Abstract
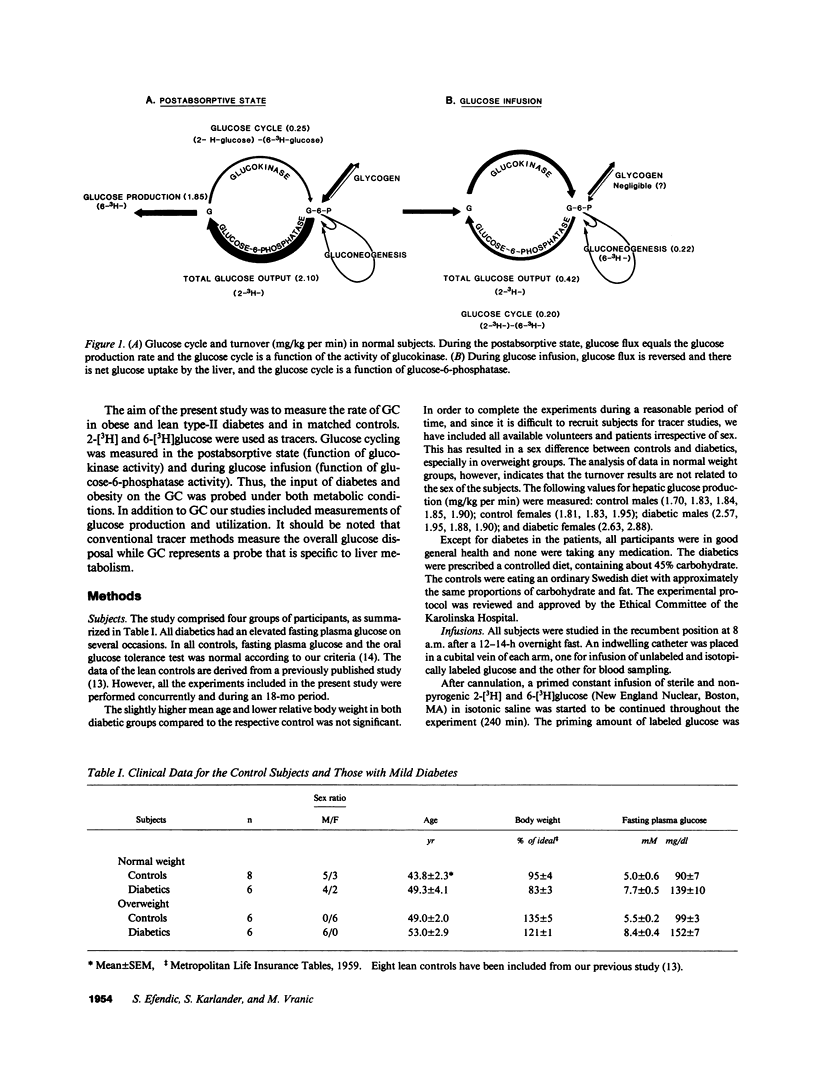
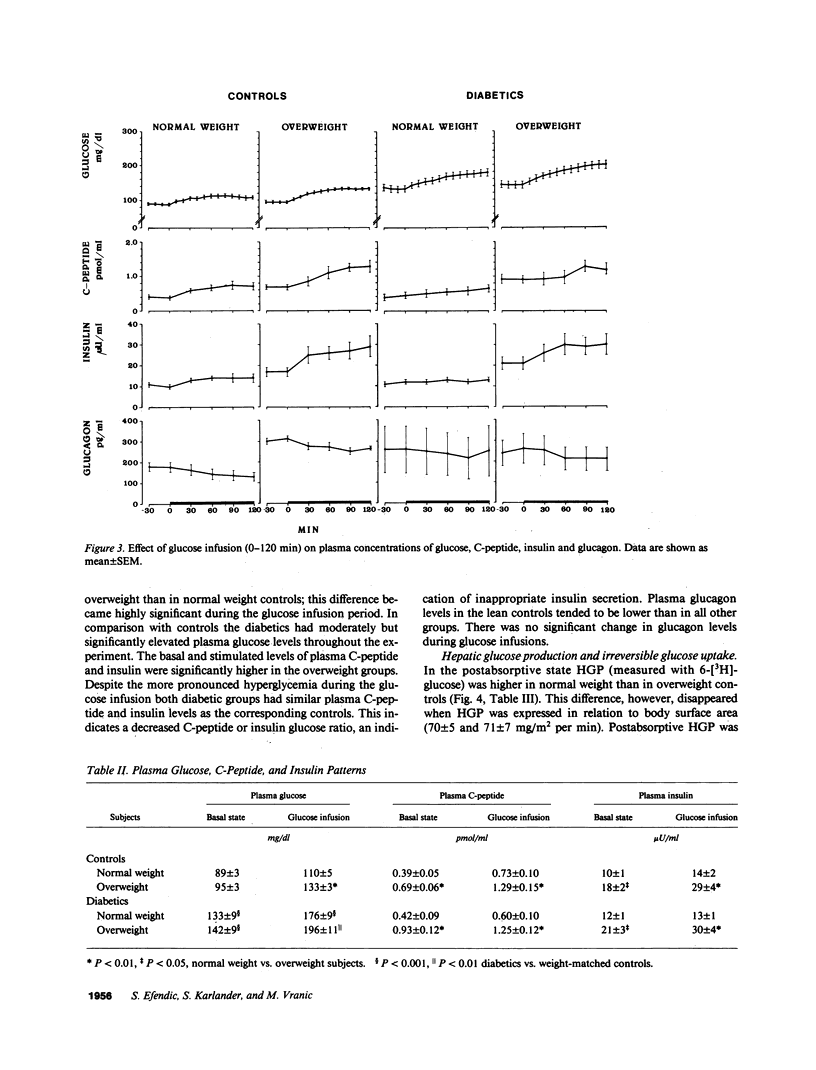
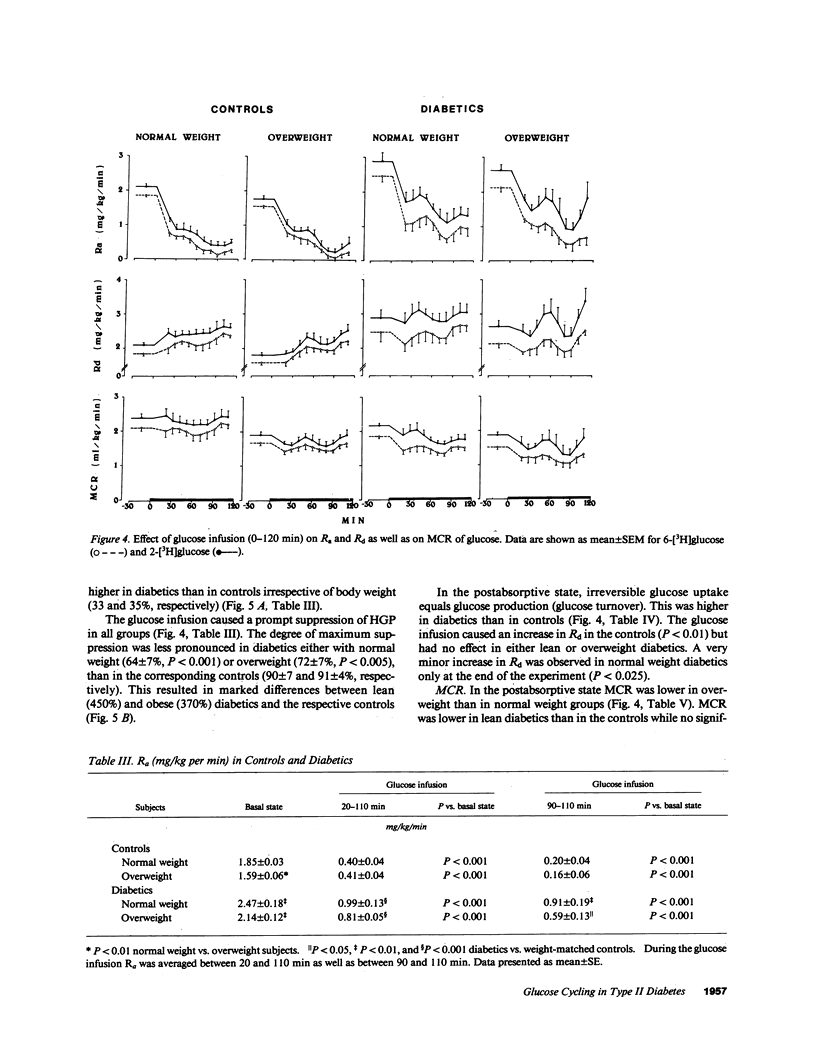
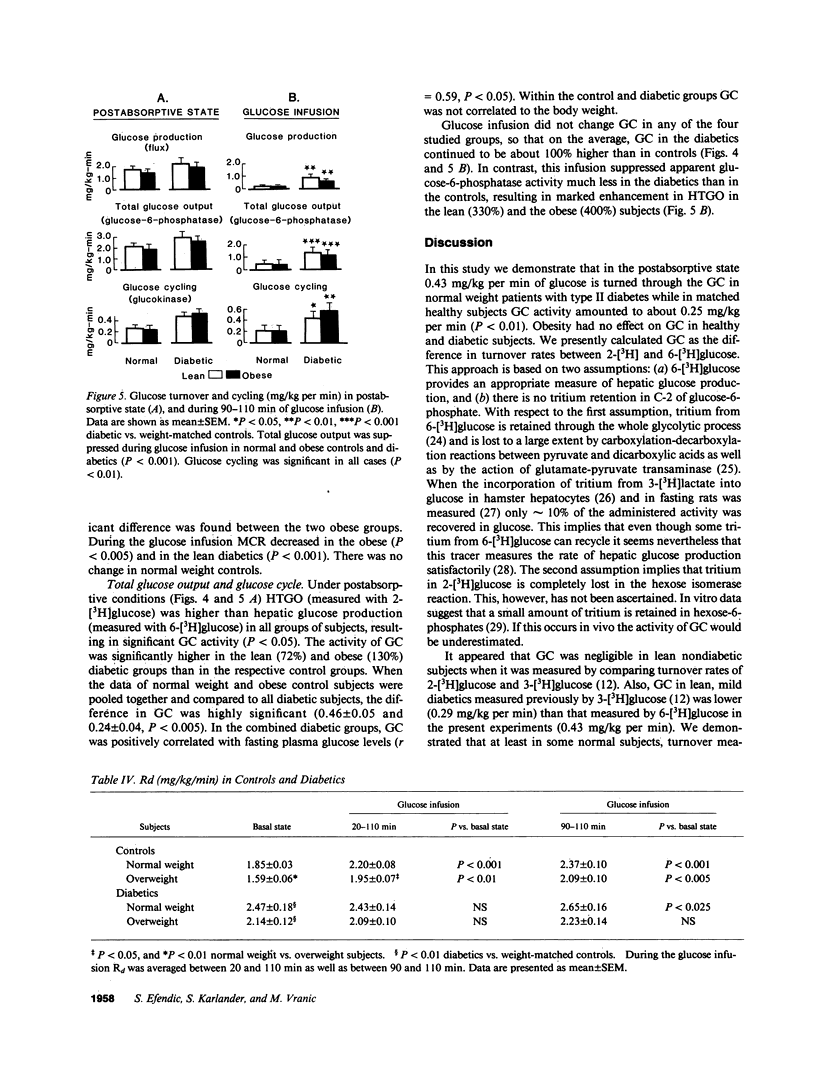
Glucose cycling (GC; G in equilibrium G6P) equals 14% of glucose production in postabsorptive man. Our aim was to determine glucose cycling in six lean and six overweight mild type II diabetics (fasting glycemia: 139 +/- 10 and 152 +/- 7 mg/dl), in postabsorptive state (PA) and during glucose infusion (2 mg/kg per min). 14 control subjects were weight and age matched. GC is a function of the enzyme that catalyzes the reaction opposite the net flux and is the difference between hepatic total glucose output (HTGO) (2-[3H]glucose) and hepatic glucose production (HGP) (6-[3H]-glucose). Postabsorptively, GC is a function of glucokinase. With glucose infusion the flux is reversed (net glucose uptake), and GC is a function of glucose 6-phosphatase. In PA, GC was increased by 100% in lean (from 0.25 +/- 0.07 to 0.43 +/- .08 mg/kg per min) and obese (from 0.22 +/- 0.05 to 0.50 +/- 0.07) diabetics. HGP and HTGO increased in lean and obese diabetics by 41 and 33%. Glucose infusion suppressed apparent phosphatase activity and gluconeogenesis much less in diabetics than controls, resulting in marked enhancement (400%) in HTGO and HGP, GC remained increased by 100%. Although the absolute responses of C-peptide and insulin were comparable to those of control subjects, they were inappropriate for hyperglycemia. Peripheral insulin resistance relates to decreased metabolic glucose clearance (MCR) and inadequate increase of uptake during glucose infusion. We conclude that increases in HGP and HTGO and a decrease of MCR are characteristic features of mild type II diabetes and are more pronounced during glucose infusion. There is also an increase in hepatic GC, a stopgap that controls changes from glucose production to uptake. Postabsorptively, this limits the increase of HGP and glycemia. In contrast, during glucose infusion, increased GC decreases hepatic glucose uptake and thus contributes to hyperglycemia. Obesity per se did not affect GC. An increase in glucose cycling and turnover indicate hepatic insulin resistance that is observed in addition to peripheral resistance. It is hypothesized that in pathogenesis of type II diabetes, augmented activity of glucose-6-phosphatase and kinase may be of importance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Chapman T. E., Gronwall R. Glucose utilization and recycling in ponies. Am J Physiol. 1976 Jan;230(1):138–142. doi: 10.1152/ajplegacy.1976.230.1.138. [DOI] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Assessment of insulin action in insulin-dependent diabetes mellitus using [6(14)C]glucose, [3(3)H]glucose, and [2(3)H]glucose. Differences in the apparent pattern of insulin resistance depending on the isotope used. J Clin Invest. 1986 Dec;78(6):1479–1486. doi: 10.1172/JCI112739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman R. N., Finegood D. T., Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Romsos D. R., Leveille G. A. Glucose turnover in the young chicken (Gallus domesticus) using variously labeled (3H, U-14C) glucose tracers. Comp Biochem Physiol B. 1977;56(4):421–425. doi: 10.1016/0305-0491(77)90242-5. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973 Jul;22(7):537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DUNN D. F., FRIEDMANN B., MAASS A. R., REICHARD G. A., WEINHOUSE S. Effects of insulin on blood glucose entry and removal rates in normal dogs. J Biol Chem. 1957 Mar;225(1):225–237. [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Cerasi E., Luft R. Insulin release, insulin sensitivity, and glucose intolerance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7425–7429. doi: 10.1073/pnas.77.12.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Vranić M. Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes. Proc Natl Acad Sci U S A. 1985 May;82(9):2965–2969. doi: 10.1073/pnas.82.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Rizza R. A. Effects of tolazamide and exogenous insulin on insulin action in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1986 May 15;314(20):1280–1286. doi: 10.1056/NEJM198605153142003. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hue L., Hers H. G. On the use of (3H, 14C)labelled glucose in the study of the so-called "futile cycles" in liver and muscle. Biochem Biophys Res Commun. 1974 Jun 4;58(3):532–539. doi: 10.1016/s0006-291x(74)80453-5. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Karlander S., Roovete A., Vranić M., Efendić S. Glucose and fructose 6-phosphate cycle in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E530–E536. doi: 10.1152/ajpendo.1986.251.5.E530. [DOI] [PubMed] [Google Scholar]
- Karlander S., Vranić M., Efendić S. Increased glucose turnover and glucose cycling in acromegalic patients with normal glucose tolerance. Diabetologia. 1986 Nov;29(11):778–783. doi: 10.1007/BF00873216. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Rognstad R. Glucose phosphorylation, glucose-6-phosphatase, and recycling in rat hepatocytes. J Biol Chem. 1978 Jul 10;253(13):4530–4536. [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickley H. L., Kemmer F. W., el-Tayeb K. M., Vranic M. Importance of glucagon in the control of futile cycling as studied in alloxan-diabetic dogs. Diabetologia. 1987 Mar;30(3):175–182. doi: 10.1007/BF00274224. [DOI] [PubMed] [Google Scholar]
- Marsh B. D., Marsh D. J., Bergman R. N. Oscillations enhance the efficiency and stability of glucose disposal. Am J Physiol. 1986 May;250(5 Pt 1):E576–E582. doi: 10.1152/ajpendo.1986.250.5.E576. [DOI] [PubMed] [Google Scholar]
- Nankervis A., Proietto J., Aitken P., Harewood M., Alford F. Differential effects of insulin therapy on hepatic and peripheral insulin sensitivity in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1982 Oct;23(4):320–325. doi: 10.1007/BF00253737. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Foster D. W., McGarry J. D. Evidence for suppression of hepatic glucose-6-phosphatase with carbohydrate feeding. Diabetes. 1984 Feb;33(2):192–195. doi: 10.2337/diab.33.2.192. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Okajima F., Chenoweth M., Rognstad R., Dunn A., Katz J. Metabolism of 3H- and 14C-labelled lactate in starved rats. Biochem J. 1981 Feb 15;194(2):525–540. doi: 10.1042/bj1940525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979 Aug 15;182(2):565–575. doi: 10.1042/bj1820565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G., Kemmer F. W., Lickley H. L., Vranic M. Importance of glucagon in mediating epinephrine-induced hyperglycemia in alloxan-diabetic dogs. Am J Physiol. 1981 Oct;241(4):E328–E335. doi: 10.1152/ajpendo.1981.241.4.E328. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Ladenson P. W., Wolfe M. H., Ridgway E. C., Wolfe R. R. Substrate cycling between gluconeogenesis and glycolysis in euthyroid, hypothyroid, and hyperthyroid man. J Clin Invest. 1985 Aug;76(2):757–764. doi: 10.1172/JCI112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Morita S., Steiner G. Insulin resistance in obesity as analyzed by the response of glucose kinetics to glucagon infusion. Diabetes. 1980 Mar;29(3):169–176. doi: 10.2337/diab.29.3.169. [DOI] [PubMed] [Google Scholar]
- Wajngot A., Roovete A., Vranić M., Luft R., Efendić S. Insulin resistance and decreased insulin response to glucose in lean type 2 diabetics. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4432–4436. doi: 10.1073/pnas.79.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J Clin Invest. 1984 Oct;74(4):1404–1413. doi: 10.1172/JCI111551. [DOI] [PMC free article] [PubMed] [Google Scholar]



