Abstract
IMPORTANCE
Type 1 diabetes has historically been associated with a significant reduction in life expectancy. Major advances in treatment of type 1 diabetes have occurred in the past 3 decades. Contemporary estimates of the effect of type 1 diabetes on life expectancy are needed.
OBJECTIVE
To examine current life expectancy in people with and without type 1 diabetes in Scotland. We also examined whether any loss of life expectancy in patients with type 1 diabetes is confined to those who develop kidney disease.
DESIGN, SETTING, AND PARTICIPANTS
Prospective cohort of all individuals alive in Scotland with type 1 diabetes who were aged 20 years or older from 2008 through 2010 and were in a nationwide register (n=24 691 contributing 67 712 person-years and 1043 deaths).
MAIN OUTCOMES AND MEASURES
Differences in life expectancy between those with and those without type 1 diabetes and the percentage of the difference due to various causes.
RESULTS
Life expectancy at an attained age of 20 years was an additional 46.2 years among men with type 1 diabetes and 57.3 years among men without it, an estimated loss in life expectancy with diabetes of 11.1 years (95% CI, 10.1-12.1). Life expectancy from age 20 years was an additional 48.1 years among women with type 1 diabetes and 61.0 years among women without it, an estimated loss with diabetes of 12.9 years (95% CI, 11.7-14.1). Even among those with type 1 diabetes with an estimated glomerular filtration rate of 90 mL/min/1.73m2 or higher, life expectancy was reduced (49.0 years in men, 53.1 years in women) giving an estimated loss from age 20 years of 8.3 years (95% CI, 6.5-10.1) for men and 7.9 years (95% CI, 5.5-10.3) for women. Overall, the largest percentage of the estimated loss in life expectancy was related to ischemic heart disease (36% in men, 31% in women) but death from diabetic coma or ketoacidosis was associated with the largest percentage of the estimated loss occurring before age 50 years (29.4% in men, 21.7% in women).
CONCLUSIONS AND RELEVANCE
Estimated life expectancy for patients with type 1 diabetes in Scotland based on data from 2008 through 2010 indicated an estimated loss of life expectancy at age 20 years of approximately 11 years for men and 13 years for women compared with the general population without type 1 diabetes.
Accurate contemporary estimates of life expectancy among patients with type 1 diabetes would be useful as a summary measure of the current effect of diabetes, as a benchmark for assessing changes in diabetes care through time, and for the setting of insurance premiums. Although there are many reports of the standardized mortality ratios for type 1 diabetes, few studies have provided life expectancy data. Diabetes charities such as Diabetes UK and the Juvenile Diabetes Research Foundation cite losses of life expectancy of between 15 and 20 years.1,2 Estimates from the United States in the 1970s reported a loss of 27 years.3 A report from 431 patients in New Zealand with type 1 diabetes during the 1980s found a loss of 16.5 years.4 In contrast, a recent analysis based on 325 deaths in participants in the Pittsburgh Epidemiology of Diabetes Complications (EDC) study5 estimated a loss of only 4 years for those diagnosed after 1965 but used extrapolation for older ages because the cohort had few people who had attained older ages.
We therefore used a large comprehensive national registry of patients with type 1 diabetes living in Scotland to provide contemporary comparisons of life expectancy with the general population without type 1 diabetes. We derived estimates that reflect death rates from 2008 through 2010 and that encompass those with older attained ages. We also examined whether there was any loss of life expectancy among those with preserved renal function as some data suggest that there may no longer be any increase in mortality rates in type 1 diabetes among those without renal disease.6-8 In addtition, we examined the contribution of various causes to the overall life expectancy differential and the differential occurring in different age strata.
Methods
Approval for use of anonymized linked data with a waiver for individual consent was obtained from the Scotland A Research Ethics Committee, National Privacy (Caldicott) Guardians, and Privacy Advisory Committee.
Data Sources
The Scottish Care Information-Diabetes Collaboration (SCI-DC) database, previously described,9 registers data on all patients assigned a diagnosis of diabetes by their clinicians for 99.5% of general practices nationally. Diagnostic coding levels are very high for adults because they are required to receive payments under the general practice United Kingdom pay-for-performance program. Registration for those older than 12 years is also ensured because the SCI-DC database is used to invite patients to the national retinopathy screening program. Because 2% or less of retinopathy screening invitations are rejected on the basis of an incorrect assignation of diabetes, the positive predictive value of registration is 98%, and specificity is high.10 Anonymized individual-level data were extracted from SCI-DC and linked with mortality data from the National Records of Scotland. We used information on all people living with type 1 diabetes at any point from 2008 through 2010. Because SCI-DC is not yet complete for pediatric data, we considered survival from an attained age of 20 years. Clinician-assigned diabetes type was accepted unless contradicted by available data on age at diagnosis and prescription history (see the Supplement).
Serum creatinine measurements captured in the SCI-DC database from the year 2000 were used to calculate estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease 4 equation. Renal replacement therapy data were obtained from the Scottish Renal Registry. Renal function status was then categorized into preserved renal function (eGFR ≥ 90/min/1.73m2) or eGFR between 60 and 89 mL/min/1.73 m2, chronic kidney disease (CKD) stage 3 (eGFR ≥30 and <60 mL/min/1.73 m2), stage 4 (eGFR ≥15 and <30 mL/min/1.73 m2), or stage 5 (a history of renal replacement therapy or 2 consecutive eGFR measures <15 mL/min/1.73m2).
Statistical Methods
Abridged period life tables for those with type 1 diabetes in Scotland aged 20 years or older were derived using the Chiang method,11,12 without adjustment for age-specific death counts of 0, using 5-year age intervals up to age 80 years and an open-ended interval thereafter, and a 3-year calendar time interval from 2008 through 2010. The calendar time interval was chosen to correspond to the most recent available Scottish general population abridged life tables from the National Records of Scotland.13 To calculate life expectancy for the general population excluding people with type 1 diabetes, we subtracted the observed number of deaths and person-years in the type 1 diabetes population, grouped by sex and fine age strata from the National Records of Scotland figures for the total general population, approximating the total person-years for the general population by the sum of the midyear population estimates. We then generated an abridged life table using these adjusted figures using the method of Chiang II.11,14
To estimate life expectancy from birth, we used the same approach but included the death counts and person-years for those younger than 20 years from the same data sources. For age strata for which there were no deaths, we assumed the mortality rate of the total general population, as is an accepted practice when computing life expectancy in populations with few deaths. In the life table calculations the number of persons lx surviving to the start of each subsequent age interval x out of a hypothetical cohort lo of 100 000 persons observed from an age interval of 20 through 24 years was estimated by lx−1.px−1 for which px−1 is the proportion expected to survive the previous age interval, in turn derived from the observed mortality rate Mx−1, the interval length nx−1, and the fraction of the last age interval lived ax−1 as
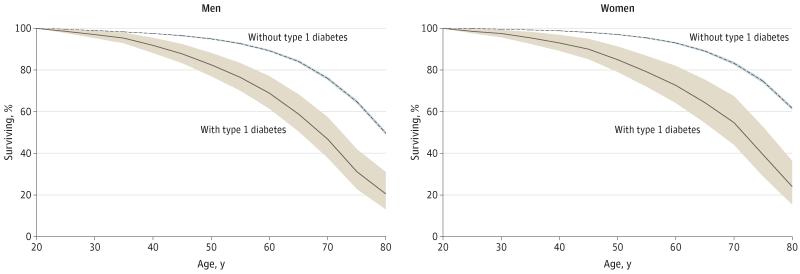
The estimated number of persons surviving as a percentage of the initial cohort was plotted by age to give a survival plot with confidence intervals obtained from the variance of a proportion (Figure).
Figure. Percentage Surviving by Age Among Those With Type 1 Diabetes Compared With the General Population Without Type 1 Diabetes.
See the Methods section for life table calculations.
A life table for those with type 1 diabetes under conditions of preserved renal function (ie, eGFR maintained at ≥90 mL/min/1.73 m2) was derived by the cause-deletion method as detailed in the supplement.15,16 In brief, a population-averaged Poisson model was fitted to the death counts to give the relative risks associated with CKD category adjusted for sex and age. These relative risks were used to estimate the population attributable fraction of deaths associated with having renal function by eGFR category and then to estimate expected mortality under conditions of preserved renal function. The overall linear trend in mortality with worsening eGFR status was reported from the model.
The underlying causes of death, which are International Classification of Disease 10 (ICD-10) coded, were grouped into 14 mutually exclusive categories. Note that ICD coding does not distinguish between coma death due to hypoglycemia and coma death due to diabetic ketoacidosis (DKA). Data for the general population were available as death counts by sex, fine-agestrata, calendar year, and underlying cause. Cause-specific mortality rates were calculated by age and sex using exact person-years in the case of the type 1 diabetes population and approximate person-years for the general population without type 1 diabetes, estimated as the sum of the 3 midyear population estimates for 2008 through 2010. The difference in additional life expectancy from age 20 years between the general population without and those with type 1 diabetes was examined by broad age strata and cause using the discrete method of Arriaga17,18 to reveal the contributions of differences in age-specific mortality rates to this total life expectancy differential as well as the contribution of specific causes.
Data analysis was carried out using Stata version 11.0 (StataCorp LP) and R version 3.0.1.19 See the eAppendix in the Supplement for further details on statistical methods.
Results
Populations Studied
We excluded the small number of patients whose diabetes was secondary to other causes including 117 with cystic fibrosis and 71 with pancreatectomy such that during the 2008-2010 period, 24 691 people registered with type 1 diabetes were observed at an age of 20 years or older, contributing 67 712 person-years of follow-up and 1043 deaths. The population at the midpoint of the study period, July 2, 2009, was 22 744 persons (43.6% were women, consistent with the known sex difference in type 1 diabetes incidence),20 the median age was 43.0 years (interquartile range [IQR], 33.0-53.0), and duration of diabetes was and 18.6 years (IQR, 9.7-28.8). Among those in the general population without type 1 diabetes aged 20 years or older, there were 161 023 deaths in 12 064 456 person-years.
Abridged Life Table
Table 1 shows the abridged life tables for men and women with type 1 diabetes. The life tables provide estimates of the estimated life expectancy or years of life remaining at each attained age interval, conditional on survival of up to at least the start of that age interval. Note that the person-time of those with later onset of type 1 diabetes was only included in the diabetes column after diabetes onset. The difference in life expectancy between those with and without type 1 diabetes declined by attained age. The point estimates for loss in life expectancy associated with type 1 diabetes were slightly greater for women than men but with overlapping confidence intervals up to age 65 years. In the general population without type 1 diabetes, 76% of men and 83% of women survived to age 70 years compared with 47% of men and 55% of women with type 1 diabetes (Figure).
Table 1. Abridged Period Life Table for Men and Women With Type 1 Diabetes vs General Population Without Type 1 Diabetes in Scotland 2008-2010.
| Age Interval, y | Observed Data in Type 1 Diabetes Population | Estimated Additional Life Expectancy, (95% CI), ya | ||||
|---|---|---|---|---|---|---|
| Observed Deaths | Population at Mid-age Intervalb | Death Rate per 1000 Person-Years | Type 1 Diabetes Population | General Population Without Type 1 Diabetesc | Difference in Life Expectancy, y | |
| Men | ||||||
| 20-24 | 10 | 1215 | 2.8 | 46.2 (45.3-47.3) | 57.3 (57.2-57.4) | 11.1 (10.1-12.1) |
| 25-29 | 12 | 1303 | 3.1 | 41.8 (40.9-42.9) | 52.5 (52.4-52.6) | 10.7 (9.7-11.7) |
| 30-34 | 14 | 1327 | 3.5 | 37.4 (36.5-38.5) | 47.8 (47.7-47.9) | 10.4 (9.4-11.4) |
| 35-39 | 36 | 1591 | 7.7 | 33.0 (32.2-34.0) | 43.1 (43.0-43.2) | 10.1 (9.2-11.0) |
| 40-44 | 45 | 1690 | 8.8 | 29.2 (28.4-30.2) | 38.4 (38.3-38.5) | 9.2 (8.3-10.1) |
| 45-49 | 62 | 1649 | 12.5 | 25.4 (24.6-26.3) | 33.8 (33.7-33.9) | 8.4 (7.5-9.3) |
| 50-54 | 59 | 1274 | 15.1 | 21.9 (21.1-22.8) | 29.3 (29.2-29.4) | 7.4 (6.5-8.3) |
| 55-59 | 60 | 902 | 21.0 | 18.4 (17.7-19.3) | 25.0 (24.9-25.0) | 6.6 (5.8-7.4) |
| 60-64 | 72 | 733 | 31.9 | 15.2 (14.5-16.0) | 20.8 (20.7-20.9) | 5.6 (4.8-6.4) |
| 65-69 | 57 | 378 | 45.3 | 12.4 (11.6-13.3) | 17.0 (16.9-17.0) | 4.6 (3.7-5.5) |
| 70-74 | 76 | 307 | 81.1 | 9.9 (9.2-10.7) | 13.5 (13.4-13.6) | 3.6 (2.8-4.4) |
| 75-79 | 44 | 167 | 82.4 | 8.8 (8.1-9.5) | 10.4 (10.4-10.5) | 1.6 (0.9-2.3) |
| ≥80 | 45 | 91 | 142.7 | 7.0 (6.5-7.7) | 7.8 (7.7-7.8) | 0.8 (0.1-1.5) |
| Women | ||||||
| 20-24 | 8 | 943 | 2.8 | 48.1 (46.9-49.3) | 61.0 (60.9-61.1) | 12.9 (11.7-14.1) |
| 25-29 | 7 | 1046 | 2.2 | 43.7 (42.7-44.8) | 56.1 (56.0-56.2) | 12.4 (11.3-13.5) |
| 30-34 | 13 | 1014 | 4.3 | 39.2 (38.1-40.3) | 51.2 (51.1-51.3) | 12.0 (10.9-13.1) |
| 35-39 | 19 | 1230 | 5.2 | 35.0 (33.9-36.0) | 46.3 (46.3-46.4) | 11.3 (10.2-12.4) |
| 40-44 | 25 | 1271 | 6.6 | 30.8 (29.8-31.8) | 41.6 (41.5-41.6) | 10.8 (9.8-11.8) |
| 45-49 | 39 | 1122 | 11.3 | 26.8 (25.8-27.7) | 36.8 (36.8-36.9) | 10.0 (9.0-11.1) |
| 50-54 | 41 | 939 | 14.4 | 23.2 (22.2-24.2) | 32.2 (32.1-32.3) | 9.0 (8.0-10.0) |
| 55-59 | 35 | 667 | 17.0 | 19.7 (18.8-20.6) | 27.7 (27.6-27.8) | 8.0 (7.1-8.9) |
| 60-64 | 40 | 540 | 24.2 | 16.3 (15.4-17.1) | 23.4 (23.3-23.5) | 7.1 (6.2-8.0) |
| 65-69 | 37 | 365 | 32.2 | 13.0 (12.3-13.7) | 19.3 (19.2-19.3) | 6.3 (5.6-7.0) |
| 70-74 | 57 | 286 | 64.1 | 9.8 (9.2-10.4) | 15.5 (15.4-15.5) | 5.7 (5.1-6.3) |
| 75-79 | 57 | 184 | 96.7 | 7.6 (7.1-8.1) | 12.0 (11.9-12.0) | 4.4 (3.9-4.9) |
| ≥80 | 73 | 127 | 171.3 | 5.8 (5.2-6.5) | 9.0 (8.9-9.0) | 3.2 (2.9-3.9) |
Additional life expectancy in years at each attained age interval conditional on survival until the start of that age interval with 95% confidence intervals.
This column gives the number of individuals in each sex and age stratum who attained the mid-age point for the interval during the study period 2008 through 2010.
General population after excluding those with type 1 diabetes.
Estimated Life Expectancy in Those With Preserved Renal Function
The subset of type 1 diabetes population observable for eGFR over the study period, a total of 21 011 persons in whom 814 deaths were observed, was used to estimate the population attributable fraction of deaths due to having an eGFR of less than 90 mL/min/1.73 m2. At entry into the study, the prevalences of the eGFR categories were 42.0% with eGFR of 90 mL/min/1.73m2 or higher and 46.7% with eGFR from 60 to 90 mL/min/1.73m2 and 9.9% with stage 3, 1.0% with stage 4, and 0.5% with stage 5 CKD. Relative risks for mortality increased with CKD stage: 1.74 (95% CI, 1.39-2.17) for stage 3, 4.70 (95% CI, 3.44-6.44) for stage 4, and 8.70 (95% CI, 6.11-12.40) for stage 5 compared with patients with an eGFR of more than 90 mL/min/1.73m2 (P for trend <.001). However, as shown in Table 2 even among those with type 1 diabetes and an eGFR of 90 mL/min/1.73 m2, the estimated loss in life expectancy remained substantial and was similar between both sexes.
Table 2. Estimates of Life Expectancy for Men and Women With Type 1 Diabetes Under Conditions of Preserved Renal Function Compared With the General Population Without Type 1 Diabetes in Scotland 2008-2010.
| Age Interval, y | Modified Death Rate per 1000 Person-Years in Type 1 Diabetes Populationb | Estimated Additional Life Expectancy, (95% CI), ya | ||
|---|---|---|---|---|
| Type 1 Diabetes With Preserved Renal Function | General Population Without Type 1 Diabetes | Difference in Life Expectancy, y | ||
| Men | ||||
| 20-24 | 2.3 | 49.0 (47.2 to 50.7) | 57.3 (57.2 to 57.4) | 8.3 (6.5 to 10.1) |
| 25-29 | 2.5 | 44.5 (42.8 to 46.2) | 52.5 (52.4 to 52.6) | 8.0 (6.3 to 9.7) |
| 30-34 | 2.9 | 40.0 (38.4 to 41.6) | 47.8 (47.7 to 47.9) | 7.8 (6.2 to 9.4) |
| 35-39 | 6.4 | 35.6 (34.0 to 37.2) | 43.1 (43.0 to 43.2) | 7.5 (5.9 to 9.1) |
| 40-44 | 7.3 | 31.6 (30.2 to 33.2) | 38.4 (38.3 to 38.5) | 6.8 (5.3 to 8.3) |
| 45-49 | 10.3 | 27.7 (26.3 to 29.2) | 33.8 (33.7 to 33.9) | 6.1 (4.6 to 7.6) |
| 50-54 | 12.5 | 24.1 (22.7 to 25.5) | 29.3 (29.2 to 29.4) | 5.2 (3.8 to 6.6) |
| 55-59 | 17.4 | 20.5 (19.2 to 21.9) | 25.0 (24.9 to 25.0) | 4.5 (3.1 to 5.9) |
| 60-64 | 26.4 | 17.1 (15.9 to 18.5) | 20.8 (20.7 to 20.9) | 3.7 (2.4 to 5.0) |
| 65-69 | 37.5 | 14.2 (13.0 to 15.5) | 17.0 (16.9 to 17.0) | 2.8 (1.6 to 4.1) |
| 70-74 | 67.1 | 11.6 (10.5 to 12.8) | 13.5 (13.4 to 13.6) | 1.9 (0.7 to 3.1) |
| 75-79 | 68.2 | 10.3 (9.2 to 11.4) | 10.4 (10.4 to 10.5) | 0.1 (−1.0 to 1.2) |
| ≥80 | 118.1 | 8.5 (7.8 to 9.2) | 7.8 (7.7 to 7.8) | −0.7 (−1.4 to 0.0) |
| Women | ||||
| 20-24 | 2.0 | 53.1 (50.7 to 55.5) | 61.0 (60.9 to 61.1) | 7.9 (5.5 to 10.3) |
| 25-29 | 1.5 | 48.6 (46.3 to 50.9) | 56.1 (56.0 to 56.2) | 7.5 (5.2 to 9.8) |
| 30-34 | 3.0 | 44.0 (41.7 to 46.2) | 51.2 (51.1 to 51.3) | 7.2 (4.9 to 9.5) |
| 35-39 | 3.6 | 39.6 (37.4 to 41.8) | 46.3 (46.3 to 46.4) | 6.7 (4.5 to 8.9) |
| 40-44 | 4.6 | 35.3 (33.1 to 37.4) | 41.6 (41.5 to 41.6) | 6.3 (4.1 to 8.5) |
| 45-49 | 7.8 | 31.0 (29.0 to 33.1) | 36.8 (36.8 to 36.9) | 5.8 (3.7 to 7.9) |
| 50-54 | 10.0 | 27.2 (25.2 to 29.1) | 32.2 (32.1 to 32.3) | 5.0 (3.0 to 7.0) |
| 55-59 | 11.7 | 23.4 (21.5 to 25.3) | 27.7 (27.6 to 27.8) | 4.3 (2.4 to 6.2) |
| 60-64 | 16.8 | 19.7 (17.9 to 21.5) | 23.4 (23.3 to 23.5) | 3.7 (1.9 to 5.5) |
| 65-69 | 22.3 | 16.2 (14.5 to 17.9) | 19.3 (19.2 to 19.3) | 3.1 (1.4 to 4.8) |
| 70-74 | 44.4 | 12.8 (11.3 to 14.3) | 15.5 (15.4 to 15.5) | 2.7 (1.2 to 4.2) |
| 75-79 | 67.0 | 10.3 (9.0 to 11.7) | 12.0 (11.9 to 12.0) | 1.7 (0.3 to 3.1) |
| ≥80 | 118.7 | 8.4 (7.7 to 9.1) | 9.0 (8.9 to 9.0) | 0.6 (−0.1 to 1.3) |
Estimated additional life expectancy in years at each attained age interval conditional on survival until the start of that age interval.
Modified death rates obtained by reducing the observed rates by the fraction of deaths attributable to having an estimated glomerular filtration rate (eGFR) less than 90 mL/min/1.73 m2, attributable to having an estimated glomerular filtration rate less than 90 mL/min/1.73 m2, based on 21 011 participants with eGFR data.
Deaths by Cause
Table 3 shows the number and percentage of deaths related to each category of underlying cause. Overall ischemic heart disease was the most commonly reported cause of death and was the most frequently assigned cause of death for men younger than 50 years, followed by deaths from diabetic coma or DKA, whereas among women younger than 50 years, these latter 2 causes were equally frequent. Renal failure was given as the underlying cause of death for 5.7% among men and 6.2% among women with type 1 diabetes, although renal disease is also an important contributor to many cardiovascular deaths.
Table 3. Underlying Cause of Death in Those With Type 1 Diabetes, Frequency by Age and Sexa.
| Age at Death, y |
||||||
|---|---|---|---|---|---|---|
| Men, No. (%) |
Women, No. (%) |
|||||
| Underlying Cause |
20-49 |
≥50 |
Total |
20-49 |
≥50 |
Total |
| All causes | 179 (100.0) | 413 (100.0) | 592 (100.0) | 111 (100.0) | 340 (100.0) | 451 (100.0) |
|
| ||||||
| Malignant neoplasms | 19 (10.6) | 72 (17.4) | 91 (15.4) | 12 (10.8) | 58 (17.1) | 70 (15.5) |
|
| ||||||
| Circulatory disease | 42 (23.5) | 203 (49.2) | 245 (41.4) | 28 (25.2) | 156 (45.9) | 184 (40.8) |
|
| ||||||
| Ischemic heart disease | 37 (20.7) | 145 (35.1) | 182 (30.7) | 17 (15.3) | 104 (30.6) | 121 (26.8) |
|
| ||||||
| Cerebrovascular disease | 0 (0) | 23 (5.6) | 23 (3.9) | 5 (4.5) | 24 (7.1) | 29 (6.4) |
|
| ||||||
| Other circulatory | 5 (2.8) | 35 (8.5) | 40 (6.8) | 6 (5.4) | 28 (8.2) | 34 (7.5) |
|
| ||||||
| Diabetes mellitus | 36 (20.1) | 28 (6.8) | 64 (10.8) | 24 (21.6) | 16 (4.7) | 40 (8.9) |
|
| ||||||
| Diabetic coma or DKAb | 29 (16.2) | 11 (2.7) | 40 (6.8) | 17 (15.3) | 0 (0) | 17 (3.8) |
|
| ||||||
| Other complications | 7 (3.9) | 17 (4.1) | 24 (4.1) | 7 (6.3) | 16 (4.7) | 23 (5.1) |
|
| ||||||
| Renal failure | 8 (4.5) | 26 (6.3) | 34 (5.7) | 8 (7.2) | 20 (5.9) | 28 (6.2) |
|
| ||||||
| Infectious/parasitic diseasec | - | - | 9 (1.5) | - | - | 12 (2.7) |
|
| ||||||
| Respiratory disease | 7 (3.9) | 29 (7.0) | 36 (6.1) | 3 (2.7) | 29 (8.5) | 32 (7.1) |
|
| ||||||
| Diseases of digestive system | 17 (9.5) | 9 (2.2) | 26 (4.4) | 8 (7.2) | 17 (5.0) | 25 (5.5) |
|
| ||||||
| Suicide/mental disorder | 19 (10.6) | 8 (1.9) | 27 (4.6) | 8 (7.2) | 12 (3.5) | 20 (4.4) |
|
| ||||||
| Other external | 13 (7.3) | 11 (2.7) | 24 (4.1) | 5 (4.5) | 6 (1.8) | 11 (2.4) |
|
| ||||||
| Disease of nervous systemd | - | - | 8 (1.4) | - | - | 10 (2.2) |
|
| ||||||
| Other causes | 12 (6.7) | 16 (3.9) | 28 (4.7) | 8 (7.2) | 11 (3.2) | 19 (4.2) |
Abbreviation: DKA, diabetic ketoacidosis.
Data are number of deaths with percentages shown in parentheses by age strata. The total number of deaths equals 1043.
Diabetic coma or DKA indicates death due to diabetic coma or diabetic ketoacidosis.
Age and sex breakdown are omitted in these cells because 1 or more cell count of 1 to 4.
There were 10 050 men ever observed while younger than 50 years and 480150 years or older; there were 7587 women ever observed while younger than 50 years and 375150 years or older. This sums to greater than the total population because some people were observed in both age strata.
Examination of the Life Expectancy Differential by Age and Cause
We found that 44.9% of the subsequent 11.1 years of loss in life expectancy at age 20 years among men occurred before age 50 years, 50.5% occurred from ages 50 years to 74 years and 4.6% at age 75 years and older. For women 40.5% of the subsequent 12.9 years of loss in life expectancy at age 20 years occurred before age 50 years, 46.1% occurred from ages 50 years to 74 years, and 13.4% at age 75 and older. Table 4 shows the percentage contribution of reported causes of death to the loss of life expectancy at an attained age of 20 years overall and also then by 3 broad age strata. Negative values can occur for certain causes when the observed mortality rates in the type 1 diabetes population for that cause are lower than in the general population without type 1 diabetes due to the competing risk from other causes that are more frequent. Differences in the mortality rate for circulatory disease, particularly ischemic heart disease, explained the largest percentage of the subsequent loss in life expectancy at an attained age of 20 years (for ischemic heart disease, 36% of men and 31% of women; for circulatory disease, 45% of men and 42% of women), with diabetes-related coma or DKA next (16% in men; 9% in women), then renal failure (9% of each sex). The relationship of different causes of death varied by age, with diabetic coma or DKA being the largest cause of the loss in life expectancy younger than age 50 years. These data can be used to give an estimate of the likely statistical effect of removing a specific cause. Thus, because diabetes-related coma or DKA deaths accounted for 16% of the loss in life expectancy in men, the difference in life expectancy would be reduced by 16% from 11.1 to 9.3 years overall if this cause were avoided. This makes the simplistic assumption that actions to avoid diabetic coma or DKA deaths have no relationship with other deaths.
Table 4. Percentage Contribution of Differences in Cause-Specific Mortality to the Loss in Additional Life Expectancy at Age 20 Years by Broad Age Strata and Sex.
| Percentage Contribution of Cause to Loss-in-Life Expectancy (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Men, Age, y |
Women, Age, y |
|||||||
| 20-49 | 50-74 | ≥75 | All Ages | 20-49 | 50-74 | ≥75 | All Ages | |
| All causes | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
|
| ||||||||
| Malignant neoplasms | 8.9 (−1.0 to 23.1) | 5.4 (−2.6 to 14.5) | −18.2 (−100.0 to 50.0) | 5.8 (−3.9 to 13.6) | 4.6 (−1.8 to 13.2) | 7.3 (−1.4 to 18.4) | 3.8 (−8.2 to 18.6) | 5.7 (0.3 to 12.6) |
|
| ||||||||
| Ischemic heart disease | 21.6 (11.9 to 40.3) | 46.4 (34.4 to 67.1) | 65.7 (−26.1 to 100.0) | 36.2 (27.5 to 54.4) | 14.3 (7.0 to 26.0) | 41.0 (29.3 to 63.4) | 43.9 (24.5 to 78.0) | 30.6 (24.0 to 43.4) |
|
| ||||||||
| Cerebrovascular disease | −1.2 (−2.2 to −0.7) | 4.4 (0.6 to 8.9) | 8.9 (−35.9 to 71.0) | 2.1 (−1.0 to 6.0) | 3.6 (0.2 to 8.7) | 3.9 (−0.3 to 9.1) | 10.6 (−0.7 to 27.1) | 4.7 (1.8 to 8.7) |
|
| ||||||||
| Other circulatory | 1.8 (−1.8 to 6.2) | 9.8 (4.8 to 17.7) | 17.1 (−23.3 to 96.7) | 6.6 (2.6 to 12.6) | 5.5 (0.9 to 13.1) | 6.0 (1.5 to 12.4) | 15.6 (4.9 to 37.1) | 7.1 (3.9 to 12.4) |
|
| ||||||||
| Diabetic coma or DKAa | 29.4 (1.0 to 100.0) | 5.3 (2.3 to 9.6) | 2.9 (−10.3 to 37.7) | 16.0 (1.8 to 71.0) | 21.7 (11.2 to 52.6) | −0.0 (−0.1 to −0.0) | −0.0 (−0.1 to −0.0) | 8.8 (4.5 to 21.3) |
|
| ||||||||
| Other diabetic complications | 5.0 (0.4 to 14.3) | 5.5 (2.3 to 10.6) | 20.2 (−34.7 to 100.0) | 6.0 (1.8 to 13.4) | 7.8 (1.7 to 25.8) | 7.7 (3.6 to 15.1) | 7.1 (1.5 to 17.0) | 7.6 (4.4 to 15.3) |
|
| ||||||||
| Renal failure | 6.2 (1.9 to 16.7) | 11.4 (6.9 to 19.0) | 7.9 (−13.7 to 50.4) | 8.9 (5.6 to 15.1) | 8.4 (2.9 to 22.5) | 9.0 (4.2 to 16.3) | 8.9 (2.1 to 19.6) | 8.8 (5.6 to 15.3) |
|
| ||||||||
| Infectious/parasitic disease | 2.0 (−0.7 to 9.3) | 2.0 (0.0 to 5.0) | −3.0 (−20.9 to 5.6) | 1.8 (−0.3 to 5.7) | 4.1 (−0.3 to 13.2) | 3.6 (0.5 to 8.5) | 1.6 (−2.1 to 7.3) | 3.5 (1.2 to 8.0) |
|
| ||||||||
| Respiratory disease | 3.8 (−0.5 to 12.0) | 3.5 (−0.8 to 8.3) | 4.1 (−48.5 to 70.9) | 3.7 (−1.0 to 9.5) | 1.5 (−1.0 to 5.2) | 7.4 (1.9 to 15.5) | 2.4 (−7.2 to 13.1) | 4.4 (1.2 to 8.6) |
|
| ||||||||
| Diseases of digestive system | 8.7 (1.3 to 22.4) | −0.9 (−4.0 to 3.1) | −7.8 (−53.0 to 14.0) | 3.1 (−1.3 to 10 to 7) | 6.4 (0.4 to 16.9) | 4.1 (−0.3 to 10.2) | 4.2 (−2.1 to 14.7) | 5.0 (1.7 to 10.2) |
|
| ||||||||
| Suicide/mental disorder | −1.9 (−26.4 to 29.8) | −0.5 (−2.7 to 2.3) | −0.3 (−26.5 to 28.8) | −1.1 (−14.2 to 13.9) | 5.2 (−2.4 to 24.2) | 2.7 (−0.4 to 7.2) | 1.4 (−6.7 to 10.4) | 3.6 (−0.2 to 11.8) |
|
| ||||||||
| Other external | 4.3 (−10.3 to 18.3) | 2.3 (−0.3 to 5.8) | 6.3 (−27.9 to 51.9) | 3.4 (−4.8 to 10.4) | 5.1 (−0.5 to 18.1) | 0.6 (−1.0 to 3.6) | 4.1 (−1.2 to 12.5) | 2.9 (0.3 to 8.4) |
|
| ||||||||
| Disease of nervous system | 1.2 (−2.3 to 7.6) | 1.0 (−1.2 to 3.8) | −6.1 (−43.8 to 11.0) | 0.7 (−2.2 to 4.9) | 3.3 (−1.1 to 12.9) | 2.5 (−0.4 to 7.9) | −1.6 (−4.2 to 2.5) | 2.3 (−0.2 to 7.8) |
|
| ||||||||
| Other causes | 10.1 (0.6 to 38.3) | 4.5 (1.2 to 8.6) | 2.4 (−26.9 to 40.2) | 6.9 (−0.3 to 20.2 | 8.5 (1.6 to 23.6) | 4.3 (0.7 to 9.7) | −2.1 (−6.7 to 3.3) | 5.1 (1.8 to 12.3) |
Abbreviations: DKA, diabetic ketoacidosis; DM, Diabetes mellitus.
Diabetic coma or DKA indicates death due to diabetic coma or diabetic ketoacidosis.
In the less complete pediatric dataset, we observed 15 deaths in 11 143 person-years younger than age 20 years during the study period and the estimate of loss in life expectancy from birth with type 1 diabetes was 11.7 years (95% CI, 10.5-12.8) in men and 13.6 years (95% CI, 12.2-15.0) in women (ie, 0.6 years higher in men and 0.7 years higher in women than the life expectancy loss from age 20 years).
eTable 1 in the Supplement shows the counts of deaths by cause and the directly standardized cause-specific mortality rates.
Discussion
In this study using contemporary death rates across a national population with type 1 diabetes, we found that at age 20 years the average man with type 1 diabetes subsequently had an estimated life expectancy loss of about 11 years and women about 13 years. In the general population without type 1 diabetes, 76% of men and 83% of women survived to age 70 years compared with 47% of men and 55% of women with type 1 diabetes. Thus, there was a substantial loss of life associated with type 1 diabetes. Our sample size was very large, so we were able to provide estimates that reflect actual contemporary death rates.
Deaths due to diabetic coma or DKA were the primary reported cause of death associated with the loss in life expectancy occurring before age 50 years in men and women followed by ischemic heart disease. At all age strata, the importance of reducing atheromatous disease risks in diabetes can be seen; more than 40% of the differential in life expectancy was attributable to circulatory disease. Another important aspect of these data was the heterogeneity in the basis of the loss of life expectancy with malignant disease and diseases of other body systems also contributing. The further reduction of both acute and chronic complications of diabetes need to remain important in type 1 diabetes management strategies. We noted a slightly higher estimate for the loss in life expectancy with type 1 diabetes in women than men. This is consistent with reports of a greater relative risk for cardiovascular disease in women than men,21 which is poorly understood but may reflect sex differences in insulin resistance prevalence in type 1 diabetes.22
An important aspect of our study was that we examined how renal disease was related to loss of life expectancy. We did this as it has been reported from other studies that only those with evidence of albuminuria or reduced renal function showed increased relative risks of death.6-8 We found that renal disease remained an important factor associated with loss in life expectancy with relative risks for death being greatly elevated in those with worse renal function. Nevertheless, our data show that in type 1 diabetes, even for those with preserved renal function, there was still a substantial difference in life expectancy compared with the general population; thus, eliminating renal disease would not by itself be expected to remove the differential in life expectancy.
We cannot directly assess whether life expectancy or the loss in life expectancy has improved with type 1 diabetes because we do not have historical life expectancy data for our population. Indeed, previous studies of any population are sparse, and none have covered the full type 1 diabetes population of a country. Goodkin3 reported that the loss in life expectancy with type 1 diabetes was 27 years in 10 538 applicants for life insurance to a company in the United States observed from 1951 to 1971. Estimates by type of diabetes or by attained age were not given but in those with onset occurring before the age of 15 years, the loss in life expectancy was 27 years. In an analysis of a diabetes register covering Canterbury, New Zealand, spanning from 1984 through 1993, the remaining life expectancy at an attained age of 20 years for those with diabetes onset before the age of 30 years was about 40 years as compared with about 47 years for the combined sexes in our study with a loss in life expectancy of 17 years.4 Compared with these earlier estimates, but in different populations, our report shows a greater life expectancy at attained age of 20 years and a smaller loss in a life expectancy compared with the general population. However, whether these study populations are sufficiently similar to ours to make comparison of life expectancy appropriate is uncertain. The difference we found was also much lower than what is currently generally cited. For example, the Diabetes UK website has until now cited a loss of about 20 years associated with type 1 diabetes, although it is not specified from what attained age.18
Our results differ substantially from a recent report from the Pittsburgh EDC and Allegheny County registry on life expectancy in type 1 diabetes.5 In that study, approximately 2000 people with diagnosis dates spanning 1950 to 1980 were followed up to 2009. There were 325 recorded deaths in the Pittsburgh EDC. The life expectancy at the attained age of 20 years was 48.9 years for those who had been diagnosed from 1965 to 1980 compared with 47 years for the sexes combined in our study. Detailed comparisons with the general population were not shown but the authors noted that the overall estimated loss in life expectancy compared with the US general population was just 4 to 6 years for those in the Pittsburgh EDC study who were diagnosed from 1965 to 1980. Because our data reflect a more recent period than that study, we would expect the life expectancy to be higher among patients in our study and the loss in life expectancy to be less. This may reflect that we are studying different populations or better outcomes in the type 1 diabetes population in the United States. However, in that study only 26 people in the Pittsburgh EDC cohort had reached attained ages older than 60 years, so the mortality beyond that was largely extrapolated. This may also affect the comparison of the 2 studies. Because our data are based on national recording of all deaths from mandatory death certification, we are certain that the deaths we report in our cohort occurred and cannot be an overestimate.
An important question is whether our findings are generalizable internationally. This cannot be directly assessed because there are no large contemporary or historical nationally representative studies from other countries. Risk factor levels, differences in hemoglobin A1c levels, and risk-factor control among those with type 1 diabetes in Scotland are similar to those in England but somewhat higher than some European countries and some US cohorts,9 which could cause a greater loss of life expectancy in Scotland than in those countries. Therefore, it would be of interest to see contemporary larger scale data from the United States and other countries too. Such studies should not simply right-censor losses to follow-up but should test the potential statistical effect of such losses being due to death and should include nationally representative samples of patients with type 1 diabetes.
Study Limitations
There are 2 types of life expectancy estimation: period life expectancy estimates, which we have calculated, describe the expected additional years of life or life expectancy that pertains for people alive today if mortality rates across all age strata observed today pertained throughout their lives; cohort life expectancy estimates use recent trends in life expectancy to project further improvements in survival in the future; we did not have enough long-term trend data as yet for this calculation because the SCI-DC registry started in the early 1990s but did not achieve national coverage until the mid-2000s. Another limitation was that coverage of those at younger ages is only now becoming complete, so we restricted our main analyses to life expectancy from an attained age of 20 years or older. Our estimates including the less complete pediatric data were very similar but should be treated as less certain than the main analyses. In addition, we could not differentiate death due to DKA or hypoglycemic coma because ICD-10 coding does not permit this. As with most mortality statistics, we relied on coded diagnoses, with their inherent uncertainty, rather than autopsy data for cause of death.
Conclusions
Estimated life expectancy for patients with type 1 diabetes in Scotland based on data from 2008 through 2010 indicated a loss of subsequent life expectancy at age 20 years of approximately 11 years for men and 13 years for women compared with the general population without type 1 diabetes.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant WT086113 from the Wellcome Trust SHIP Grant and from the Chief Scientist Office of the Scottish Government.
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank the members of the Scottish Diabetes Research Network, the Scottish Renal Registry, and the SCI-DC team.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Petrie reported receiving personal fees from Novo Nordisk; grants, personal fees, and nonfinancial support from sanofi-aventis; grants and nonfinancial support from Merck Serono; grants and personal fees from AstraZeneca; personal fees from Alere; personal fees from Lilly; nonfinancial support from Itamar Medical; grants from Juvenile Diabetes Research Foundation; and grants and personal fees from Quintiles. Dr Lindsay reported receiving personal fees from Eli Lilly Limited and personal fees from Novo Nordisk Limited. Dr Colhoun reported receiving grants, personal fees, and costs for advisory panel participation from Pfizer Inc; costs for advisory panel and contribution to sponsored clinical trial from sanofi-aventis and Novartis Pharmaceuticals; costs for advisory panel, and contribution to sponsored clinical trial and research support from Eli Lilly & Co; grants and research support from and shareholder with Roche Pharmaceuticals; grants from Boehringer Ingelheim; grants from AstraZeneca LP. No other disclosures were reported.
Footnotes
REFERENCES
- 1. [Accessed July 31, 2014];Diabetes in the UK 2010: Key statistics on diabetes. Diabetes UK. http://www.diabetes.org.uk/Documents/Reports/Diabetes_in_the_UK_2010.pdf.
- 2.Juvenile Diabetes Research Foundation Ltd. [Accessed July 31, 2014];What is the life expectancy of someone with type 1 diabetes? http://www.jdrf.org.uk/life-with-type-1-diabetes/faq-about-type-1-diabetes/what-is-the-life-expectancy-of-someone-with-type-1-diabetes.
- 3.Goodkin G. Mortality factors in diabetes: a 20 year mortality study. J Occup Med. 1975;17(11):716–721. [PubMed] [Google Scholar]
- 4.Brown LJ, Scott RS, Moir CL. All-cause mortality in the Canterbury (New Zealand) insulin-treated Diabetic Registry population. Diabetes Care. 2001;24(1):56–63. doi: 10.2337/diacare.24.1.56. [DOI] [PubMed] [Google Scholar]
- 5.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61(11):2987–2992. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jørgensen ME, Almdal TP, Carstensen B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia. 2013;56(11):2401–2404. doi: 10.1007/s00125-013-3025-7. [DOI] [PubMed] [Google Scholar]
- 9.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9(10):e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed November 13, 2014];Scottish Care Information Diabetes Collaboration (SCI-DC) website. http://www.sci-diabetes.scot.nhs.uk/
- 11.Chiang CL. The life table and its construction. In: Chiang CL, editor. Introduction to Stochastic Processes in Biostatistics. Wiley & Sons; New York, NY: 1968. pp. 189–214. [Google Scholar]
- 12.Newell C. Methods and Models in Demography. Guilford Press; New York, NY: 1988. [Google Scholar]
- 13.Life expectancy for areas in Scotland, 2008-2010. General Register Office for Scotland; [Accessed July 31, 2014]. http://www.gro-scotland.gov.uk/statistics/theme/life-expectancy/scottish-areas/2008-2010/tables.html. [Google Scholar]
- 14.National Records of Scotland . Life Expectancy for Scotland: Methodology Guide. National Statistics; [Accessed October16, 2014]. 2014. http://www.gro-scotland.gov.uk/files2/stats/life-expectancy-areas-in-scotland/2010-2012/le-methodology-paper-april-2014.pdf [Google Scholar]
- 15.Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol. 2004;160(4):331–338. doi: 10.1093/aje/kwh222. [DOI] [PubMed] [Google Scholar]
- 16.Katzmarzyk PT, Lee IM. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arriaga EE. Measuring and explaining the change in life expectancies. Demography. 1984;21(1):83–96. [PubMed] [Google Scholar]
- 18.Ponnapalli KM. A comparison of different methods for decomposition of changes in expectation of life at birth and differentials in life expectancy at birth. Demogr Res. 2005;12(7):141–172. [Google Scholar]
- 19.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: [Accessed November 13, 2014]. 2013. http://www.r-project.org/ [Google Scholar]
- 20.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 21.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29(4):798–804. doi: 10.2337/diacare.29.04.06.dc05-1433. [DOI] [PubMed] [Google Scholar]
- 22.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843–2863. doi: 10.2337/dc14-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.