy, it was not technically possible to measure Ca2+ levels in the very thin myoepithelial cells in the contracting alveoli. Thus, we cannot rule out that the significant longer delay to the onset of contraction in comparison with the onset of Ca2+ signaling may result in part from diffusion into the more complex structure of the whole tissue preparation. However, such a diffusion delay cannot explain the lag time in the alveolar unit contraction in Orai1−/− tissue, which was over twice that observed for wild-type tissue (Fig. 4J). This indicates that the initial global, massive release of stored Ca2+ is insufficient in itself to facilitate effective myoepithelial cell signaling; rather, this initial response may serve predominately as an initiator of Ca2+ store depletion and the development of Orai1-dependent irregular Ca2+ oscillations that appear to drive alveolar unit contractions in the mammary gland. There are two important implications from this conclusion. First, it seems unlikely that myoepithelial cell-driven alveolar contraction results from a simple and direct Ca2+ activation of contractile proteins. The significant disconnect between the timing of the [Ca2+]i signals and alveolar unit contractions implies that more complex pathways are activated linking Ca2+ entry through store-operated Orai1 channels to coordinate alveolar unit contractile behavior and the expulsion of stored milk. Second, the specific link to the small Ca2+ oscillations implies tight compartmentalization of the Orai1-dependent Ca2+ signaling, such as has been demonstrated previously for other Ca2+ influx-driven cellular responses (24). Finally, we point out that during suckling, bursts of oxytocin are released cyclically from the pituitary gland, and after a delay, each of these episodes of oxytocin release is followed by an increase in intramammary pressure, causing milk expulsion (25). Thus, the asynchronous Ca2+ oscillations and alveolar unit contractions observed in this study, in response to a sustained application of oxytocin, may not exactly reproduce the temporal characteristics of physiological lactation. Nonetheless, our experimental model clearly reveals the tight link between Orai1-mediated myoepithelial Ca2+ entry and alveolar unit contraction, especially when the cellular data are considered in light of the phenotypes of the Orai1 deletion mouse models.
In summary, using genetically modified mouse models, the current study has shed new light on the fundamental cell biology of lactation by identifying two indispensible roles for the store-operated Ca2+ channel subunit Orai1. Orai1 channels provide a major conduit for transporting Ca2+ into milk and also constitute an essential channel for signaling milk expulsion through myoepithelial cell contractility. Deficiencies in SOCE underlie failure of other exocrine glands in mouse models (26) and in humans (27), and similar mechanisms may cause these potentially debilitating channelopathies.
Materials and Methods
Methods using standard and previously published techniques are detailed in SI Materials and Methods, including reagents, genotyping primers, single-cell Ca2+ measurements, real-time RT-PCR, histology and whole-mount analysis, and statistics.
Animal Models.
Mice carrying Orai1 null alleles (Orai1−/−) were kindly provided by Jean-Pierre Kinet (Harvard Medical School, Boston) and were generated using gene trap mutagenesis (14). These mice had a high incidence of perinatal lethality, which was improved by further outbreeding this line (C57/DBA/129 background) with Institute of Cancer Research (ICR) mice [Harlan Laboratories Inc., strain Hsd:ICR (CD-1)] and by delaying weaning in potential KO animals (26). cKO mice (Orai1fl/fl) were generated by flanking exons 2 and 3 of Orai1 with loxP sites. Mice were generated as described in ref. 28 and provided by S.F. To delete Orai1 in mammary epithelial cells in vivo, mice with one null Orai1 allele (Orai1+/−) were crossed with mice expressing Cre-recombinase under the control of the mouse mammary tumor virus (MMTV) long terminal repeat promoter (line D) (29), to achieve heterozygosity for both floxed Orai1 and MMTV-Cre. These animals were subsequently bred to Orai1fl/fl mice to generate mammary Orai1 KO (cKO; Orai1fl/−;MMTV-Cre) or control (Orai1fl/+;MMTV-Cre and Orai1fl/−) animals. The MMTV-Cre line was selected for these studies due to the extensive expression of Cre under the control of this promoter in both luminal and myoepithelial cells of the mammary gland; however, MMTV-mediated recombination is not restricted to the mammary gland and also occurs in other tissues (29). Although some MMTV-Cre lines are associated with a lactation defect, MMTV-Cre line D mothers show overtly normal mammary gland development and no statistically significant difference in pup weight gain or survival (30).
To produce lactating female mice for these studies, female Orai1+/+, Orai1+/−, and Orai1−/− mice were mated with Orai1+/+ sires. Mammary cKO and control mice were mated with CD-1 sires. Females were monitored daily for copulatory plugs and moved to individual cages with nesting material and heating pads for delivery and nursing. All studies were performed between lactation days 1–4 to minimize suffering and adverse health events in pups nursed by mothers with a lactation defect, as assessed by veterinary staff at the National Institute of Environmental Health Sciences. All animal procedures were reviewed and approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee. Animals were housed, cared for, and used in compliance with the Guide for the Care and Use of Laboratory Animals (31) in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited program.
Mouse Milking, Serum Collection, and Fostering.
Lactating dams were removed from the nest 2 h before milking. Mice were lightly sedated with isofluorane (2.5%). Oxytocin (2 IU) was given by i.p. injection, and milking was initiated after 2 min (32). Milk was expressed by manually massaging the mammary gland and was immediately collected from the tip of the nipple with a pipette.
Milk output was measured by recording pup weights on lactation days 1–4 (17). For studies using traditional KO and control animals, litter size was not standardized; the average litter size was 11 (range 8–13) for Orai1+/+, 8 (range 6–11) for Orai1+/−, and 9 (range 8–11) for Orai1−/− dams. Litter size was standardized to six pups per litter for mammary cKO and control mice. A timed milk volume estimate was obtained by removing day 2 lactating dams from actively nursing pups for 3 h. Six pups were weighed and immediately placed back with the dam. Pups were reweighed 30 min after the dam returned to the nest, and the change in pup weight was used as an estimate of the weight of the ingested milk (17). For fostering experiments, mouse pups were fostered on PND1 to lactating CD-1 foster mothers. Blood samples were collected immediately after euthanasia by cardiac puncture. Serum was separated by centrifugation in serum separation tubes (BD Biosciences).
Enzymatic Dissociation and Flow Cytometric Analysis and Sorting of Mouse Mammary Epithelial Cells.
To evaluate gene expression of Orai1 and Ca2+ responses in specific cell types, abdominal and inguinal mammary glands were excised from four virgin female mice per genotype (euthanized by CO2 inhalation) and incubated overnight at 37 °C in collagenase (1 mg/mL) and hyaluronidase (100 U/mL). A single-cell suspension of mammary epithelial cells was prepared as described by Prater et al. (33). Briefly, cells were treated with ammonium chloride (0.64%) for lysis of red blood cells, followed by trypsin (0.25%), dispase (4.5 U/mL), and DNase (0.09 mg/mL) treatments with repeat centrifugation steps. Cells were filtered through a 40-μm cell strainer, and mammary epithelial cells were isolated by immunomagnetic negative selection. Cells were prepared for flow cytometry by preblocking with normal rat serum (10%) and staining with propidium iodide, CD49f-AF488 (2.5 μg/106 cells, BioLegend 313608), EpCAM-AF647 (0.25 μg/106 cells, BioLegend 118212), or isotype controls (BioLegend 400525 and 400526). Flow cytometric analysis and sorting was performed as previously described (33) and shown in Fig. S6. Cells were plated overnight and used within 24 h.
Ex Vivo, Live Mammary Contraction Assay.
Pups from day 4 lactating dams were removed from the nest and euthanized. Four hours later, dams were euthanized and mammary tissue excised, dissected into 1–2 mm3 tissue pieces, and incubated at 37 °C in mammary growth medium. All tissue was used within 10 h postdissection. Tissue pieces were loaded with CellTracker dye (1–4 μM) in growth medium at 37 °C for 30 min. Dye-loaded tissue pieces were washed in HBSS, immobilized on glass coverslips, and bathed in fresh HBSS containing Ca2+ (2 mM). Mammary alveoli were treated with oxytocin (50 nM), and alveolar unit contractions were visualized in real time using a Zeiss 780 or Zeiss 710 NLO microscope with a Plan-Apochromat 20×/0.8 objective. Time-lapse experiments were performed over 60 min with a sample rate dt of 6 s and recording in four focal planes over a total thickness of ∼20 μm. For analysis of alveolar unit contractions, a maximal intensity projection of the Z-stack time lapse was created, and 10 regions of interest (∼320 μm2) were selected from the surface image for each genotype. Time-dependent intensity curves were imported into SigmaPlot (Systat Software Inc.). The percentage response, contraction frequency, and contraction lag times were analyzed using a macro developed in-house using SigmaPlot with derivatives for semi-Fourier analysis. To help illustrate the complex movements of alveolar unit contraction, we developed a method for visualization based on grouping projected image stacks into 60-s data bins, each containing three images (at 20, 40, and 60 s). Each image within a data bin was assigned a primary color (red, green, blue), and a merged image from each 60-s bin was subsequently generated. Regions that did not move during the 60-s period have red, green, and blue pixels superimposed and therefore appear white. Regions where significant tissue movement/contraction has occurred appear red, green, blue, or a combination of two primary colors.
Supplementary Material
Acknowledgments
We thank Jeff Tucker, Page Myers, John Brodie, Maria Sifre, Pamela Ovwigho, the Pathology Support Group, and Julie Foley for technical assistance. This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences; NIH Grant AI097302 (to S.F.); and National Health and Medical Research Council Grant 631347 (to G.R.M. and S.J.R.-T.).
Footnotes
Conflict of interest statement: S.F. is a cofounder of Calcimedica.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502264112/-/DCSupplemental.
References
- 1.Watson CJ, Khaled WT. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development. 2008;135(6):995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 2.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 3.Linzell JL. The silver staining of myoepithelial cells, particularly in the mammary gland, and their relation to the ejection of milk. J Anat. 1952;86(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: Targets for breast cancer. Biochim Biophys Acta. 2006;1765(2):235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Mamillapalli R, et al. Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology. 2013;154(9):3031–3042. doi: 10.1210/en.2012-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279(41):42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol. 1999;276(4 Pt 1):C796–C802. doi: 10.1152/ajpcell.1999.276.4.C796. [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol. 2000;279(5):C1595–C1602. doi: 10.1152/ajpcell.2000.279.5.C1595. [DOI] [PubMed] [Google Scholar]
- 9.Faddy HM, et al. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008;369(3):977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: A mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148(12):5943–5954. doi: 10.1210/en.2007-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAndrew D, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10(3):448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 12.Ross DG, Smart CE, Azimi I, Roberts-Thomson SJ, Monteith GR. Assessment of ORAI1-mediated basal calcium influx in mammary epithelial cells. BMC Cell Biol. 2013;14:57. doi: 10.1186/1471-2121-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross BM, Hack A, Reinhardt TA, Rao R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS ONE. 2013;8(6):e67348. doi: 10.1371/journal.pone.0067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9(1):89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motiani RK, et al. Orai3 is an estrogen receptor α-regulated Ca²⁺ channel that promotes tumorigenesis. FASEB J. 2013;27(1):63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanHouten J, et al. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci USA. 2010;107(25):11405–11410. doi: 10.1073/pnas.0911186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer CA, Neville MC, Anderson SM, McManaman JL. Analysis of lactation defects in transgenic mice. J Mammary Gland Biol Neoplasia. 2006;11(3-4):269–282. doi: 10.1007/s10911-006-9023-3. [DOI] [PubMed] [Google Scholar]
- 18.Raymond K, et al. Control of mammary myoepithelial cell contractile function by α3β1 integrin signalling. EMBO J. 2011;30(10):1896–1906. doi: 10.1038/emboj.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haaksma CJ, Schwartz RJ, Tomasek JJ. Myoepithelial cell contraction and milk ejection are impaired in mammary glands of mice lacking smooth muscle alpha-actin. Biol Reprod. 2011;85(1):13–21. doi: 10.1095/biolreprod.110.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimori K, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 22.Mikoshiba K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J Neurochem. 2007;102(5):1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 23.Bird GS, et al. Mechanisms of phospholipase C-regulated calcium entry. Curr Mol Med. 2004;4(3):291–301. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- 24.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: Impact on cell function. J Physiol. 2008;586(13):3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev. 1992;13(1):33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 26.Xing J, et al. Role of Orai1 and store-operated calcium entry in mouse lacrimal gland signalling and function. J Physiol. 2014;592(Pt 5):927–939. doi: 10.1113/jphysiol.2013.267740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarl CA, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124(6):1311–1318, e7. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somasundaram A, et al. Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J Neurosci. 2014;34(27):9107–9123. doi: 10.1523/JNEUROSCI.0263-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner KU, et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10(6):545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- 30.Robinson GW, Hennighausen L. MMTV-Cre transgenes can adversely affect lactation: Considerations for conditional gene deletion in mammary tissue. Anal Biochem. 2011;412(1):92–95. doi: 10.1016/j.ab.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed. Available at grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf. Accessed April 7, 2015.
- 32.DePeters EJ, Hovey RC. Methods for collecting milk from mice. J Mammary Gland Biol Neoplasia. 2009;14(4):397–400. doi: 10.1007/s10911-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prater M, Shehata M, Watson CJ, Stingl J. Enzymatic dissociation, flow cytometric analysis, and culture of normal mouse mammary tissue. Methods Mol Biol. 2013;946:395–409. doi: 10.1007/978-1-62703-128-8_25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.