Abstract
Objective
To determine the frequency of hyperintense cortical signal (HCS) on T1-weighted pre-contrast MRI in progressive multifocal leukoencephalopathy (PML) patients, its association with seizure risk and immune reconstitution inflammatory syndrome (IRIS), and its pathologic correlate.
Methods
We reviewed clinical data including seizure history, presence of IRIS, and MRI scans from PML patients evaluated at our institution between 2003 and 2012. Cases that were diagnosed either by CSF JC Virus (JCV) PCR, brain biopsy or autopsy, and who had MRI images available were included in the analysis (n=49). We characterized pathologic findings in areas of the brain displaying HCS in two patients and compared them with isointense cortex in the same individuals.
Results
Of 49 patients, 17 (34.7%) had seizures and 30 (61.2%) had HCS adjacent to subcortical PML lesions on MRI. Of the 17 PML patients with seizures, 15 (88.2%) had HCS compared to 15/32 (46.9%) patients without seizures (p= 0.006). HCS was associated with seizure development with a relative risk (RR) of 4.75 (95% confidence interval of 1.2 to 18.5; p=0.006). Of the 20 patients with IRIS, 16 (80.0%) had HCS compared to 14/29 (49.3%) of those without IRIS (p=0.04). On histological examination, HCS areas were associated with striking JCV-associated demyelination of cortical and sub-cortical U-fibers, significant macrophage infiltration and a pronounced reactive gliosis in the deep cortical layers.
Interpretation
Seizures are a frequent complication in PML. HCS is associated with seizures as well as IRIS, and correlates histologically with JCV focal leukocortical encephalitis (JCV FLE).
Introduction
Progressive multifocal leukoencephalopathy (PML) is a debilitating and often fatal disease caused by JC virus (JCV) in immunocompromised individuals. JCV causes a lytic infection of oligodendrocytes, and PML is characterized histologically by confluent lesions commonly located in subcortical white matter. These lesions harbor a triad of demyelination, infiltrating macrophages involved in clearing myelin debris, and reactive astrocytes. Recently, we and others have described cortical involvement of PML and extension of the lesions into the adjacent gray matter.1, 2 In addition, we have shown that JCV can infect cortical neurons in classic PML cases and in a separate entity restricted to cortical gray matter, named JCV encephalopathy.3 When immunosuppression is reversed, patients with PML frequently develop an immune reconstitution inflammatory syndrome (IRIS) accompanied by paradoxical worsening of neurological symptoms.4 IRIS is also suspected to be a risk factor for seizures in natalizumab-associated PML cases.5
Moreover, seizures – considered to be of cortical origin– occur in up to 18% of non-natalizumab PML patients, and are associated with white matter lesion location immediately adjacent to the cortex.6, 7 However, with the growing evidence of gray matter involvement in PML, a more direct mechanism of cortical irritation might explain this phenomenon.
Hyperintense cortical signal (HCS) in pre-contrast T1-weighted images on MRI has been described in a variety of CNS conditions and most notably in hypoxia/ischemia, status epilepticus, hypoglycemia, osmotic demyelination syndrome, and mitochondrial disorders. Less frequent associations have been made with immunologic disorders such as systemic lupus erythematousus or subacute sclerosing panencephalitis, and meningoencephalitis.8-13 There is no systematic description of this radiologic feature in PML, but it has been noted in two recent reports.14, 15 This finding is commonly thought to be a radiologic marker of cortical laminar necrosis, which is a process of neuronal loss and death with resultant gliosis and atrophy of the energy dependent 3rd and 5th cortical layers.16, 17 However, the anatomic substrate causing this hyperintense signal remains unclear. We sought to determine the frequency of this hyperintense cortical signal (HCS) on pre-contrast T1-weighted MR images in patients with PML and whether it is a predictor of seizures and if it is associated with IRIS. Furthermore, we carried out a histological correlation to understand the histopathologic substrate of this finding in PML.
Methods
Clinical data
We performed a retrospective analysis of PML patients evaluated over the past ten years at our neuro-infectious disease clinic (2003-2012). Forty-nine cases of PML proven either by CSF JCV PCR, brain biopsy, or autopsy, and who had any available MR images were included in the analysis. Cases classified as ‘possible’ PML, with radiologic and clinical presentation consistent with PML but without histologic or virologic confirmation, were excluded (6 cases).18 Patients were also excluded from the analysis if they had a history of epilepsy prior or unrelated to the diagnosis of PML. Seizures occurring less than 24 hours after brain biopsy were considered a post-surgical complication and were not included (1 case). The presence of IRIS was determined based on criteria including (1) worsening of neurologic signs and symptoms in the setting of an improved immunologic and virologic response, (2) evidence of immune reconstitution demonstrated by an increase of CD4+ T-cell counts and a decrease in HIV plasma RNA after starting on combined antiretroviral treatment (cART) in HIV+ patients or after discontinuation of immunosuppressive medications in HIV- individuals, and (3) an absence of other pathogens, inflammatory processes or tumors. In several cases, these criteria were further supported by the presence of mass effect or contrast enhancement (CE) on MRI. Patients surviving beyond one year from PML onset were considered PML-survivors (PML-S) and those who passed away within a year from PML onset were labeled as PML-progressors (PML-P)19.
MRI
We reviewed the T1, FLAIR, and when available, the diffusion weighted imaging (DWI), gradient echo (GRE), and susceptibility weighted imaging (SWI) sequences of the 49 subjects. The imaging studies were obtained at various facilities using different MRI protocols. The presence or absence of HCS adjacent to subcortical PML lesions was determined by comparison to normal-appearing cortical gray matter in the same image and was examined independently by two neurologists (M.K., I.K.). Eighty-three T1-weighted images of 49 patients were retrospectively reviewed and relevant images were examined separately by a neuroradiologist (D.H.) who was blinded to all clinical information. There was agreement on 80/83 (96%) images on the presence or absence of HCS between the neurologists and neuroradiologist. The 3 discrepant images were reviewed together by a neurologist and a neuroradiologist who agreed upon a final classification. HCS was considered to be present, if it was seen at least once in patients who had multiple MRIs.
Pathology
Post mortem analysis was performed on 2 patients with PML, within 12 days and 5 months from last MRI showing evidence of HCS, respectively.
At autopsy the whole brain was immediately fixed in 10% neutral buffered formalin. Brain dissection was performed axially, guided by MR images to target PML lesions. Tissue was then embedded in paraffin and sectioned at 5 microns. H&E stains were performed on deparaffinized tissue with a progressive Meyer's hematoxylin and counterstaining with Eosin Y. Additionally, Luxol Fast Blue for the detection of myelin with an H&E counterstain (LFB-H&E) was done on deparaffinized slides overnight at 60°C in 0.1 LFB in 95% ethanol. Differentiation was achieved with 0.5% lithium carbonate in water, and then followed up with a standard Meyer's H&E staining. Immunohistochemical stains for macrophages/microglia (CD68), glial acidic fibrillary protein (GFAP), and JC virus T and VP1 proteins were performed on selected tissue blocks including areas with HCS and contralateral and non-HCS containing areas as controls.
Statistical analysis
We recorded relevant demographic, clinical data, and radiologic findings as well as incidence of seizures and IRIS in this cohort. Mann–Whitney was used to compare age between PML patients with and without seizures or IRIS. Fisher's exact test was used to compare the remainder of the patient characteristics, as well as evaluate for an association between HCS and seizures, HCS and IRIS, HCS and HIV status, seizures and IRIS. Relative risk analysis was used to determine the association of HCS with development of seizures as well as that of IRIS with seizures.
Results
Patients' characteristics
The clinical characteristics of all 49 patients with PML are listed in Table 1.
Table 1. PML patients' characteristics.
| CLINICAL CHARACTERISTICS OF 49 PML PATIENTS | |
|---|---|
| Mean Age, y (range) | 54.51 (21-89) |
| Gender M:F | 33 (67.3 %):16 (32.7 %) |
| CSF PCR diagnosis | 39 (79.6 %) |
| Histologic diagnosis | 10 (20.4 %) |
| Underlying immunosuppression | |
| HIV infection | 19 (38.8 %) |
| Hematologic malignancy* | 13 (26.5 %) |
| Hematologic disease- other† | 7 (14.3 %) |
| Autoimmune disease‡ | 6 (12.2 %) |
| Other§ | 4 (8.2 %) |
| IRIS at the time of MRI | 20 (40.8 %) |
| PML-S:PML-P | 26 (53.1 %):23 (46.9 %) |
| HIV+ PML-S | 13 (73%) |
| HIV- PML-S | 12 (40%) |
Abbreviations: PML = progressive multifocal leukoencephalopathy; PCR = polymerase chain reaction; HIV = human immunodeficiency virus; IRIS = immune reconstitution inflammatory syndrome; PML-S = PML-survivors; PML-P = PML-progressors
Chronic lymphocytic leukemia (n=9), non-Hodgkin lymphoma (n=2), NK cell leukemia (n=1), follicular center cell lymphoma (n=1)
Idiopathic lymphocytopenia (n=2), lymphomatoid granulomatosis (n=1), common variable immunodeficiency (n=1), Waldenstrom macroglobulinemia (n=2), Good syndrome (n=1)
Multiple sclerosis treated with natalizumab (n=3), lupus erythematosus (n=1), dermatomyositis (n=1), rheumatoid arthritis (n=1)
brainstem glioma (n=1), cirrhosis (n=2), lung transplant (n=1)
Seizures in PML
Seizures occur in a third of PML patients
Of 49 PML patients, 17 (34.7%) had seizures. Patients with seizures tended to be younger (mean age 48.9) than those who did not have seizures (mean age 57.5); p=0.07. Seizure occurrence was not associated with HIV seropositivity (p= 0.54) or with clinical outcome (p=0.13). The time from PML symptom onset to first seizure ranged from <1 month to 55 months with a median of 4 months.
T1 MRI characteristics in PML
Hyperintense cortical signal on pre-contrast T1-weighted images is present in a majority of PML patients
Of 49 patients, 30 (61.2%) had HCS, which was always adjacent to subcortical PML lesions and the cortical area affected was atrophic in 29 (96.7%) of them. In 4 cases (13.3%), HCS was found in non-atrophic cortex, including 3 with HCS in atrophic cortex as well. HCS was often seen in the deeper layers of the cortex, close to the demyelinated subcortical white matter (Fig 1). Of 43 patients who had an MRI within 3 months from PML symptom onset, the time to development of HCS ranged from < 1 month to 11 months with a median of 2 months. All of the 13 HIV+ PML patients with HCS, were on cART at the time of initial HCS identification. Amongst these 13 patients, there were 4 patients who were not on cART at the time of the first available MRI. Those patients also did not have HCS on their initial MRI, but had HCS on the subsequent MRI done 2 to 5 months later. The 3 MS patients were already off natalizumab at the time of the first MRI showing HCS, and 2 of them had initial MRI available without HCS, but showing lesions of PML, leading to discontinuation of natalizumab. Thirteen patients (5 HIV+, 2 MS, 3 hematologic malignancies, 2 hematologic diseases and 1 brainstem glioma) with HCS had 1-4 follow up MRIs within 1 to 47 months. All HIV+ patients were on cART and HIV- patients were already off immunosuppressive medications at the time of the first MRI showing HCS. HCS remained apparent in the follow up scans, but decreased in intensity with time. In patients with HCS and where GRE/SWI (15 patients) or DWI sequences (12 patients) were completed, none had susceptibility artifact or restricted diffusion abnormalities in the affected cortical areas.
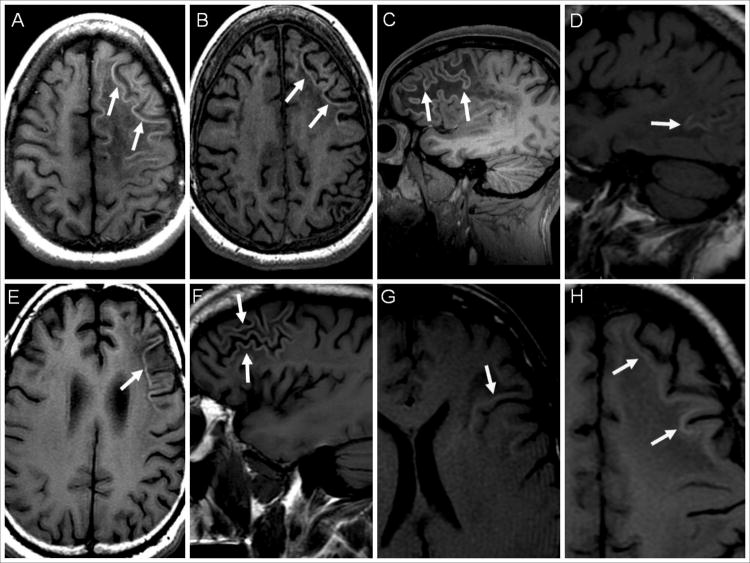
Figure 1. Hyperintense cortical signal on pre-contrast T1-weighted images in PML patients.
T1-weighted MR images of PML patients are shown (A-H). (A) Hyperintense cortical signal (HCS) in a patient with multiple sclerosis treated with natalizumab (arrows). The patient developed immune reconstitution inflammatory syndrome (IRIS) after natalizumab withdrawl. Within one month of IRIS, the patient had seizures requiring long term anti-epileptic drug (AED) use. (B) Four months later, the same patient demonstrates a persistence of the HCS although slightly less prominent (arrows). (C) Patient with congenital HIV with HCS in the right frontal lobe (arrows). The patient had PML-IRIS and developed seizures within a month of the MRI requiring AED use. (D) HCS in a patient with chronic lymphocytic leukemia who did not have IRIS or seizures (arrows). (E) Axial and (F) sagittal images of a patient with HIV and new onset seizures who had PML-IRIS six months earlier, showing HCS (arrows). The patient required long term AED. (G) Patient with HIV who had PML-IRIS one month earlier but did not develop seizures. The HCS affects the deeper cortical layers (arrows). (H) HCS (arrows) in the deep cortical layers in an HIV+ patient who, three months earlier, developed IRIS and seizures requiring AEDs.
Hyperintense cortical signal on pre-contrast T1-weighted images is associated with seizures
Of the 17 PML patients who developed seizures (Table 2), 15 (88.2%) had HCS compared to 15/32 (46.9%) patients without seizures (p= 0.006). The unadjusted relative risk (RR) of developing seizures in patients with HCS was 4.75 (95% confidence interval (CI) of 1.2 to 18.5; p=0.006). Of 15 patients with HCS and seizures, 6 had HCS on MRI before their first seizure and 2 did not, but developed HCS on subsequent MRI done within days of seizure onset. The other 7 patients did not have an MRI available for review before their first seizure, which in most cases was among the inaugural symptoms of PML, but all had HCS on the subsequent MRI performed within 0 to 3 months.
Table 2. Occurrence of HCS in all PML subpopulations.
| HCS+ | HCS - | |
|---|---|---|
| Total number of patients | 30 | 19 |
| HIV+ patients | 13 | 6 |
| HIV- patients: total | 17 | 13 |
| Multiple sclerosis | 3 | 0 |
| Hematologic malignancies | 7* | 6** |
| Hematologic disease other | 4† | 3†† |
| Auto-immune disease | 1‡ | 2‡‡ |
| Other | 2§ | 2§§ |
Abbreviations: HCS= hyperintense cortical signal; PML = progressive multifocal leukoencephalopathy; HIV = human immunodeficiency virus
Chronic lymphocytic leukemia (n=5), non-Hodgkin lymphoma (n=1), follicular center cell lymphoma (n=1)
Chronic lymphocytic leukemia(n=4), non-Hodgkin lymphoma(n=1), NK cell leukemia(n=1)
Good syndrome(n=1), Idiopathic lymphocytopenia(n=2), lymphomatoid granulomatosis(n=1)
Waldenstrom macroglobulinemia(n=2), common variable immunodeficiency(n=1)
lupus erythematosus(n=1)
dermatomyositis(n=1), rheumatoid arthritis(n=1)
brainstem glioma(n=1), lung transplant(n=1)
cirrhosis(n=2)
Hyperintense cortical signal on pre-contrast T1-weighted images is not associated with HIV serostatus
Of the 19 HIV+ patients, 13 demonstrated HCS (68.4%); while 17 of the 30 HIV- patients (56.7%) had HCS. This difference was not significant (p=0.55). The occurrence of HCS in each PML subpopulation is shown in Table 2.
PML IRIS
Seizures are associated with a history of PML-IRIS
Of 20 patients with PML-IRIS, 12 (60.0%) developed seizures compared to 5/29 (17.2%) of PML patients without IRIS. The unadjusted RR of developing seizures in patients with IRIS was 3.48 (95% CI of 1.5 to 8.3; p= 0.005). Of the 12 patients with PML-IRIS who developed seizures (Table 3), 8 (66.7%) had their initial seizure within 35 days of IRIS onset. The time from IRIS onset to first seizure ranged from <1 month to 54 months with a median of 1 month.
Table 3. Clinical characteristics of PML patients with seizures.
| Patient No. | HCS | IRIS | peri-IRIS seizures (35 days) | HIV | PML-S/P | continued AED requirement |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | + | + | + | + | S | - |
| 2 | + | + | + | -* | P | na |
| 3 | + | + | + | -† | P | na |
| 4 | + | + | + | -‡ | S | U |
| 5 | + | + | + | -‡ | S | + |
| 6 | + | + | + | + | S | + |
| 7 | + | + | + | -* | P | na |
| 8 | + | + | - | + | S | + |
| 9 | + | + | - | + | S | + |
| 10 | + | + | - | + | S | + |
| 11 | + | + | - | + | S | + |
| 12 | + | - | na | -§ | S | + |
| 13 | + | - | na | -¶ | P | na |
| 14 | + | - | na | + | S | + |
| 15 | + | - | na | -¶ | S | U |
| 16 | - | + | + | + | S | + |
| 17 | - | - | na | -** | P | na |
Abbreviations: AED= anti-epileptic drug; HCS= hyperintense cortical T1-signal; HIV= human immunodeficiency virus; IRIS= immune reconstitution inflammatory syndrome; na= not applicable; PML= progressive multifocal leukoencephalopathy progressor; PML-P= PML-progressor; PML-S= PML-survivor; U= unknown/lost to follow up
Chronic Lymphocytic Leukemia treated with rituximab
Chronic Lymphocytic Leukemia treated with regimen that did not include rituximab
Multiple sclerosis treated with natalizumab
Non-Hodgkins Lymphoma treated with rituximab
lymphomatoid granulomatosis treated with rituximab
CD4 lymphopenia
Common variable immunodeficiency
Hyperintense cortical signal on pre-contrast T1-weighted images is associated with IRIS but not with PML outcome
Of the 20 patients with IRIS, 16 (80.0%) had HCS compared to 14/29 (49.3%) of those without IRIS, (p=0.04). Of the 30 patients with HCS, 17 (56.7 %) were PML-S and 13 (43.3%) were PML-P; while of the 19 patients without HCS, 9 (47.4%) were PML-S and 10 (52.6%) were PML-P; p=0.57.
Histopathological evaluation
Detailed histopathological analyses were performed on the post mortem brain samples of two HIV-negative PML cases, within 12 days and 5 months from their last MRI. These included one natalizumab-treated multiple sclerosis (MS) patient who developed IRIS (Fig 2) and one with idiopathic CD4+ lymphocytopenia (Fig 3).
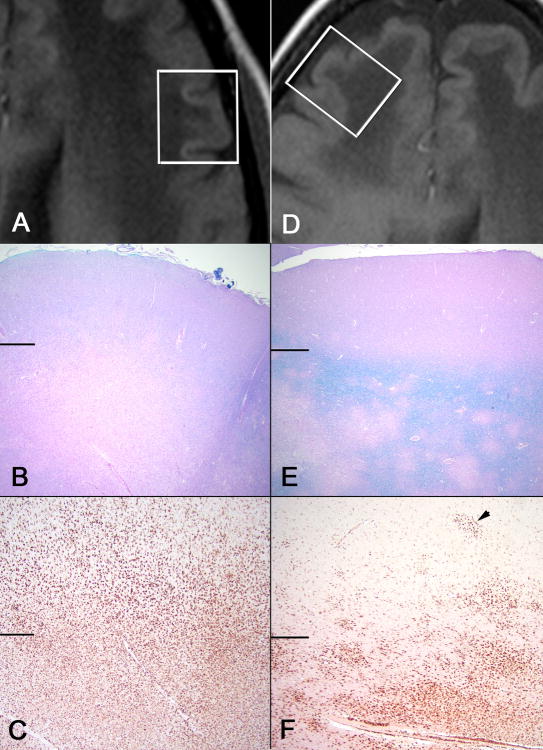
Figure 2. Pathologic findings in a PML-IRIS patient with multiple sclerosis treated with natalizumab.
Area of HCS (A, B, C) compared to isointense cortex (D, E, F) in the same patient. (A) T1-weighted image demonstrating hyperintense cortical signal adjacent to a PML lesion, and (D) isointense cortex on T1-weighted image adjacent to a PML lesion. White boxes represent histologic sampling. Horizontal lines in B, C, E, F separate cortex (above) from white matter (below) (B) Myelin stain in HCS area demonstrates a near complete demyelination in white matter and loss of demarcation of gray-white junction, while isointense area (E) shows patchy demyelination with an intact rim of myelin at the gray-white junction (B and E, LFB-H&E 20x). (C) CD68 stain highlights prominent infiltration of macrophages in white matter extending deep into cortex in HCS area; while the isointense area shows prominent CD68 staining mostly limited to the white matter (F) with only scattered macrophages and microglial nodules (arrow head) involving deep cortical layers (C and F, CD68 40x)
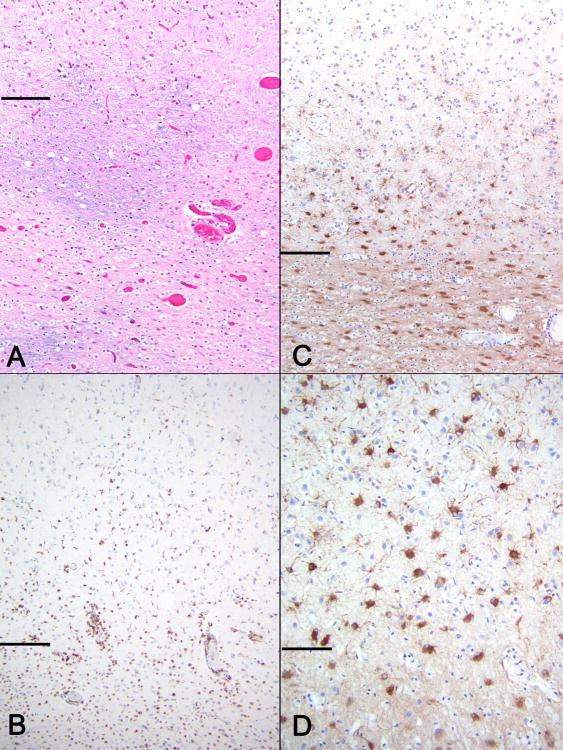
Figure 3. Area of HCS in a PML patient with idiopathic CD4+ lymphocytopenia.
Horizontal lines separate cortex (above) from white matter (below) (A) Myelin stain demonstrates nearly complete demyelination with rare relatively preserved areas (LFB-H&E, 100x). (B) CD68 staining shows perivascular and interstitial macrophages and activated microglia present in white matter as well as deep cortex (CD68, 100x) GFAP staining at low and high magnifications (C and D) highlights prominent astrogliosis in white matter and JCV focal leukocortical encephalitis in deeper cortical layers (C-100x, D-400x).
PML lesions in subcortical white matter show demyelination, macrophage infiltrate and reactive astrocytes
Characteristic punctuate and confluent areas of white matter demyelination with variable numbers of foamy macrophages were present in both cases. Macrophages had a blue-staining granular content on LFB-H&E stains, consistent with lipid rich myelin debris. CD68 stains highlighted prominent perivascular macrophage clusters, a diffuse parenchymal macrophage infiltrate, as well as a prominent reactive population of microglia, including scattered cortical microglial nodules. Variable, reactive astrogliosis, related to size and chronicity of the lesions, was highlighted by GFAP immunostains. Marked astrogliosis was present in larger, confluent lesions. Infected glial cells were also variably present, with more recognizable nuclear viral cytopathic changes in smaller lesions. Monoclonal antibodies specific for JCV proteins, showed significant staining at the periphery of confluent lesions. Very few cells within the gray matter or along the gray white junction stained for JCV proteins, consistent with complete demyelination of these areas.
Cortical areas affected by HCS show demyelination, macrophage infiltrate and reactive astrocytes
Areas displaying HCS on MRI (Fig 2A) were compared to areas of isointense cortex (Fig 2D) and revealed three significant differences. The first notable change was a prominent compromise of sub-cortical and cortical U-fibers by demyelination (Fig 2B) as compared to isointense areas, which had variable preservation of U-fibers (Fig 2E). A second prominent change was a dramatic difference in the distribution of macrophages/reactive microglia between the two areas. HCS areas displayed a striking infiltrate of macrophages extending to the deep half of the cortex (Fig 2C). Isointense areas, on the other hand, had macrophages primarily limited to the perivascular space and white matter with minimal involvement of the overlying cortex. The cortical involvement was limited to scattered deep cortical macrophage clusters and scattered microglial nodules (Fig 2F). Of note, in this patient with MS, there were no MS lesions in the immediate subcortical white matter or in the cortical areas of HCS. Detailed histological findings in a HCS area of the PML patient with idiopathic CD4+ lymphocytopenia is shown in Fig 3. Extensive demyelination in the immediate subcortical area (Fig 3A) and macrophage infiltration (Fig 3B) can be seen. A third characteristic finding of HCS areas was a much more pronounced reactive astrogliosis, highlighted by GFAP immunostain, particularly in deep cortical areas, blending with the prominent white matter astrogliosis (Figs 3C, D). This finding was only minimal in isointense areas. Therefore, the histological substrate of HCS appears to be caused by JCV infection of oligodendrocytes and demyelination in the lower layers of the cortex, triggering an infiltrate of phagocytic macrophages involved in clearing the myelin debris and a reactive astrogliosis. This triad bears striking similarity to that of subcortical PML lesions, except for the location in the deep layers of the cortex. We therefore decided to call it JCV-associated focal leukocortical encephalitis (JCV FLE).
Discussion
Seizures are an underappreciated complication of PML; a disease that is largely recognized as a white matter process as its name implies. In this study, we demonstrated that seizures are more frequent than previously reported. In addition, we described a novel MRI marker, HCS, which if verified in a prospective study, could be used as a predictor of seizures. Finally, we characterized the histopathologic correlate of this radiologic marker, and named it JCV FLE.
Increased frequency of seizures in PML and association with IRIS and HCS
Incidence of seizures in PML was 35 %, which is higher than 18% reported at our institution from 1995 to 2005.7 Unlike the previous report, this study only included patients with histologically or virologically proven PML. However, the ‘possible-PML’ group consisted of a small percentage in the prior study. Furthermore, there was no difference in length of follow up between the two cohorts that could account for the increase in incidence of seizures. Finally, the previous study did not investigate a relationship between IRIS and seizures. Recently, Dalhaus et al found a 53% incidence of seizures in patients with natalizumab-associated PML-IRIS.5 Our results also support a relationship between seizures and IRIS as well as HCS and IRIS. Interestingly, in our new data set, the incidence of seizures would have been 22% if the eight patients who developed seizures within 35 days of IRIS diagnosis had been excluded from the analysis, which is similar to the 18% reported in 2006. It is possible that the recent increase of seizure diagnosis in PML is due to a greater incidence of IRIS as a result of more effective cART regimens and monoclonal antibody use that were not readily available during the previous study.
Frequency of HCS in PML and correlation with JCV focal leukocortical encephalitis (FLE)
A hyperintense cortex, as seen on pre-contrast T1-weighted MR images, has been described in a variety of neurologic conditions. Our study indicates that HCS is common in PML, since 61% of our cases demonstrated this finding. The hyperintense cortex has been described as a marker of cortical laminar necrosis.17, 20, 21 Cortical laminar necrosis is a process typically affecting cortical layers (3rd and 5th) that are highly vulnerable to energy depletion (eg: hypoxia) or high energy demand (eg: status epilepticus).11, 22, 23 On histology, laminar necrosis exhibits neuronal death and necrosis, followed by macrophage infiltrate and astrogliosis, tissue loss, and cortical atrophy. However, on pathological review of two HIV- PML cases with HCS, we found a histologic pattern that differs from cortical laminar necrosis. This pattern consists of the following triad: 1) complete demyelination of the subcortical area and cortical U fibers; 2) intense macrophagic infiltrate in the layers 5 and 6 of the cortex affected by demyelination; 3) reactive astrogliosis in the same cortical areas. This triad recapitulates the histological findings classically described in the subcortical white matter lesions of PML, and shows that the same process can also affect the cortical white matter. We therefore decided to name it JCV focal leukocortical encephalitis (JCV FLE). We propose that these areas of damaged cortex serve as a seizure focus in PML patients.
Various substrates responsible for the hyperintense signal on T1-weighted images in cortical laminar necrosis have been hypothesized, including deposition of iron, calcium, lipids, or generation of paramagnetic free radicals.2, 3, 24, 25 In our cases, Prussian blue staining did not reveal iron deposition (data not shown). Moreover, the GRE/SWI MRI sequences and histological sections did not demonstrate evidence of microhemorrhages. Calcifications were not observed on histologic sections. There were many lipid-laden macrophages along the affected cortex. Furthermore, activated microglia and some microglial nodules were seen in these areas of affected cortex. Interestingly phagocytic macrophages and microglia are capable of generating free radicals which have been postulated as a possible cause of hyperintense signal on T1-weighted images reported in some MS lesions. 25 Microglial nodules can be seen in HIV infection as well as early MS.26, 27 However, the two brain samples described above that demonstrated microglial nodules were in HIV- individuals. Additionally, there was no significant increase in HCS in the HIV+ population compared to the HIV- group.
Study limitations
There are several limitations to the study. First, this is a retrospective analysis and MRIs were performed at inconsistent intervals depending on the patients' clinical course. In several cases, MRIs were not available immediately before the initial seizure and therefore, establishing an exact temporal relationship between HCS and seizure was not feasible. Given the fact that 6 out of 8 PML patients had HCS on MRI before the seizure and in view of the strong association between HCS and seizures, it appears more likely that HCS preceded seizures rather than seizures causing HCS. In addition, 15 patients had HCS without seizures. Furthermore, HCS was an early event in the course of PML. However, a prospective study focused on the temporal relationship of HCS and seizures in PML will be needed to resolve this issue. In addition, because of the small sample size largely due to the low incidence of PML, multivariable modeling was not feasible. A larger, prospective study would allow for an assessment of various confounders. Secondly, PML is a rare disease and since we sought to be inclusive in the collection of our cases, we enrolled patients with several different underlying conditions. However, HCS, IRIS and seizures were found in HIV+ and HIV- PML patients, which justifies studying them as a group. Thirdly, MRIs were completed at various sites since some patients were referred to the neuroinfectious disease clinic after a workup including an initial MRI. In theory, different MRI machines could produce cuts at various interval lengths, which might have missed slices demonstrating HCS. However, this effect would be minimal since most HCS lesions were large enough to span multiple slices. Moreover, MRIs of poor quality or with significant artifact were not included in the study. It would have been preferable to have included diffusion and T2* imaging for all patients. However, the striking absence of unique findings on these sequences when they were performed suggests that little was lost by using studies from a variety of sites.
Of all PML patients, only 53% survived beyond one year. This likely reflects the high proportion of HIV- patients in our cohort. Indeed, only 40% of those were survivors, compared to 73% of HIV+ patients, which is consistent with more than 60% survival reported in recent studies28, 29. However, it is possible that some of the patients seen at our PML referral center have more severe disease. Because of the inclusion criteria in this pilot study, our results only apply to biopsy or PCR proven PML and need to be replicated in patients with possible PML. However, previous studies of possible PML cases have shown that they are similar to proven PML cases with regard to their age of onset, gender, CD4+ T-cell counts, seizure prevalence and mortality.7,28
Lastly, histological correlate of HCS could only be verified in a couple of brain samples which were sectioned axially to mirror MRI images, rather than coronally, as is customary. Modification of pathological protocols allowing coregistration of MRI findings and histological samples will be required to confirm our findings in a larger group of patients.
Clinical implications
These findings shed a new light on the pathophysiology of PML and seizures and also reaffirm the gray matter involvement in this disease. HCS is easy to identify on routine MR images and does not require the injection of intravenous contrast material. This radiological sign will be helpful to clinicians managing patients with PML, since they will be aware of increased risk of seizures. Prospective studies will be needed to evaluate the benefit of prophylactic antiepileptic drugs (AED) in this group of patients. Moreover, additional studies will be needed to investigate the occurrence of this radiological marker and its relationship with epilepsy and AED requirements in other disease processes that allow a larger sample size and longer survival periods. This will be particularly valuable in pathologies that can affect the juxtacortical tissue including stroke, sarcoidosis, encephalitides such as HSV, HHV6, paraneoplastic disorders, and other demyelinating disorders including ADEM and multiple sclerosis.
Acknowledgments
We thank Sarah Gheuens for helpful discussion and Elizabeth Norton for technical assistance.
The funding source had no direct involvement in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Study Funding: Funded in part by NIH grants R01 NS 047029, R01 NS 074995, and K24 NS 060950 to I.J.K. and R01 NS081916 and R21 MH100868 to M.P.A.
Disclosures: M.N.K. was funded by BU-CHART/NIH T32 AI052074 (NIAID)
D.C.A. receives institutional royalties for MRI inventions unrelated to the work in this manuscript from GE Healthcare and Philips Healthcare, both vendors of MRI scanners. He also receives research support from GE Healthcare for studies unrelated to the topic of this manuscript.
M.P.A. is funded by NIH grants R01 NS081916 and R21 MH100868
I.J.K. is funded by NIH grants R01 NS 047029, R01 NS 074995, and K24 NS 060950
Footnotes
S.P.A. reports no disclosures.
R.P. reports no disclosures
C.W. reports no disclosures
M.H. reports no disclosures
D. H. reports no disclosures
L.N. reports no disclosures
Authors and their contributions: M.N.K. conceptualized and designed the study, acquired data, analyzed and interpreted the data and drafted and revised the manuscript, including medical writing.
M.H. acquired data, analyzed and interpreted the data.
D.H. acquired data, analyzed and interpreted the data.
L.N. analyzed and interpreted the data.
M.A. acquired data, analyzed and interpreted the data.
I.J.K. conceptualized and designed the study, acquired data, analyzed and interpreted the data and drafted and revised the manuscript.
References
- 1.Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: Similarities and differences. Neurology. 2008 Jan 29;70(5):336–43. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- 2.Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012 Jan;71(1):54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang X, Wuthrich C, Gordon J, Sawa H, Koralnik IJ. JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant. PLoS One. 2012;7(4):e35793. doi: 10.1371/journal.pone.0035793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009 Apr 28;72(17):1458–64. doi: 10.1212/01.wnl.0000343510.08643.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013 Apr 19; doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badawy RA, Lai A, Vogrin SJ, Cook MJ. Subcortical epilepsy? Neurology. 2013 May 14;80(20):1901–7. doi: 10.1212/WNL.0b013e3182929f4f. [DOI] [PubMed] [Google Scholar]
- 7.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006 Jan 24;66(2):262–4. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 8.Donaire A, Carreno M, Gomez B, et al. Cortical laminar necrosis related to prolonged focal status epilepticus. J Neurol Neurosurg Psychiatry. 2006 Jan;77(1):104–6. doi: 10.1136/jnnp.2004.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho AH, Choi CG, Lee SA. Cortical Laminar Necrosis associated with Osmotic Demyelination Syndrome. J Clin Neurol. 2005 Oct;1(2):174–6. doi: 10.3988/jcn.2005.1.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh JH, Kim JH, Oh K, Kim SG, Park KW, Kim BJ. Cortical laminar necrosis caused by rapidly corrected hyponatremia. J Neuroimaging. 2009 Apr;19(2):185–7. doi: 10.1111/j.1552-6569.2008.00244.x. [DOI] [PubMed] [Google Scholar]
- 11.Finsterer J. Laminar cortical necrosis in mitochondrial disorders. Clin Neurol Neurosurg. 2009 Oct;111(8):655–8. doi: 10.1016/j.clineuro.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Kashihara K, Fukase S, Kohira I, Abe K. Laminar cortical necrosis in central nervous system lupus: sequential changes in MR images. Clin Neurol Neurosurg. 1999 Jun;101(2):145–7. doi: 10.1016/s0303-8467(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 13.Niwa T, Aida N, Shishikura A, Fujita K, Inoue T. Susceptibility-weighted imaging findings of cortical laminar necrosis in pediatric patients. AJNR Am J Neuroradiol. 2008 Oct;29(9):1795–8. doi: 10.3174/ajnr.A1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horger M, Beschorner R, Beck R, et al. Common and uncommon imaging findings in progressive multifocal leukoencephalopathy (PML) with differential diagnostic considerations. Clin Neurol Neurosurg. 2012 Oct;114(8):1123–30. doi: 10.1016/j.clineuro.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012 Nov;72(5):779–87. doi: 10.1002/ana.23676. [DOI] [PubMed] [Google Scholar]
- 16.Siskas N, Lefkopoulos A, Ioannidis I, Charitandi A, Dimitriadis AS. Cortical laminar necrosis in brain infarcts: serial MRI. Neuroradiology. 2003 May;45(5):283–8. doi: 10.1007/s00234-002-0887-7. [DOI] [PubMed] [Google Scholar]
- 17.Castillo M, Scatliff JH, Kwock L, et al. Postmortem MR imaging of lobar cerebral infarction with pathologic and in vivo correlation. Radiographics. 1996 Mar;16(2):241–50. doi: 10.1148/radiographics.16.2.8966284. [DOI] [PubMed] [Google Scholar]
- 18.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013 Apr 9;80(15):1430–8. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009 Nov 10;73(19):1551–8. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyko OB, Burger PC, Shelburne JD, Ingram P. Non-heme mechanisms for T1 shortening: pathologic, CT, and MR elucidation. AJNR Am J Neuroradiol. 1992 Sep-Oct;13(5):1439–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi S, Higano S, Ishii K, et al. Hypoxic brain damage: cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology. 1993 Nov;189(2):449–56. doi: 10.1148/radiology.189.2.8210374. [DOI] [PubMed] [Google Scholar]
- 22.Lovblad KO, Wetzel SG, Somon T, et al. Diffusion-weighted MRI in cortical ischaemia. Neuroradiology. 2004 Mar;46(3):175–82. doi: 10.1007/s00234-003-1133-7. [DOI] [PubMed] [Google Scholar]
- 23.Brierley JB, G D. Hypoxia and vascular disorders of the central nervous system. In: Adams H, C J, Duchen LW, editors. Greenfield's Neuropathology. 4th. New York: John Wiley & Sons; 1984. pp. 125–207. [Google Scholar]
- 24.Karimi S, Hatzoglou V, Punia V, Partovi S, Abrey LE, Deangelis LM. Post-treatment T1 shortening in primary CNS lymphoma. J Neurooncol. 2013 Jan;111(1):25–31. doi: 10.1007/s11060-012-0984-3. [DOI] [PubMed] [Google Scholar]
- 25.Janardhan V, Suri S, Bakshi R. Multiple sclerosis: hyperintense lesions in the brain on nonenhanced T1-weighted MR images evidenced as areas of T1 shortening. Radiology. 2007 Sep;244(3):823–31. doi: 10.1148/radiol.2443051171. [DOI] [PubMed] [Google Scholar]
- 26.Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80(4):393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Metz I, Amor S, van der Valk P, Stadelmann C, Bruck W. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 2013 Apr;125(4):595–608. doi: 10.1007/s00401-013-1082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009 Jan 1;199(1):77–83. doi: 10.1086/595299. [DOI] [PubMed] [Google Scholar]
- 29.Casado JL, Corral I, Garcia J, et al. Continued declining incidence and improved survival of progressive multifocal leukoencephalopathy in HIV/AIDS patients in the current era. Eur J Clin Microbiol Infect Dis. 2014 Feb;33(2):179–87. doi: 10.1007/s10096-013-1941-6. [DOI] [PubMed] [Google Scholar]