Calcium mineral deposits are radiographically evident in the majority of significant atherosclerotic lesions.1 These deposits generally consist of a non-homogeneous composite containing hydroxyapatite mineral nanocrystals embedded in an organic matrix including type I collagen nanofibers. The morphometry varies over a spectrum. Amorphous deposits lack organization at the light-microscopic scale while bone-like deposits have varying degrees of architectural macro-organization similar to those of developing and mature skeletal bone, which also consist of a non-homogeneous composite containing hydroxyapatite nanocrystals embedded in a collagenous organic matrix. Based on histopathological features, the organized, bone-like deposits appear to arise from angiogenic invasion of the amorphous deposits.2 This is consistent with the known sequence of events that leads to bone formation in fracture repair, embryonic intramembranous ossification, and in embryonic endochondral ossification, where bone arises from a cartilage scaffold.
The similarities between atherosclerotic calcification and osteogenesis are more than skin deep. Several investigative groups have demonstrated osteogenic features at the cellular and molecular levels, including dynamic osteogenic gene expression in vitro and in vivo as reviewed recently by Towler and colleagues.3 In this issue of ATVB, Duer and colleagues4 take this comparison to the next level -- nanoscale architecture. Using solid-state nuclear magnetic resonance (NMR) techniques, they examined the mineral-organic interface in calcium deposits from atherosclerotic plaque and skeletal bone and found marked similarities. The NMR method (rotational echo double resonance) has the capacity to isolate the nanoscale features because it generates forces that act only within distances less than 1 nm. Results showed that the interface in atherosclerotic mineral is also a bonded nanocomposite rather than a simple mixture, and, interestingly, that it is enriched in glycosaminoglycans.
In bone, the organic matrix has both physical and chemical effects on the mineral. Mechanically, the protein nanofibers in skeletal bone tissue contribute to physical integrity in a manner comparable to steel in reinforced concrete or straw in adobe, one providing compressile, and the other tensile, strength. Chemically, the interaction between the inorganic mineral and organic proteins appears to be complex, with the proteins, such as osteopontin, osteocalcin and bone sialoprotein having biphasic effects on the crystal initiation and propagation. Indeed, many of the macromolecules believed to initiate mineralization are also implicated in restricting it. Bone proteins interact with the mineral components via electrostatic interactions between negatively charged domains (such as phosphorylated and gamma-carboxylated amino acid groups) and the positively charged mineral surface, forming a biologically and chemically bonded composite, rather than a mere mixture.5 The forces in this bonding also allow the organic matrix to constrain the pattern of crystal formation. For example, the nanocrystal organization, which has some degrees of freedom, may be entrained to the known characteristic axial and helical periodicities of collagen I fibers.6 Thus, atherosclerotic calcium deposits may gain their mineral features from a basic template pattern generated by the organic matrix at a molecular or nanoscale level.
The findings of Duer et al.4 suggest that the regulatory mechanisms of osteogenesis are recapitulated in atherosclerotic calcification and ossification. This evidence for governance in atherosclerotic calcification conflicts with the older views of the process as “dystrophic,” accidental, or maladaptive, instead suggesting that vascular calcification is no accident, but a regulated process. The body appears to have every intention of producing mineral deposits in the plaque, though the purpose is unclear. One possibility is that soft tissue mineralization evolved as an adaptive response to chronic infectious or inflammatory foci. The ultimate immune response to tuberculous infection in a wide variety of soft tissues is a Ghon focus, containment by a capsule of osseous tissue. The fact that it requires an intact immune system suggests that this shell of bone surrounding the focus is no accident.7 Walls of ectopic bone also form around chronic parasitic infections, foreign bodies, and tumors, including schistosomiasis, silicosis, and breast cancer. In each of these cases, ordinary cellular and humoral immune mechanisms fail.
Clinically, the presence of calcium deposits around tuberculous nodules, is believed to confer stability, and the containment may explain how tuberculosis can be clinically “dormant” and recur if the wall is breached by mineral resorption. Some have suggested that calcification may protect plaque from rupture8, but others suggest it may have the opposite effect.9, 10
The walling-off by bone may represent an immune response of last resort, but how the osteogenic programs are activated is not known. One possibility is that soft tissue calcification around chronic infectious foci is triggered by bacterial cell walls directly or through the inflammatory cytokines they induce. The bacterial wall contains lipids that may undergo oxidative modification either by nonenzymatic means or possibly by reactive oxygen species released by white cells. Perhaps atherosclerotic calcification is simply another example of an attempt to wall-off a soft tissue focus of chronic inflammation. The concept that atherosclerotic plaque induces immunological responses is well established. When lipoprotein particles are retained long enough in the subendothelial matrix of plaque, they undergo oxidative modification. These modified lipids may give lipoproteins enough similarity to bacteria as to trigger the same osteogenic events as the Ghon focus.
The finding of glycosaminoglycans (GAGs) at the organic-inorganic interface is intriguing (Figure). These long repeating sugar chains, which often decorate proteins to form proteoglycans, are a signature feature of cartilage matrix, which further supports the relationship between atherosclerotic calcification and endochondral ossification. As with other bone-related proteins, GAGs have dual functionality in biomineralization: a direct role in initiating apatite nanocrystal formation and, later, a direct role in restraining nanocrystal propagation. They block crystal growth by linking via calcium ion bridges to growth sites on the crystal surface.11, 12 When hydroxyapatite mineral crystallizes in the presence of chondroitin sulfate in vitro, it forms highly ordered arrays of nanocrystals.13
Figure.
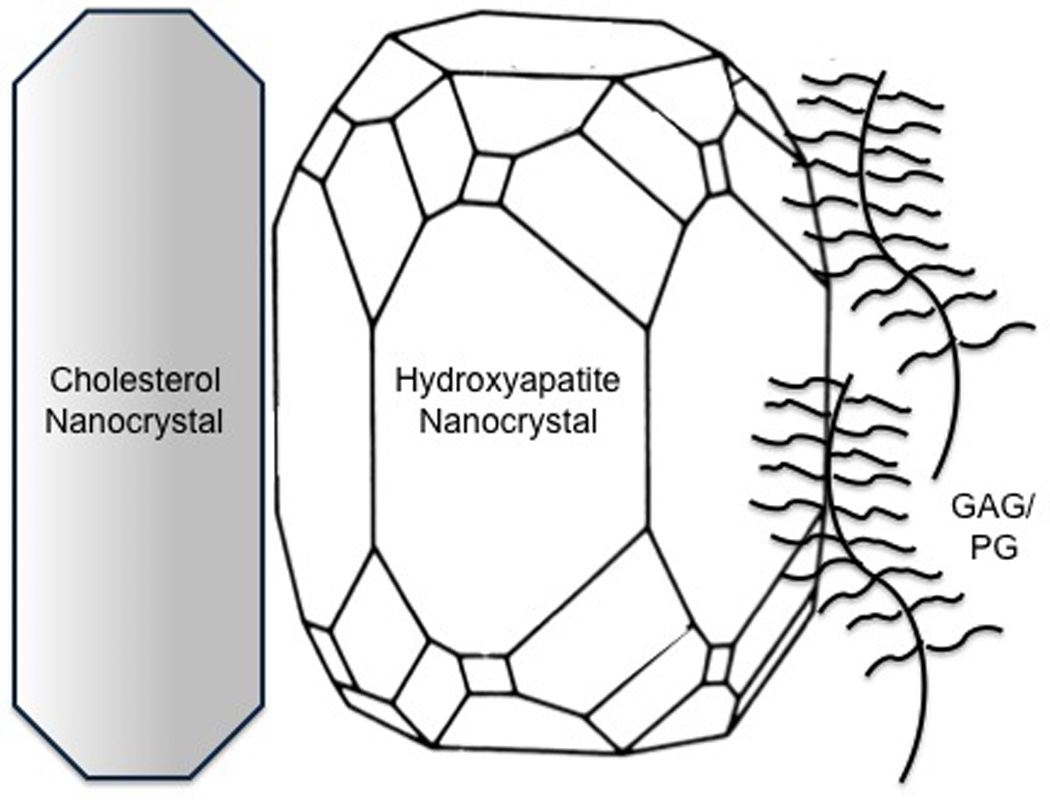
Schematic diagram of possible interfaces between hydroxyapatite and cholesterol nanocrystals and proteoglycans (GAG/PG).
Thus, the glycosaminoglycans at the interface may represent a nanoscale template for mineral crystallization. Interestingly, certain glycosaminoglycans, such as chondroitin sulfate, not only interact with mineral but also with lipoprotein particles via chemical “bridges”.14 As Duer et al.4 suggest, this binding of glycosaminoglycans to lipid may initiate injury and death of some vascular smooth muscle cells leading to osteogenic differentiation of others. Notably, since oxidative stress alone induces osteogenic differentiation in vascular cells, this may occur even without cell injury or death.3, 15, 16
Kirton and colleagues17 showed that the abundance of negatively charged GAGs in vascular cell cultures is regulated by the Wnt/beta-catenin pathway, a pathway shown by Towler and colleagues to serve a central regulatory role in vascular mineralization.18 At the same time, as shown by Hirsch and colleagues, apatite crystals also interact at a nanoscale level with cholesterol crystallites both in vitro and in human atherosclerotic plaque.19 Such an interaction may further modulate the order and pattern of mineral crystals formed within the cholesterol-rich environment of atherosclerotic lesions (Figure).
In conclusion, the nanoscale evidence provided by Duer et al. in this issue underscore the macroscale evidence that atherosclerotic plaque develop in an ordered manner similar to the highly regulated process of bone formation. Such nanotechnological approaches appear to have the capacity to answer many more questions about the fundamental physicochemical mechanisms involved in vascular calcification such as the effects of protein periodicity and cholesterol nanocrystals.
Acknowledgement
The authors thank Jeffrey Hsu, Howard Hughes Medical Institute Medical Student Fellow, for providing the figure. The authors were supported in part by NIH grants HL069261 (L.L.D.) and DK076009 (Y.T.) as well as by an American Heart Association Post-Doctoral Fellowship Award 0825033F (A.P.S.).
References
- 1.Honye J, Mahon DJ, Jain A, White CJ, Ramee SR, Wallis JB, al-Zarka A, Tobis JM. Morphological effects of coronary balloon angioplasty in vivo assessed by intravascular ultrasound imaging. Circulation. 1992;85:1012–1025. doi: 10.1161/01.cir.85.3.1012. [DOI] [PubMed] [Google Scholar]
- 2.Bunting CH. The formation of true bone with cellular (red) marrow in a sclerotic aorta. Journal of Experimental Medicine. 1906;8:365–376. doi: 10.1084/jem.8.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 4.Duer MJ, Friscic T, Proudfoot D, Reid DG, Schoppet M, Shanahan CM, Skepper JN, Wise ER. Mineral Surface in Calcified Plaque Is Like That of Bone. Further Evidence for Regulated Mineralization. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.172387. [DOI] [PubMed] [Google Scholar]
- 5.Chang MC, Douglas WH, Tanaka J. Organic-inorganic interaction and the growth mechanism of hydroxyapatite crystals in gelatin matrices between 37 and 80 degrees C. J Mater Sci Mater Med. 2006;17:387–396. doi: 10.1007/s10856-006-8243-9. [DOI] [PubMed] [Google Scholar]
- 6.Yokobori AT, Jr., Miyasaka Y, Sakurai M. Theory of osteogenesis behavior based on calcium diffusion theory. Biomed Mater Eng. 1995;5:209–217. [PubMed] [Google Scholar]
- 7.Bittar N. Microbiology. Elsevier; 1997. p. 149. [Google Scholar]
- 8.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 9.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Schmermund A. Clinical significance of coronary calcification. Arterioscler Thromb Vasc Biol. 2004;24:e172. doi: 10.1161/01.ATV.0000142384.70383.ea. author reply e172. [DOI] [PubMed] [Google Scholar]
- 11.Eanes ED, Hailer AW, Midura RJ, Hascall VC. Proteoglycan inhibition of calcium phosphate precipitation in liposomal suspensions. Glycobiology. 1992;2:571–578. doi: 10.1093/glycob/2.6.571. [DOI] [PubMed] [Google Scholar]
- 12.Wu LN, Genge BR, Wuthier RE. Association between proteoglycans and matrix vesicles in the extracellular matrix of growth plate cartilage. J Biol Chem. 1991;266:1187–1194. [PubMed] [Google Scholar]
- 13.Best SM, Duer MJ, Reid DG, Wise ER, Zou S. Towards a model of the mineral-organic interface in bone: NMR of the structure of synthetic glycosaminoglycan- and polyaspartate-calcium phosphate composites. Magnetic Resonance iin Chemistry. 2008;46:323–329. doi: 10.1002/mrc.2168. [DOI] [PubMed] [Google Scholar]
- 14.Camejo G, Hurt E, Wiklund O, Rosengren B, Lopez F, Bondjers G. Modifications of low-density lipoprotein induced by arterial proteoglycans and chondroitin-6-sulfate. Biochim Biophys Acta. 1991;1096:253–261. doi: 10.1016/0925-4439(91)90013-y. [DOI] [PubMed] [Google Scholar]
- 15.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 16.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 18.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch D, Azoury R, Sarig S, Kruth HS. Colocalization of cholesterol and hydroxyapatite in human atherosclerotic lesions. Calcif Tissue Int. 1993;52:94–98. doi: 10.1007/BF00308315. [DOI] [PubMed] [Google Scholar]


