Abstract
MicroRNA are important regulators of CD4 T cell differentiation, altering the balance between the immunogenic and tolerogenic pathways. Studies in mice with microRNA-deficient T cells have revealed defects in differentiation into the regulatory T cell lineage; however, the individual microRNA responsible have remained elusive. A recent paper in The EMBO Journal uses a systematic screen to find a novel cooperative action between an inducible and a constitutive microRNA in aiding regulatory T cell induction.
See also: SC Warth et al (May 2015)
The key decision of the immune system—to trigger inflammation or to maintain tolerance—is heavily regulated by CD4 T cells. Naïve CD4 T cells have the capacity to differentiate into either regulatory T cells (Tregs) with suppressive capacity or inflammatory effector T cells (such as the IL-17-expressing Th17 cell). The choice of which of these dichotomous pathways a naïve T cell takes is heavily influenced by the molecular milieu; for example, TGFβ promotes both Treg and Th17 differentiation; however, the presence of either retinoic acid or IL-6 can tip the balance in favour of Tregs or Th17 cells, respectively (Fig1A). A newly recognised class of regulators for this process is microRNA (miR), as loss of the miR network both retards the process and skews it against Treg differentiation, resulting in autoimmunity (Muljo et al, 2005; Tian et al, 2012).
Figure 1. Systematic screening for miR function reveals a cooperative role for miR-99a and miR-150 in Treg differentiation.
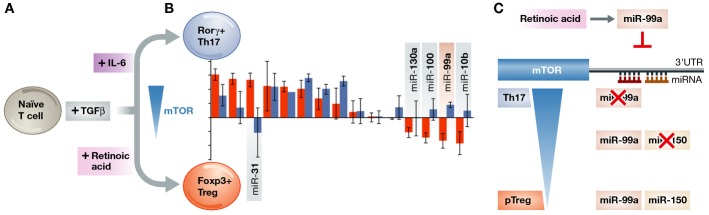
(A) Treg and Th17 represent opposing differentiation pathways from a common precursor. Both common (TCR signalling, TGFβ) and polarising (IL-6 versus retinoic acid) factors regulate this process, including the expression level of mTOR. (B) In a systematic overexpression screen, Warth and colleagues identified those miR capable of favouring either the Treg or Th17 outcome following in vitro stimulation with TCR signalling and TGFβ. (C) miR-99a is upregulated following retinoic acid exposure. miR-99a binds the 3′ UTR of Mtor, reducing expression and aiding polarisation towards the Treg lineage. The constitutively expressed miR-150 can also bind the 3′ UTR of Mtor, however repression is poor in the absence of miR-99a. Coexpression of miR-99a and miR-150 results in cooperative binding to Mtor mRNA, allowing for efficient Treg induction.
miR are small RNA species that undergo a maturational processing from an immature pri-miR to a functional miR of ∽22 nt in length. Mature miR are then integrated into the RNA-induced silencing complex (RISC), which uses the “seed sequence” of the miR component to target specific mRNA for degradation or silencing. This regulation of mRNA by miR has a critical role in shaping the immune system. While many miR-regulated processes have been found for CD4 T cell differentiation (Dooley et al, 2013), much of this research has been led by the availability of research tools rather than being performed in an unbiased and systematic manner. Key questions have been left unanswered on both cellular function, such as which miR are required for Treg differentiation, and molecular mechanism, such as the relationship between induced and constitutive miR. In this issue of The EMBO Journal, Warth et al (2015) systematically screened 130 miR for the capacity to regulate the Treg–Th17 lineage division and identified a novel cooperative regulation between induced and constitutive miR in the process.
In this study, Heissmeyer and colleagues took advantage of a screening platform they previously generated (Warth & Heissmeyer, 2013), where modified T cells are transduced with high efficiency by adenoviruses that drive the expression of pri-miR. The 130 screened miR were chosen from among those normally expressed in Tregs. Following adenoviral overexpression in naïve T cells, cells were exposed to TGFβ and TCR stimulation to drive Treg differentiation, and the effect of each miR on the process was quantified. This screening process picked up both known negative regulators of Treg differentiation (miR-31 and miR-17∽19a) and novel positive regulators (miR-10b, miR-130a, miR-320, miR-99a, miR-146b, miR-296, miR-505, miR-150 and miR-195/497). In a secondary screen, candidate miR were tested for the capacity to regulate both the Treg and Th17 fate in vitro (Fig1B). Intriguingly, the authors found a consistent difference between the positive and negative miR regulators: negative regulators impeded both Treg and Th17 induction (suggestive of general inhibition of T cell differentiation), while most positive regulators of Treg fate were also negative regulators of Th17 fate (indicating a function in the lineage polarisation).
Following the findings of the screen, the authors focused on one candidate, miR-99a. miR-99a is highly upregulated upon Treg induction, indicating a physiological role for the pro-Treg inductive capacity. Bioinformatic analysis of putative miR-99a targets identified a binding site in the 3′ untranslated region (UTR) of the mammalian target of rapamycin (mTOR) mRNA. mTOR is an attractive target for miR-99a, as it influences the metabolic control of T cells. Tregs preferentially utilise the lipid oxidation metabolic pathway, while T helper cells rely on aerobic glycolysis. The latter is regulated by mTOR, and mTOR deletion or suppression promotes a shift of T cell differentiation towards Treg (Delgoffe et al, 2009). Interestingly, the 3′ UTR of the Mtor mRNA contains a binding sequence for a second candidate, mi-R150, in close proximity to one of the miR-99a binding regions.
Both miR-99a and miR-150 were validated as negative regulators of Mtor mRNA in this study, a finding which is sufficient to explain the positive effect these miR have on Treg induction. In further investigation, the authors stumbled upon the unusual observation that miR-150 was only able to regulate Mtor in the presence of miR-99a. This fascinating observation was found using a system free from endogenous miR. Dicer-deficient cells are incapable of processing pre-miR into mature miR, with the exception of a handful of Dicer-independent pre-miR (such as miR-451). By reverse-engineering the mature sequences for miR-99a and miR-150 into the backbone of pre-miR-451, and using Dicer-deficient cells for reconstitution, the authors could study the function of these two miR in isolation and in combination. This series of well-designed miR suppression assays revealed a novel concept in which the binding of one miR (here the abundantly expressed miR-150) is abolished in the absence of a second miR (here miR-99a), although notably miR-99a showed no reciprocal dependence (Fig1C).
Together, these results reveal a new molecular pathway for Treg induction. Naïve T cells, expressing miR-150 but not miR-99a, will maintain high mTOR and will be prone to differentiate into Th17 cells. However, the addition of retinoic acid, known to aid Treg induction (Mucida et al, 2007), causes an upregulation of miR-99a, allowing miR-150 and miR-99a to cooperatively suppress mTOR expression. With lower mTOR activity, the naïve T cells become biased towards differentiating into the Treg lineage, allowing the generation of peripheral Tregs and the suppression of autoimmunity.
The significance of these findings, however, reaches beyond the understanding of peripheral Treg development. While cooperative effects of miR have been observed before (Grimson et al, 2007; Saetrom et al, 2007), this is the first to use a system free of endogenous miR to extensively study the interactions. The complexity of interaction seen goes beyond a simple cooperative binding. The unidirectional cooperative binding observed demonstrates the principle that one miR can “license” a second miR to bind a mRNA, in effect creating a functional dependency of one miR upon the other. While in this particular example the licensing came through induction of the cooperative miR, it is also possible that licensing could be generated through alternative splicing of the 3′ UTR bringing a second miR binding site to within close proximity of the first. It is highly probable that miR regulation is far more complex than the simple miR:mRNA interactions typically studied and that the regulatory impacts of any given miR are heavily modified by the composition of the miR and mRNA networks in which they function. This study provides both proof of principle and also development of tools to unravel the complexity of miR regulation.
References
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J, Linterman MA, Liston A. MicroRNA regulation of T-cell development. Immunol Rev. 2013;253:53–64. doi: 10.1111/imr.12049. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetrom P, Heale BS, Snove O, Jr, Aagaard L, Alluin J, Rossi JJ. Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res. 2007;35:2333–2342. doi: 10.1093/nar/gkm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, De Hertogh G, Fedeli M, Staats KA, Schonefeldt S, Humblet-Baron S, Van Den Bosch L, Dellabona P, Dooley J, Liston A. Loss of T cell microRNA provides systemic protection against autoimmune pathology in mice. J Autoimmun. 2012;38:39–48. doi: 10.1016/j.jaut.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Warth SC, Heissmeyer V. Adenoviral transduction of naive CD4 T cells to study Treg differentiation. J Vis Exp. 2013 doi: 10.3791/50455. 78: e50455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth SC, Hoefig KP, Hiekel A, Schallenberg S, Jovanoivc K, Klein L, Kretschmer K, Ansel KM, Heissmeyer V. Induced miR-99a expression represses Mtor cooperatively with miR-150 to promote regulatory T-cell differentiation. EMBO J. 2015;34:1195–1213. doi: 10.15252/embj.201489589. [DOI] [PMC free article] [PubMed] [Google Scholar]


