Abstract
Adult organisms have to adapt to survive, and the same is true for their tissues. Rates and types of cell production must be rapidly and reversibly adjusted to meet tissue demands in response to both local and systemic challenges. Recent work reveals how stem cell (SC) populations meet these requirements by switching between functional states tuned to homoeostasis or regeneration. This plasticity extends to differentiating cells, which are capable of reverting to SCs after injury. The concept of the niche, the micro-environment that sustains and regulates stem cells, is broadening, with a new appreciation of the role of physical factors and hormonal signals. Here, we review different functions of SCs, the cellular mechanisms that underlie them and the signals that bias the fate of SCs as they switch between roles.
Keywords: differentiation, niche, regeneration, signal transduction, stem cells
Introduction
Tissues are diverse not only in their structure and function but also in the dynamics of their constituent cells (Leblond & Walker, 1956). SC turnover rates in adult mouse lineages vary by orders of magnitude (Fig1). Epithelial and male germ SCs cycle rapidly, while in tissues such as liver, kidney and muscle, proliferation is scarcely detectable. In homoeostasis, on average, one SC and one differentiating cell are produced per SC division, and the rate of SC proliferation is matched with the rate of loss of cells from the differentiated lineage(s) it supports. Following damage, however, SCs must rapidly and reversibly switch to produce an excess of proliferating cells to regenerate the lost tissue. Here, we will define SCs in purely functional terms as cell populations that sustain long-term tissue turnover and/or contribute to tissue repair after injury. We first survey SC behaviour in high turnover and quiescent tissues in homoeostasis, then consider how SCs respond to injury and conclude by considering the physical and molecular signals which switch SC behaviour to meet the requirements of the organism.
Figure 1. SC turnover across mouse tissues.

Average cycle times for SCs in the lineages indicated.
The functional repertoire of SCs
There are two cellular mechanisms by which SCs may maintain homoeostasis (Watt & Hogan, 2000). A population of SCs may behave so that on average, 50% of their progeny are themselves SCs and 50% differentiating cells. Such ‘population asymmetry’ may result from the cell autonomous properties of SCs or external regulation, for example by a niche of restricted size in which SCs can only divide when a cell differentiates and leaves the niche. Alternatively, each individual SC may divide asymmetrically. Advances in lineage tracing and live imaging reveal population asymmetry emerges as the predominant mechanism in tissues with a high rate of turnover (Alcolea & Jones, 2013; Rompolas & Greco, 2014). We will focus on tissues in mice where robust evidence on SC behaviour is available, before turning to other species.
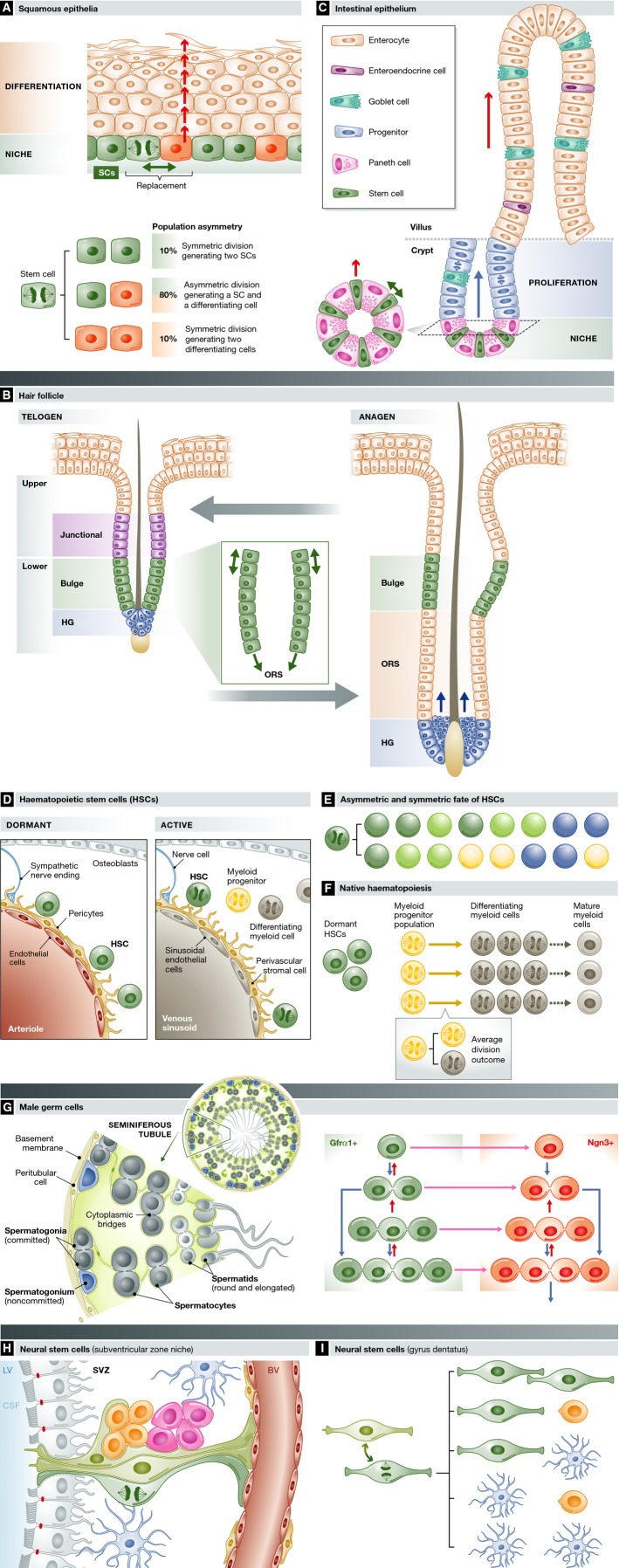
Some of the first insights into SC dynamics in vivo came from studies of squamous epithelia, which cover the skin, mouth and oesophagus and predominantly consist of layers of keratinocytes. Proliferation is confined to the deepest, basal cell layer, which forms the SC niche (Fig2A). When dividing cells commit to differentiation, they withdraw from the cell cycle and migrate out of the basal layer (Alcolea & Jones, 2014). Large-scale lineage tracing has revealed that cellular homoeostasis is achieved by a single population of keratinocyte SCs in the basal cell layer (Clayton et al, 2007; Doupe et al, 2010, 2012; Mascre et al, 2012; Lim et al, 2013). The outcome of individual cell divisions is unpredictable, generating two SCs, two differentiating cells or one cell of each type (Alcolea & Jones, 2013) (Fig2A). However, the probabilities of each division outcome are balanced, so an equal number of SCs and differentiating cells are generated across the basal layer, an example of ‘population asymmetry’ (Watt & Hogan, 2000; Clayton et al, 2007). The random element in SC fate appears at odds with histological features such as the ordered columns of differentiating cells seen in mouse epidermis, but these arise simply from the physical packing properties of suprabasal cells rather than reflecting deterministic SC behaviour (Doupe et al, 2010). Analysis of lineage-tracing data argues that the balanced stochastic fate of squamous SCs is a cell-intrinsic property rather than a consequence of external regulation, but the molecular machinery that achieves this is unknown (Clayton et al, 2007; Jones & Simons, 2008).
Figure 2. SC dynamics across lineages.
(A) Squamous epithelia consist of layers of keratinocytes, SCs (green) reside in the basal cell layer, along with post-mitotic cells waiting to stratify (red). When a differentiating cell leaves the niche (arrow), a nearby SC divides with one of the three division outcomes shown to maintain constant cell density in the niche. The probabilities of each outcome (expressed as per cent, oesophageal epithelium shown) are balanced, so equal numbers of SCs and differentiated cells are produced across the population. (B) The hair follicle cycles between a resting stage (telogen) and expansion of the lower follicle (anagen). Multiple SCs have been identified, in the junctional zone (purple), the bulge (green) and the hair germ (blue), which lie in contact with the mesenchymal cells of the dermal papilla (blue). The hair shaft (black) is surrounded by concentric layers of inner root sheath cells that have been omitted for clarity. In the transition into anagen, hair germ and upper bulge cells self-duplicate. In the bulge, divisions are aligned parallel with the axis of the hair shaft (inset). Lower bulge cells contribute differentiating progeny to greatly expand the root sheath. Later in anagen, hair germ cells assemble around the dermal papilla and then generate inner root sheath cells (arrows). Self-renewal and differentiation are balanced, so the numbers of SCs in each compartment is maintained at a constant level across multiple hair cycles. (C) Intestinal epithelium contains four lineages sustained by SCs (green) that lie between Paneth cells in the crypt base. Differentiating cells migrate through a progenitor compartment in the upper crypt from which post-mitotic cells populate the villus, from which they are shed. Inset shows a simplified top-down view of the niche. As a differentiating SC exits the niche, it is replaced by the self-duplicating division of an immediately adjacent SC. (D) Heamatopoietic SCs reside close to blood vessels in the bone marrow. Dormant SCs (green) lie close to arterioles, receiving paracrine signals from endothelial cells (red), perivascular cells expressing Ng2 and sympathetic nerve endings (blue). On activation, SCs migrate to be close to venous sinusoids that support Lepr-expressing perivascular cells. (E) Asymmetric fate and symmetric fate of haematopoietic SCs. Transplantation of daughter cells of a single SC reveals self-duplicating and asymmetric divisions: dark green: long-term-reconstituting SCs; light green: short- or intermediate-term-reconstituting SCs; yellow: megakaryocyte progenitor; and blue: common myeloid progenitor. (F) Native haematopoiesis. Lineage tracing in homoeostasis suggests that myeloid lineages may be maintained by self-sustaining progenitor cells (yellow) exhibiting ‘population asymmetry’ by generating equal proportions of progenitor and differentiating cells (grey) (see inset). Haematopoietic SCs (green) make negligible contribution to myelopoiesis in homoeostasis, but function as ‘reserve’ cells. (G) Male germ cell SCs are diverse in appearance but functionally equivalent. Male germ cells expressing GFRα1 reside in the outermost layer of the seminiferous tubule. SCs (green) exist as singles or 2–4 cell syncytia connected by cytoplasmic bridges, which may self-duplicate (blue arrows) to generate two SCs or undergo fragmentation (short red arrows). Upon differentiation (pink arrow) into Ngn3-positive cells (red), the same behaviour continues, but Ngn3+ cells are unlikely to revert to GFRα1+ SCs in homoeostasis. Once in the Ngn3 compartment, syncytia larger than 4 cells form which may undergo further differentiation into cKit-expressing cells (not shown), which are even less likely to revert to GFRα1-positive cells. (H) Neural SCs exhibit symmetric and asymmetric cell divisions. Quiescent neural SCs (light green) reside in the subventricular zone niche, extending processes into cerebrospinal fluid (blue) that fills the lateral ventricles (LV) and underlying endothelial cells (red) lining blood vessels (BV). Endothelial cells that line the LV are shown in grey. Differentiating transit amplifying cells (pink) and neural precursor cells (orange) lie adjacent to the SCs. Figure after Silva-Vargas et al (2013). (I) Division outcomes of neural SCs in the dentate gyrus inferred from lineage tracing. Neural SCs interconvert between quiescent (light green) and proliferating (dark green) states. Division of SCs has a range of symmetric and asymmetric outcomes as shown, generating neural SCs, neuroblasts (orange) or differentiated astrocytes (blue).
The epidermis is punctuated by appendages in the form of hair follicles (HFs) and sweat ducts, each a self-maintaining mini-organ containing multiple cell lineages (Schepeler et al, 2014). HFs are split into functionally discrete compartments (Page et al, 2013). The lower hair follicle undergoes cyclical expansion (anagen) and contraction (catagen) followed by a period of quiescence (telogen) (Fig2B). The HF is homoeostatic in SC numbers, and tissue organisation remains the same at telogen phase of each successive hair cycle. HFs are sustained by SC populations in a region known as the bulge and the adjacent hair germ (Rompolas & Greco, 2014). Lineage tracing and cell culture studies have revealed a bewildering range of apparently overlapping candidate SCs in HFs, though it is unclear whether variations in gene expression mark functionally distinct SC populations (Rompolas & Greco, 2014; Schepeler et al, 2014).
The HF bulge, which marks the upper limit of the lower follicle, was first identified as a distinct SC niche as it contains slow-cycling cells (Cotsarelis et al, 1990; Tumbar et al, 2004) (Fig2B). Bulge SCs differentiate into multiple lineages when sorted and transplanted, and undergo both symmetric and asymmetric divisions (Blanpain et al, 2004; Waghmare et al, 2008; Zhang et al, 2009). More recently, lineage tracing and intra-vital imaging have shown that SCs in the hair germ are the first cells to be mobilised as HFs enter anagen, undergoing apparently symmetric self-duplicating divisions aligned with the long axis of the HF (Greco et al, 2009; Rompolas et al, 2012). Only when the exponentially expanding hair germ has morphed to envelop a bud of mesenchyme called the dermal papilla (DP) SCs differentiate into ‘transit amplifying’ cells that will form the inner root sheath that surrounds the hair shaft (Rompolas et al, 2012). Signals from the DP are essential for the progression of the hair cycle; indeed, the number of cells in the DP determines hair size and shape (Rompolas et al, 2012; Chi et al, 2013). In the bulge, SCs remain in slow cycling or quiescent, until a proportion of cells are recruited into division by signals from TA cells. These induce SCs to first contribute to the outer root sheath and then undergo self-renewal until the bulge is repopulated with SCs, when quiescence resumes (Hsu et al, 2014) (Fig2B). HF expansion is followed by involution (catagen), and by the time the telogen phase is reached, bulge and hair germ SCs are restored, achieving homoeostasis over the entire cycle (Hsu et al, 2014).
The glandular epithelium that lines the intestine is also rapidly turned over but has a distinctive tissue organisation that contributes to SC regulation. The differentiated cells on the finger-like villi are shed rapidly from the villus tip. Cell replacement is achieved by SCs and progenitor cells located in pits known as crypts (Clevers, 2013) (Fig2C). Cycling SCs lie at the crypt base among cells expressing the Wnt target gene Lgr5, maintaining four lineages of differentiated cells via the lineage-committed progenitor cells that populate the upper crypt (Barker et al, 2007). Differentiated Paneth cells lie next to SCs and secrete signals to sustain SCs in the niche (Sato et al, 2011b). The requirement for paracrine signals restricts the size of the SC pool and ensures homoeostasis. Lineage tracing indicates that as a SC differentiates, it migrates out of the niche and is replaced by the self-duplicating division of a neighbouring SC (Fig2C) (Lopez-Garcia et al, 2010; Snippert et al, 2010). It was initially thought that all Lgr5 + cells were cycling functionally equivalent SCs, but a recent study has shown only 5 of the 14–15 Lgr5 + cells in each crypt base are proliferating at any one time (Kozar et al, 2013). Live imaging further suggests the likelihood of a SC differentiation depends on its location, increasing in the uppermost part of the niche (Ritsma et al, 2014). Analysis of spontaneous clones carrying mitochondrial mutations argues similar SC behaviour maintains human colonic epithelium (Baker et al, 2014). Intestinal homoeostasis is thus achieved by linking differentiation to self-duplicating SC division within a niche of restricted size.
It has long been argued that the prodigious cell turnover of the haematopoietic system is sustained by rare SCs, found both in the circulation and in a specific niche environment in the bone marrow. Heamopoietic SCs (HSCs) are assayed by their ability to reconstitute the blood system in the long term following transplantation into irradiated host animals (Babovic & Eaves, 2014; Ema et al, 2014). Highly purified HSCs expressing cell surface identical markers may be cycling or dormant, but only the latter group has long-term transplant potential (Fig1) (Wilson et al, 2008; Foudi et al, 2009; Catlin et al, 2011; Anjos-Afonso et al, 2013; Oguro et al, 2013). Dormant HSCs reside in a distinct niche environment, next to arterioles, and migrate to lie close to venous sinusoids on activation (Fig2D) (Kunisaki et al, 2013; Mendelson & Frenette, 2014). Transplant assays argue that haematopoiesis is a hierarchy, with HSCs continually generating progenitors that become progressively restricted in both lineage and self-renewal potentials as they differentiate, but recent discoveries are challenging this view. Transplantation of daughters from a single HSC division reveals self-duplicating and asymmetric divisions which generate a variety of differentiating progenitors directly from HSCs, challenging the view of an ordered differentiation programme (Yamamoto et al, 2013) (Fig2E). More remains to be discovered about the apparent heterogeneity among HSCs with long-term transplant potential. Some of this may result from HSCs commuting between different states that vary in their ability to survive the rigours of transplantation (Ema et al, 2014; van Galen et al, 2014). Recently, transposon-based lineage tracing has revealed native haematopoiesis is very different from that in transplanted animals. Rather than myeloid lineages deriving from a few HSCs, as is seen after transplantation, numerous short-lived progenitor clones are observed, consistent with myelopoiesis being maintained by a self-sustaining progenitor pool exhibiting population asymmetry (Fig2F) (Sun et al, 2014b). This argues for a separation of roles in haemopoiesis, with a pool of progenitors maintaining the myeloid lineage in homoeostasis and quiescent HSCs functioning as a ‘reserve’, mobilised to expand the progenitor population when the demand for myeloid cells rises (Wilson et al, 2008; Sun et al, 2014b). Different SC functions may thus be devolved to discrete populations in some tissues.
The final high turnover lineage we will consider is that of male germ cells. Spermatogenesis continues throughout life, sustained by a population of SCs, which reside in the basal cell layer of the seminiferous tubule, express GFRα1 and are negative for the differentiation marker Ngn3. Spermatogonia undergo incomplete cell division, generating syncytial clusters of cells linked by cytoplasmic bridges (Yoshida, 2012) (Fig2G). It was thought that only single GFRα1+ cells were SCs, but combined live imaging and lineage tracing have revealed that all GFRα1+ cells, whether single or in syncytia of up to 4 cells, are functionally equivalent SCs (Hara et al, 2014). Quantitative modelling argues SCs divide symmetrically producing two SC daughters, while the stochastic differentiation of a SC into a Ngn3+ cell appears linked to syncytial fragmentation by an as yet unknown mechanism. In combination, these mechanisms achieve population asymmetry as SC division is balanced with differentiation across the GFRα1+ compartment (Klein et al, 2010; Hara et al, 2014). Ngn3+ cells have a high probability of undergoing differentiation into cKit-expressing cells, but infrequently revert back to GFRα1 SC. Almost all cKit cells undergo terminal differentiation. The spermatogenesis paradigm supports the concept that SC function may reside in a pool of heterogeneous cells which interconvert between different states, so that there are multiple reversible paths by which a given SC may differentiate (Nakagawa et al, 2010)(Hara et al, 2014).
Turning to tissues with a low rate of turnover, it is now clear that both rodent and human brains, once thought to be post-mitotic, continue to produce new neurons throughout life which contribute to specific forms of learning and memory and responding to stress (Snyder et al, 2011; Spalding et al, 2013; Aimone et al, 2014; Ernst et al, 2014). Adult neural SCs are found in the subventricular zone (SVZ) lining the lateral ventricles and in the dentate gyrus (DG) of the hippocampus and are largely quiescent (Fig2H). Lineage tracing in transgenic mice reveals that proliferating DG SCs are capable of both asymmetric and symmetric divisions generating two SCs or two differentiating astroglial or neural progenitor daughters (Bonaguidi et al, 2011) (Fig2I). After division, some SCs return to a quiescent state, negative for proliferation markers, paralleling the transition between dormant and active states seen in heamatopoietic SCs.
Even establishing the existence of SCs in adult tissues with a very low rate of turnover is challenging. Studies in mouse and human support a slow rate of turnover of differentiated hepatocytes in the liver and glomerular and tubular cells in the kidney, though the existence of a discrete stem cell population in these tissues is controversial (Magami et al, 2002; Rodins et al, 2002; Fellous et al, 2009; Kusaba et al, 2014; Miyajima et al, 2014). In contrast, adult skeletal muscle contains a well-defined quiescent SC population, residing among the satellite cells that lie beneath the plasma membrane of terminally differentiated multinucleate muscle fibres (Brack & Rando, 2012). Muscle SCs typify the ‘reserve’ role of SCs, actively maintained in a quiescent state until they are mobilised following muscle injury (Cheung et al, 2012; Cheung & Rando, 2013).
In summary, there are two cellular mechanisms of population asymmetry in mammalian tissues with a high turnover (Watt & Hogan, 2000; Klein & Simons, 2011). The first, exemplified by squamous epithelia, is SCs within an ‘open plan’ niche with an apparently cell-intrinsic mechanism which balances the probabilities of generating SCs or differentiating progeny (Clayton et al, 2007; Alcolea & Jones, 2014). An alternative strategy seen in the intestine is having a niche of limited size in which self-duplicating SC division occurs as a differentiating cell exits the niche (Lopez-Garcia et al, 2010; Vermeulen & Snippert, 2014). In both these examples, the robust linkage of division and the exit of a nearby cell maintain a constant cellular density within the niche. Another common theme is that while the fate of an individual SC is unpredictable, homoeostasis is achieved across the SC population. The male germ cell lineage illustrates the unreliability of using marker gene expression or cellular morphology to define SCs, as such characteristics may vary within a functionally equivalent pool of SCs. Likewise, active SCs may coexist with those within a dormant state in the hair follicle, bone marrow and intestinal crypt.
While the discussion above has focussed on mammalian systems, population asymmetry has also been observed in Drosophila, where neutral competition for a niche of fixed size is seen in ovarian SCs, while a balanced three way division outcome reminiscent of mammalian squamous epithelia is a feature of midgut SCs (de Navascues et al, 2012; Kronen et al, 2014). Intriguingly transgenic lineage tracing reveals that neural retina SCs of the medaka fish, Orizias latipes, have fixed asymmetric cell division in homoeostasis, the first observation of such behaviour in an adult tissue (Centanin et al, 2014).
In summary, for the mammalian and Drosophila lineages where SC dynamics has been resolved, cellular homoeostasis is achieved by population asymmetry, which may be cell intrinsic or niche specified. The fate of individual cells is unpredictable, but the probabilities of self-renewal and differentiation are balanced across the SC population, so equal proportions of SCs and differentiating cells are generated. Evolution has also sampled another mechanism of tissue maintenance, fixed asymmetric SC division, but to date, this has only been observed in fish retina.
Regeneration: using all the options
Tissue injury is an inevitable part of life. It is becoming clear that following damage, SCs and their differentiating progeny exhibit hitherto unexpected plasticity in their behaviour (Doupe & Jones, 2013). We first consider how cycling SC populations respond to injury before turning to the activation of quiescent SCs in low turnover tissues.
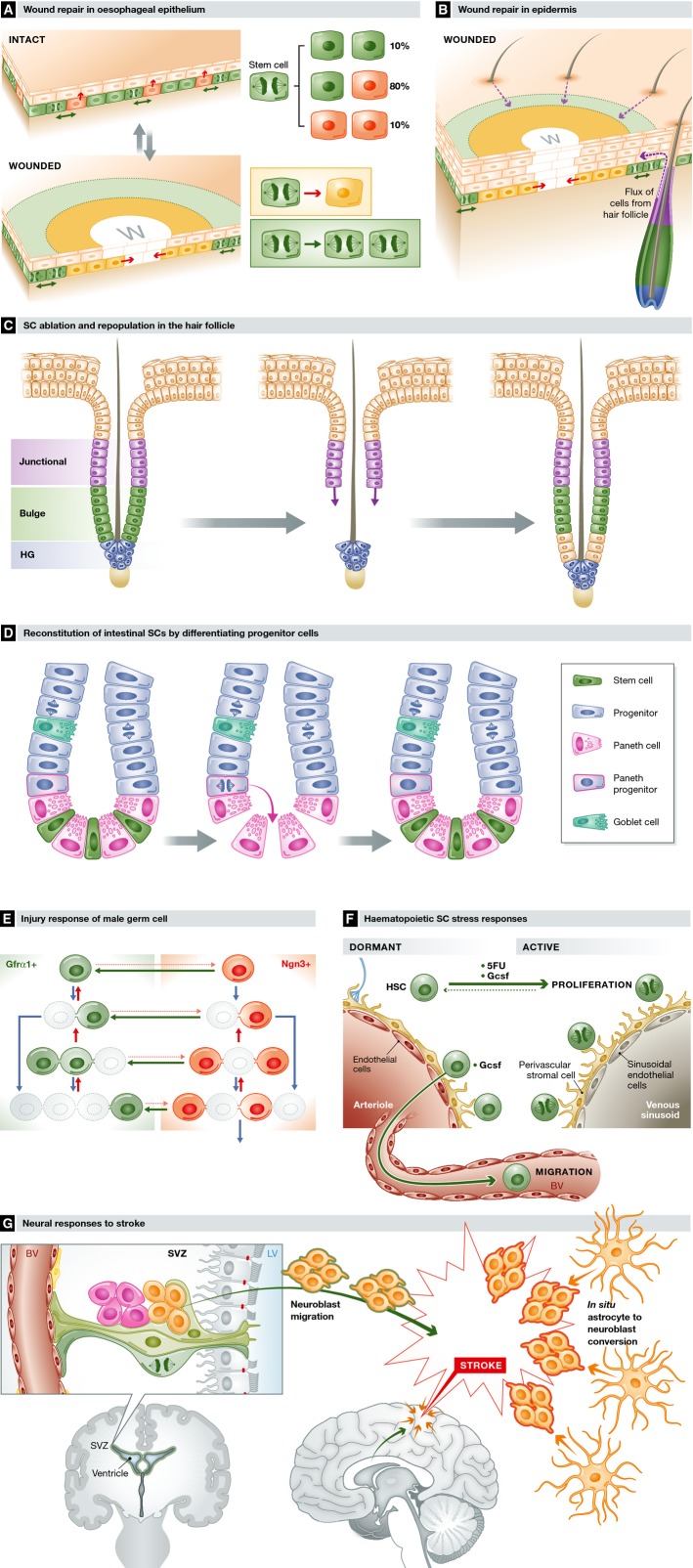
Wounding is frequent in surface epithelia. In the squamous epithelium of the oesophagus and the epidermis of the paw, SCs adjacent to a wound rapidly and reversibly switch to producing an excess of SCs, reverting to homoeostatic behaviour once the defect is closed (Fig3A) (Doupe et al, 2012; Lim et al, 2013). The need for such flexibility may be one of the reasons for three division outcomes in squamous epithelial SCs. Pure asymmetric division achieves cellular homoeostasis but is inflexible, while adjusting the probability of the two types of symmetric division allows rapid modulation of cell production.
Figure 3. Stem cell responses to stress.
(A) Wound repair in oesophageal epithelium. Following wounding, progenitor cells next to the wound (w) exit the cell cycle and migrate towards the defect (yellow area and inset). Behind this ‘migrating front’, cycling progenitors undergo divisions heavily biased to self-duplication (green area and inset), expanding the progenitor compartment to generate the excess cells required to repair the epithelium. Once the wound has closed, progenitor behaviour switches back to homoeostasis. (B) Wound repair in epidermis. In the epidermis, progenitor cells change their behaviour as in the oesophagus, but wound repair is supported by a flux of cells into the epidermis from hair follicles (purple arrows) and, in tail skin, mobilisation of quiescent reserve cells in the epidermis. Migration explains the appearance of radial clones around a healed wound seen in lineage-tracing experiments. (C) HF ablation and repopulation. Following ablation of SCs in the bulge (green) of telogen HFs, cells in the upper follicle (purple) migrate and proliferate (purple arrows) to reconstitute the bulge SC population, enabling the hair cycle to proceed normally. (D) Reconstitution of intestinal SCs by differentiating progenitor cells. Following ablation of Lgr5-expressing SCs in the crypt base (green), Paneth cell precursors (blue with pink border) dedifferentiate and colonise the crypt base niche with SCs functionally equivalent to the original population. (E) Injury response of male germ SCs. Following treatment with the cytotoxic agent busulphan, the GFRα1+ SC population is depleted (grey outlines) but then reconstituted by surviving Ngn3+ cells reverting into GFRα1+ cells (green arrows) and a decrease in the probability of GFRα1+ cells transferring to the Ngn3+ compartment (pink dotted arrows). (F) Haematopoietic SC stress responses. Dormant SCs are recruited into cycle by treatment after damage to the haematopoietic system from a cytotoxic drug (5FU) or cytokines, such as Gcsf, released after infection. Gcsf also triggers dormant SCs to migrate into the circulation. (G) Neural SC responses to stroke. Following a CNS stroke, which results in cell death due to ischaemia, clusters of neuroblasts (orange) are seen in the injured region. Lineage tracing argues these derive both from mobilisation of neural SCs in the SVC followed by migration to the site of injury (orange with green border) and from differentiated astrocytes within the area of the stroke entering a neurogenic programme (orange with red borders).
Most of the epidermis has a dense array of HFs, which normally make negligible contribution to homoeostasis. Following injury, however, the progeny of bulge and upper follicle stem cells migrate into the epidermis and contribute extensively to wound repair (Ito et al, 2005; Levy et al, 2005; Page et al, 2013) (Fig3B). In the specialised tail epidermis, which is sparse in HFs, rare, slow-cycling ‘reserve’ SCs are mobilised, while in hairless skin, sweat duct-derived cells contribute to wound healing (Lu et al, 2012; Mascre et al, 2012; Roshan & Jones, 2012). In humans, similar principles apply, but sweat ducts make a larger contribution to epidermal repair than HFs (Rittie et al, 2013).
Injury also induces the appearance of new HFs by a non-cell autonomous route. The infiltration of specialised gamma delta T cells following epidermal injury activates Wnt signalling via Fgf9 signalling (Gay et al, 2013). Wnt has been shown to alter the axis of division of bulge stem cells to generate a new hair follicle alongside an existing one (Deschene et al, 2014).
Recent developments in live imaging combined with laser ablation to delete cell subpopulations have also revealed unexpected plasticity in HFs. Following ablation, bulge SCs are replaced by the progeny from cells in the upper HF which normally make no contribution to the hair cycle (Fig3C; Rompolas et al, 2013; Rompolas & Greco, 2014). Similarly, the hair germ can be replaced if the bulge SCs remain intact. Only destruction of the niche cells surrounding the bulge or separation of the DP from the hair germ halts hair regeneration, indicating the niche plays an essential role in hair regeneration.
Cellular plasticity extends beyond SCs crossing normal compartmental barriers. Following ablation of the Lgr5-positive cells in the intestinal crypt, differentiating lineage-committed precursor cells above the crypt base dedifferentiate into functional SCs which repopulate the crypt (Fig3D) (van Es et al, 2012; Buczacki et al, 2013; Ritsma et al, 2014). In lung and gastric epithelia, reversion of post-mitotic differentiated cells into SCs is observed following injury (Stange et al, 2013; Tata et al, 2013). In non-epithelial tissues, evidence for dedifferentiation is more limited, but following cytotoxic drug treatment, the depleted male germ cell GFRα1+ SC pool is regenerated by the combination of decreasing the probability of reversion of GFRα1+ differentiation and reversion of Ngn3+ cells, most of which differentiate in homoeostasis, back into GFRα1+ SCs (Fig3E; Nakagawa et al, 2010)(Hara et al, 2014).
Tissue damage or stress mobilises slow-cycling or quiescent SCs, which function as ‘reserve’ cells, such as dormant haematopoietic SCs that are rapidly recruited into cycle following treatment with the cytotoxic drug 5 fluorouracil or the cytokine Gcsf, the latter also triggering SC migration into the circulation (Wilson et al, 2008; Cheung & Rando, 2013) (Fig3F). The liver is an intriguing organ, capable of extensive regeneration after a variety of insults (Miyajima et al, 2014). Lineage tracing in different models of injury indicates surviving hepatocytes re-enter the cell cycle to restore their own lineage (Schaub et al, 2014; Tarlow et al, 2014; Yanger et al, 2014). Tissue repair can thus be achieved by dedifferentiation alone in the absence of a reserve stem cell population. Differentiated cells have also been observed to generate cycling progenitors of a different lineage. Following vascular stroke in mouse brain, lineage tracing provides evidence for differentiated astrocytes generating proliferating neuroblasts, supplementing neuroblasts derived from mobilised neural SCs in the SVZ which have migrated into the zone of injury (Magnusson et al, 2014) (Fig3G).
The ultimate test of the regenerative potential of a SC is to reconstitute a tissue from a single cell, a feat that requires an exponential increase in SC number and the generation of differentiated cells. Transplantation into immune-suppressed mice has long been a standard SC assay, but more recently, SC culture methods have been developed which reveal the ability of a single SC to reconstitute a tissue-like ‘organoid’ in defined culture conditions (Sato et al, 2009; Sato & Clevers, 2013). This was first demonstrated with human epidermal SCs that generate large colonies containing multiple SCs and differentiating keratinocytes, which fuse into stratified sheets (Rheinwald & Green, 1975; Jones et al, 1995). When this cultured epidermis is grafted back onto burnt patients, it persists over many years (Gallico et al, 1984; Compton et al, 1989). More recently, 3-dimensional culture systems were established for intestinal and prostatic SCs from both mice and humans (Sato et al, 2009, 2011a; Chua et al, 2014; Karthaus et al, 2014). Disaggregation and re-culturing reveal each organoid contains self-renewing SCs capable of multiple rounds of organoid formation (Sato et al, 2009). The ability of SCs and their progeny to assemble themselves into a niche within a tissue-like array of differentiated cells in the absence of external spatial cues is remarkable and is most graphically illustrated in the formation of complex brain-like structures in 3D cell culture (Lancaster et al, 2013; Lancaster & Knoblich, 2014).
If this technology can be used to expand SC populations ex vivo which successfully reconstitute and sustain damaged tissues in vivo, it will have great potential for regenerative medicine (Huch et al, 2013a,b; Sato & Clevers, 2013; Dorrell et al, 2014). In combination with genome editing, ex vivo stem cell expansion may be particularly powerful for treating genetic diseases with tissue-restricted phenotypes, where engrafted SCs can compete with host SCs on at least even terms (Schwank et al, 2013). The factors required to culture organoids give insight into the nature of niche signals for SC survival and proliferation, an area we will explore below (Sato et al, 2011b).
To summarise, recent in vivo studies have revealed dramatic plasticity following tissue damage. SCs are recruited into cycle and/or change their fate to increase cell production. Progenitors and even differentiated cells may dedifferentiate to speed repair. Spatial compartments and lineage boundaries are crossed. Advances in cell culture are further revealing the self-organising ability of SCs and their progeny in generating organ-like structures in the absence of tissue cues.
Autonomy and instruction: regulating SC states
We now turn to examine recent insights into the regulation of SC dynamics in homoeostasis and regeneration, critical in tuning cell production to the requirements of the tissue and whole organism. Potentially adjustable parameters include the rate of SC division and the balance of the resulting SC and differentiating progeny. In tissues with minimal cell turnover, SCs are actively maintained in a quiescent state and conditionally activated. We will consider the relative contributions of cell-intrinsic factors, paracrine signalling in the SC microenvironment, the physical characteristics of the niche and ‘remote’ signals such as hormones and neural regulation in switching SC behaviour.
For squamous SCs, population asymmetry requires balancing the probabilities of three potential division outcomes (Clayton et al, 2007; Doupe et al, 2012). This process requires Notch signalling, which was long known to drive the differentiation of post-mitotic keratinocytes (Lowell et al, 2000; Rangarajan et al, 2001; Nicolas et al, 2003; Blanpain et al, 2006). Transgenic inhibition of Notch signalling in individual oesophageal SCs accelerates the rate of cell division and blocks the symmetric differentiation division outcome (Alcolea et al, 2014). As a result, eventually, the entire epithelium is replaced by Notch-inhibited cells. Intriguingly, as this occurs, the basal layer becomes crowded and the symmetric differentiation division outcome is restored (Alcolea et al, 2014). In culture, cell crowding promotes keratinocyte differentiation, suggesting the reinstatement of homoeostasis may be a consequence of increased cell density within the basal layer (Watt et al, 1988). Regulation of cell density is a robust and widespread phenomenon, and potentially is a defence against SCs carrying any mutation that enhances proliferation and results in crowding in the niche (Eisenhoffer et al, 2012; Marinari et al, 2012; Frede et al, 2014).
The regulation of squamous SC proliferation also involves highly conserved cell–cell and paracrine signalling pathways. Epidermal SCs secrete both Wnt ligands and Wnt inhibitory Dkk proteins, resulting in the activation of Wnt target genes in basal cells (Choi et al, 2013; Lim et al, 2013). Blockade of Wnt signalling in SCs inhibits their proliferation, resulting in a thinned epidermis (Choi et al, 2013; Lim et al, 2013). Autocrine Wnt signalling is thus an essential part of the squamous SC niche, providing permission to proliferate (Frede & Jones, 2013).
The remodelling of HFs during the hair cycle makes dissection of SC regulation in the fast-changing niche especially challenging, but cell autonomous factors and paracrine signalling again emerge as crucial SC regulators. Expression of the transcription factor Sox9 in HF SCs is essential for their survival and normal differentiation, but this is achieved at least in part by regulation of paracrine activin/TGFβ signalling by Sox9 target genes (Kadaja et al, 2014). Wnt signalling again plays a key role. If Wnt signals are inhibited, HF SCs enter a state of ‘suspended animation’ in which they persist long term but do not proliferate, re-entering the cell cycle when the Wnt activity is reinstated (Choi et al, 2013). In contrast, activating the Wnt pathway triggers SC expansion and tilts the axis of cell division to generate new HFs (Deschene et al, 2014). As with the epidermis, there is evidence for paracrine signalling, as SCs with high Wnt activity recruit adjacent SCs by secreting Wnt ligands (Deschene et al, 2014). Hedgehog signalling mediates the activation of quiescent SCs by differentiating TA cells (Hsu et al, 2014).
The short range of Wnt signals from Paneth cells, essential for SCs remaining in an undifferentiated state, restricts the number of SCs in the intestinal niche (Sato et al, 2011b). Expansion of the Paneth cell population is a feature of benign intestinal tumours, whose formation is driven by Wnt-activating Apc mutations (Schepers et al, 2012). Apc-mutant SCs eventually colonise the crypt through their enhanced fitness over wild-type cells, as a differentiating cell is more likely to be replaced by a mutant than a wild-type cell (Vermeulen et al, 2013). Loss of Lrig1, which negatively regulates ErbB family growth factor signalling, causes crypt expansion, while activation of Ras, which lies downstream of ErbB receptors, in intestinal SCs can trigger a process called crypt fission in which a mutant crypt splits into two allowing a mutant stem cell clone to spread through the intestine (Wong et al, 2012; Amoyel et al, 2014). Thus, while limiting niche size is a simple means of achieving homoeostasis, it is not invulnerable to mutations that confer a clonal fitness advantage on SCs.
The morphology of the niche has long been recognised as of pivotal importance in plants, whose architecture is determined by the spatial arrangement of the meristem (Heidstra & Sabatini, 2014). The size and shape of hair can be manipulated by altering the number of cells in the dermal papilla that forms the niche for hair germ SCs (Chi et al, 2013). Within the bone marrow, HSCs reside in close proximity to stromal cells adjacent to blood vessels and the bone surface (Mendelson & Frenette, 2014). Each of these cell types makes a distinct paracrine contribution to the niche (Ding et al, 2012). For example, the chemokine Cxcl12, which is essential for maintaining HSCs, derives primarily from the perivascular stromal cells that lie directly adjacent to quiescent HSCs (Ding & Morrison, 2013; Greenbaum et al, 2013). Paracrine niche signals also regulate neural SCs in the SVZ, which extend cellular processes into the cerebrospinal fluid (CSF) that fills the ventricles and forms contacts with underlying endothelial cells (Fig2H) (Silva-Vargas et al, 2013; Codega et al, 2014). These contacts allow regulation of neural SCs via both secreted factors in the CSF and signals from the vasculature. For example, endothelium-derived Ntf3 and G protein-coupled receptor ligands in the CSF promote SC quiescence (Codega et al, 2014; Delgado et al, 2014).
Seemingly at odds with SCs residing in an ordered niche is their ability to recapitulate the morphology of a tissue in culture (Rheinwald & Green, 1975; Sato et al, 2009; Stange et al, 2013; Karthaus et al, 2014). Feeder cells or exogenous growth factors and ligands in the media are able to substitute for the paracrine environment provided by the niche, but in isotropic 3-dimensional culture systems, morphogenic gradients are initially absent. A common feature of many protocols is the requirement for the Rock kinase inhibitor Y26732 during establishment of the culture. This suggests that manipulation of cytoskeletal signalling is required for isolated SCs to enter a state that allows survival and promotes proliferation in the absence of niche cells (Sato et al, 2009; Huch et al, 2013a,b; Karthaus et al, 2014). Rather than being determined by SCs, the resemblance of organoids to their parent tissue may rest on the ability of differentiating cells to self-assemble into a tissue-like array by virtue of cell shape-defined packing considerations and differential adhesion (Watt & Green, 1982; Honda et al, 1996).
Thus, recent studies are revealing more of the micro-architecture of the SC niche and the highly conserved paracrine signals, such as Wnt, which regulate cell behaviour across a wide range of lineages. While the niche may not ‘micromanage’ the outcome of each individual SC division, it has an essential role in sustaining SC populations in a proliferating or quiescent state. Understanding how cell-intrinsic factors, autocrine signals and exogenous factors combine to sustain SCs in culture will likely give new understanding of the niche in vivo.
Remote control: integrating SC dynamics and whole body physiology
Stem cell behaviour must not only match local requirements in the tissue, but also needs across the organism (Fig4). One example of such distant regulation is the circadian control of SCs. Proliferation varies dramatically in a diurnal cycle. For example in the mouse oesophagus, 10-fold more SCs undergo mitosis in the early diurnal than in the early nocturnal phase (Burns et al, 1976). In the epidermis, hair follicle bulge SCs vary in their transcription of the Per1 gene, arguing they are in different phases of the circadian cycle. Cells high in Per1 expression have increased clonogenicity in vitro, suggesting variation in circadian phase may contribute to functional heterogeneity in bulge SCs (Janich et al, 2011). In the hematopoietic system, SCs regularly leave the bone marrow and enter the circulation before returning to their niche. This migration also has a circadian rhythm with twice as many circulating SCs in the diurnal than in the nocturnal phase. The exit of SCs is controlled centrally from the suprachiasmatic nucleus in the brain, via release of noradrenaline from nerve endings in the bone marrow, which in turn regulates the transcription of Cxcl12, a chemokine that directs HSC migration (Mendez-Ferrer et al, 2008).
Figure 4. SC regulation from outside the niche.
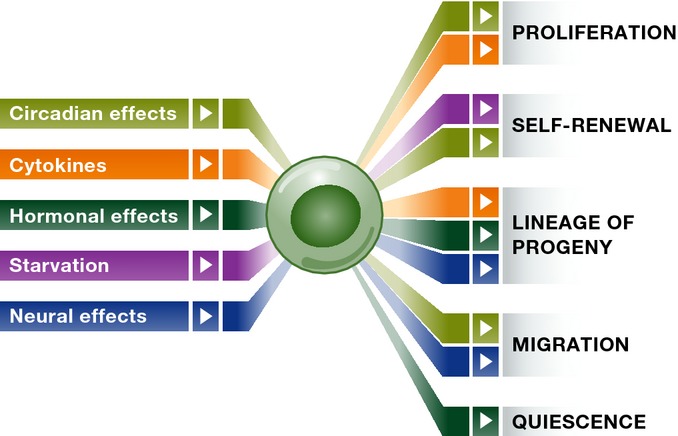
Summary of systemic influences on SC fate. Fate outcome is mapped by colour to distinct systemic factors. See the text for more detail.
Sex hormone regulation of reproductive and mammary SCs is well documented, but recently has been found to extend to SCs in other tissues. For example, HSCs are subject to positive regulation of proliferation and self-renewing cell divisions by oestrogen in female mice. In pregnancy, oestrogen levels rise, the number of cycling HSCs increases and more erythroid progenitor cells are produced, meeting the physiological requirement of the animal for increased oxygen delivery to tissues (Nakada et al, 2014). Another example is the hair cycle, which is arrested during in pregnancy by prolactin driving HF SCs into a quiescent state (Goldstein et al, 2014).
A significant evolutionary pressure on organisms and SCs is starvation, which induces a body-wide adaptive response that includes effects on SCs (Longo & Mattson, 2014; Mihaylova et al, 2014). The midgut of fasted Drosophila expands dramatically after feeding, underpinned by a switch in the mode of SC division from balanced cell production to self-duplication in response to insulin (O'Brien et al, 2011). In the murine small intestine, fasting reduces Mtorc1 signalling in the Paneth cells that form the SC niche (Yilmaz et al, 2012). In turn, this increases paracrine signalling to SCs via cyclic ADP ribose, resulting in an increased efficiency of organoid formation in cultures from fasted animals (Yilmaz et al, 2012). While not definitive, this result suggests starvation may expand the SC population by acting on the niche. Starvation also impacts haematopoiesis, acting via IGF1 signalling to increase the number of transplantable HSCs consistent with an enhanced probability of self-renewing division (Cheng et al, 2014).
Another route for physiological SC regulation is via the nervous system, recently revealed as having a key role in HSC regulation. Chronic psychological stress induces increased levels of leucocytes. As with circadian control, this effect is mediated by noradrenaline release from sympathetic neurons in the bone marrow, which decreases production of Cxcl12 in niche cells triggering HSC proliferation (Heidt et al, 2014). The importance of this mechanism is illustrated in the neoplastic myeloproliferative disorder, which is caused by JAK2 mutations in HSCs (Arranz et al, 2014). Secretion of interleukin 1b by the mutant HSCs induces neuropathy in bone marrow, decreasing CXCL12 levels and triggering HSC proliferation and disease progression. The development of the disease is halted by restoring adrenergic signalling by treatment with β3-adrenergic agonists (Arranz et al, 2014).
HSCs also illustrate how systemic signals interact with cell-intrinsic factors to promote the generation of a specific lineage from a multipotent SC population. For example, the cytokine M-CSF, which is released in high levels during infection, induces the master myeloid transcription factor PU-1, increasing the probability of HSCs differentiating into myeloid progenitors to meet the increased requirement for leucocytes (Mossadegh-Keller et al, 2013).
Drawing these studies together reveals a new aspect of SC biology that goes beyond single lineage or tissue. Understanding how neural, endocrine and cytokine signals regulate cell production to meet the requirements of an organism is a key challenge for SC research.
Sleeping, waking and sleeping again: regulating SC quiescence
Maintaining the quiescence of SCs is an active process (Cheung & Rando, 2013). For example, muscle satellite cells are maintained in a quiescent state by specific miRNAs and transcription factors downstream of Notch, loss of which results in depletion of the SC population (Fukada et al, 2011; Cheung et al, 2012; Crist et al, 2012). In the liver, the hepatocytes that fulfill the role of ‘reserve’ SCs are actively maintained in a quiescent and differentiated state by Hippo signalling acting via the Notch pathway (Yimlamai et al, 2014).
When a tissue is stressed or injured, quiescent cells must be mobilised. Entry into cycle may require specific transcription factors, such as Ascl1, which is required for neural SCs to exit the quiescent state (Andersen et al, 2014). The transition from quiescence also involves the induction of cellular biogenesis. In muscle SCs, the rapid upregulation of translation is achieved by post-translational mechanisms involving the sequestration of regulatory miRNAs in mRNP granules (Crist et al, 2012). Following a remote injury, circulating factors acting via HGF signalling and the mTorc1 signalling node prime quiescent SCs enhancing their regenerative potential. Once activated, some SCs contribute to differentiated muscle, while others re-enter the quiescent state to maintain the SC population (Shea et al, 2010). Return to quiescence requires multiple factors including Sprouty1, a negative regulator of receptor tyrosine kinase signalling and active Notch signalling (Shea et al, 2010; Gopinath et al, 2014).
It is becoming clear that the quiescent state is far from being a protected state as used to be thought. While cell competition results in the loss of mutant SCs in proliferating tissues, quiescent SCs accumulate epigenetic and genetic damage, resulting in impaired function as they age (Liu et al, 2013; Martins et al, 2014; Sun et al, 2014a). In quiescent HSCs, the error-prone non-homologous end-joining DNA repair alterations progressively accumulate (Mohrin et al, 2010; Beerman et al, 2014). Recruitment of HSCs into cycle is accompanied by induction of a broad range of DNA repair genes mobilised to remedy genomic alterations (Beerman et al, 2014). As well as intrinsic factors, age-associated inflammatory signalling acting via the Jak/Stat pathway impairs muscle SC function (Price et al, 2014; Tierney et al, 2014). Intriguingly, administration of a single circulating growth factor, Gdf11, seems able to reverse the defects in ageing muscle SCs (Sinha et al, 2014). Gdf11 also benefits neurogenesis by ageing neural SCs, possibly acting via effects on the vasculature in the SVZ (Katsimpardi et al, 2014). Understanding how quiescent SCs accumulate molecular damage and the ways in which this may be reversed is a central question in the biology of ageing.
Conclusion
Adult SCs have evolved a diverse repertoire of SC behaviours to meet tissue-specific requirements. In cycling tissues, homoeostatic balance between self-duplication and differentiation may be achieved by cell-intrinsic mechanisms, a spatially constrained niche, or exceptionally invariant asymmetric cell division. In each case, SC division is restrained by the requirement to maintain a constant cell density within the niche. Repair is also achieved by diverse strategies, with mobilisation of reserve SCs, plasticity of SC behaviour and dedifferentiation emerging as common themes. While specific growth factors may bias cell-intrinsic lineage selection, a shared paracrine signalling toolkit sustains SCs across multiple lineages (Clevers et al, 2014). Stochastic cell-intrinsic processes may be biased by extrinsic signals, including ‘long-distance’ signals from nerves and hormones. The application of powerful imaging and transgenic tools to reveal the dynamics of SCs within their niche offers the prospect of integrating molecular and cellular mechanisms to gain a broader perspective on the functional plasticity of SCs in the context of the whole organism.
Acknowledgments
We thank J. Fowler, J. Frede, P. Greulich, A.M. Klein, K. Murai, B.D. Simons and D.J. Winton for discussions. We acknowledge the support of the Medical Research Council, the Wellcome Trust (Project grant WT090334MA, P.H.J. and PhD studentship Programme in Stem Cell Biology & Medicine, A.W.) and Cancer Research UK (Programme Grant C609/A17257, P.H.J.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, Jones PH. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol. 2014;16:615–622. doi: 10.1038/ncb2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea MP, Jones PH. Tracking cells in their native habitat: lineage tracing in epithelial neoplasia. Nat Rev Cancer. 2013;13:161–171. doi: 10.1038/nrc3460. [DOI] [PubMed] [Google Scholar]
- Alcolea MP, Jones PH. Lineage analysis of epidermal stem cells. Cold Spring Harb Perspect Med. 2014;4:a015206. doi: 10.1101/cshperspect.a015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Simons BD, Bach EA. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J. 2014;33:2295–2313. doi: 10.15252/embj.201387500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Goritz C, Frisen J, Nakafuku M, Guillemot F. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 2014;83:1085–1097. doi: 10.1016/j.neuron.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjos-Afonso F, Currie E, Palmer HG, Foster KE, Taussig DC, Bonnet D. CD34(-) cells at the apex of the human hematopoietic stem cell hierarchy have distinctive cellular and molecular signatures. Cell Stem Cell. 2013;13:161–174. doi: 10.1016/j.stem.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntion S, Tzeng YS, Lai DM, Schwaller J, Skoda RC, Mendez-Ferrer S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- Babovic S, Eaves CJ. Hierarchical organization of fetal and adult hematopoietic stem cells. Exp Cell Res. 2014;329:185–191. doi: 10.1016/j.yexcr.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Baker AM, Cereser B, Melton S, Fletcher AG, Rodriguez-Justo M, Tadrous PJ, Humphries A, Elia G, McDonald SA, Wright NA, Simons BD, Jansen M, Graham TA. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 2014;8:940–947. doi: 10.1016/j.celrep.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beerman I, Seita J, Inlay Matthew A, Weissman Irving L, Rossi Derrick J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15:37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Burns ER, Scheving LE, Fawcett DF, Gibbs WM, Galatzan RE. Circadian influence on the frequency of labeled mitoses method in the stratified squamous epithelium of the mouse esophagus and tongue. Anat Rec. 1976;184:265–273. doi: 10.1002/ar.1091840302. [DOI] [PubMed] [Google Scholar]
- Catlin SN, Busque L, Gale RE, Guttorp P, Abkowitz JL. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117:4460–4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanin L, Ander JJ, Hoeckendorf B, Lust K, Kellner T, Kraemer I, Urbany C, Hasel E, Harris WA, Simons BD, Wittbrodt J. Exclusive multipotency and preferential asymmetric divisions in post-embryonic neural stem cells of the fish retina. Development. 2014;141:3472–3482. doi: 10.1242/dev.109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, Cotsarelis G, Andl T, Morrisey EE, Millar SE. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, McKiernan JM, Benson MC, Hibshoosh H, Shen MM. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol. 2014;16:951–961. doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Codega P, Silva-Vargas V, Paul A, Maldonado-Soto Angel R, DeLeo AnninaM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton CC, Gill JM, Bradford DA, Regauer S, Gallico GG, O'Connor NE. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989;60:600–612. [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crist CG, Montarras D, Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P, Farinas I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, Saotome I, Greco V. beta-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science. 2014;343:1353–1356. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Tarlow B, Wang Y, Canaday PS, Haft A, Schug J, Streeter PR, Finegold MJ, Shenje LT, Kaestner KH, Grompe M. The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res. 2014;13:275–283. doi: 10.1016/j.scr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, Jones PH. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Morita Y, Suda T. Heterogeneity and hierarchy of hematopoietic stem cells. Exp Hematol. 2014;42:74–82. doi: 10.1016/j.exphem.2013.11.004. .e2. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A, Clevers H. Dll1(+) secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous TG, Islam S, Tadrous PJ, Elia G, Kocher HM, Bhattacharya S, Mears L, Turnbull DM, Taylor RW, Greaves LC, Chinnery PF, Taylor G, McDonald SA, Wright NA, Alison MR. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–1663. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede J, Adams DJ, Jones PH. Mutation, clonal fitness and field change in epithelial carcinogenesis. J Pathol. 2014;234:296–301. doi: 10.1002/path.4409. [DOI] [PubMed] [Google Scholar]
- Frede J, Jones PH. Development. Permission to proliferate. Science. 2013;342:1183–1184. doi: 10.1126/science.1248274. [DOI] [PubMed] [Google Scholar]
- Fukada S, Yamaguchi M, Kokubo H, Ogawa R, Uezumi A, Yoneda T, Matev MM, Motohashi N, Ito T, Zolkiewska A, Johnson RL, Saga Y, Miyagoe-Suzuki Y, Tsujikawa K, Takeda S, Yamamoto H. Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development. 2011;138:4609–4619. doi: 10.1242/dev.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, Xie S, Laurenti E, Hermans K, Eppert K, Marciniak SJ, Goodall JC, Green AR, Wouters BG, Wienholds E, Dick JE. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–272. doi: 10.1038/nature13228. [DOI] [PubMed] [Google Scholar]
- Gallico GG, 3rd, O'Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, Nace A, Zhang X, Baratono S, Wang F, Ornitz DM, Millar SE, Cotsarelis G. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:1515. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the Process of self-renewal. Stem Cell Reports. 2014;2:414–426. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, Simons BD, Yoshida S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–672. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra R, Sabatini S. Plant and animal stem cells: similar yet different. Nat Rev Mol Cell Biol. 2014;15:301–312. doi: 10.1038/nrm3790. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Tanemura M, Imayama S. Spontaneous architectural organization of mammalian epidermis from random cell packing. J Invest Dermatol. 1996;106:312–315. doi: 10.1111/1523-1747.ep12342964. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013a;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013b;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Janich P, Pascual G, Merlos-Suarez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- Jones P, Simons BD. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat Rev Mol Cell Biol. 2008;9:82–88. doi: 10.1038/nrm2292. [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, Stokes N, Zheng D, Fuchs E. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28:328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, Vries RG, Cuppen E, Chen Y, Sawyers CL, Clevers HC. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159:163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7:214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavare S, Vermeulen L, Winton DJ. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell. 2013;13:626–633. doi: 10.1016/j.stem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Kronen MR, Schoenfelder KP, Klein AM, Nystul TG. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS ONE. 2014;9:e101085. doi: 10.1371/journal.pone.0101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:17. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Walker BE. Renewal of cell populations. Physiol Rev. 1956;36:255–276. doi: 10.1152/physrev.1956.36.2.255. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magami Y, Azuma T, Inokuchi H, Kokuno S, Moriyasu F, Kawai K, Hattori T. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver. 2002;22:419–425. doi: 10.1034/j.1600-0676.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, Lasitschka F, Mastitsky SE, Brors B, Hielscher T, Fehling HJ, Rodewald H-R. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014;509:465–470. doi: 10.1038/nature13317. [DOI] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Sabatini DM, Yilmaz OH. Dietary and metabolic control of stem cell function in physiology and cancer. Cell Stem Cell. 2014;14:292–305. doi: 10.1016/j.stem.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegue E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Navascues J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martinez-Arias A, Simons BD. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31:2473–2485. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;182:163–171. doi: 10.1016/j.ajpath.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodins K, Cheale M, Coleman N, Fox SB. Minichromosome maintenance protein 2 expression in normal kidney and renal cell carcinomas: relationship to tumor dormancy and potential clinical utility. Clin Cancer Res. 2002;8:1075–1081. [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014;25–26:34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan A, Jones PH. Act your age: tuning cell behavior to tissue requirements in interfollicular epidermis. Semin Cell Dev Biol. 2012;23:884–889. doi: 10.1016/j.semcdb.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011a;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011b;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepeler T, Page ME, Jensen KB. Heterogeneity and plasticity of epidermal stem cells. Development. 2014;141:2559–2567. doi: 10.1242/dev.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, Wang H, Le T, Faull KF, Chen R, Gu H, Bock C, Meissner A, Gottgens B, Darlington GJ, Li W, Goodell MA. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014a;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014b;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–289. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, Sacco A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavare S, Winton DJ. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–998. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer. 2014;14:468–480. doi: 10.1038/nrc3744. [DOI] [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982;295:434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Watt FM, Jordan PW, O'Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci USA. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction. 2012;144:293–302. doi: 10.1530/REP-11-0320. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]