Abstract
Background Context
MRI is the standard imaging modality for the assessment of the cervical spinal cord; however, MRI assessment of the spinal cord in cervical spondylotic myelopathy (CSM) patients has not demonstrated a consistent association with neurological function or outcome after surgical or medical intervention. Thus, there is a need for sensitive imaging biomarkers that can predict functional impairment in patients with advanced cervical spondylosis.
Purpose
The purpose of this study was to implement diffusion tensor imaging (DTI) as an imaging biomarker for microstructural integrity and functional impairment in patients with cervical spondylosis.
Study Design/Setting
Non-randomized, single institution study.
Patient Sample
Forty-eight cervical spondylosis patients with or without spinal cord signal
change underwent DTI of the spinal cord along with functional assessment.
Outcome Measures
Functional measures of neurological function via mJOA score
Methods
This study was supported by the NIH and there are no perceived conflicts of interest. A zoomed-EPI technique and 2D spatially selective RF excitation pulse were used for DTI measurement. Fractional anisotropy (FA), mean diffusivity (MD), radial and axial diffusion coefficient (RD and AD), axial diffusion anisotropy, ψ, defined as AD-MD, and the standard deviation of primary eigenvector orientation were evaluated at the site of compression.
Results
Results suggest average FA, tADC, ψ, and standard deviation of primary eigenvector orientation at the spinal level of highest compression were linearly correlated with modified Japanese Orthopedic Association (mJOA) score. Receiver-operator characteristic (ROC) analysis suggested FA and ψ could identify stenosis patients with mild to moderate symptoms with a relatively high sensitivity and specificity.
Conclusion
The results of this study support the potential use of DTI as a biomarker for predicting functional impairment in patients with cervical spondylosis.
Keywords: Diffusion tensor imaging, DTI, spinal cord, cervical spondylotic myelopathy, biomarker, CSM, stenosis, mJOA
Introduction
Cervical spondylosis is a spinal disorder characterized by degeneration of vertebral bodies, intervertebral disks, facet joints, and associated ligaments typically resulting in formation of bony spurs and, in some cases, myelopathy. Cervical spondylosis, with or without myelopathy, occurs more frequently with increasing age and is seen in as many as 95% of men and 70% of women between 60 and 65 years old [1]. Myelopathy from cervical spondylosis is the most common diagnosis for patients with spinal cord disorders over the age of 64 [2] and is thought to occur primarily from spinal canal stenosis resulting in compromise of spinal cord tissue. Studies have shown that a prolonged duration of symptoms may be associated with a poor surgical outcome [3, 4], and therefore early surgical intervention is commonly advocated based on imaging features before severe symptoms manifest.
MRI is the standard imaging modality for the assessment of cervical spinal cord health. Assessment of the spinal cord for cervical spondylotic myelopathy (CSM) often includes morphology measurements, such as canal size or Torg-Pavlov ratio [5] and T1/T2-weighted MR signal changes; however, morphometry [6–8] and abnormal MR signal change [9–16] have not demonstrated consistent associations with neurological function or outcome after surgical intervention. Thus, there is a need for reliable radiological criteria and imaging biomarkers for identifying patients who will benefit from surgical intervention [17].
DTI, a MRI technique sensitive to the magnitude and orientation of water self-diffusion, is sensitive to spinal cord tissue microarchitecture [18] and is superior to routine T2-weighted MRI in detecting subtle changes in spinal cord integrity after injury [19–21]. Preliminary reports have found differing diffusion characteristics between normal volunteers and patients with cervical spondylosis and myelopathy, suggesting that this technique might be of diagnostic utility [10, 19, 20, 22]. Although studies have explored the use of DTI as a tool to study cervical spondylotic myelopathy and DTI has shown promise in characterizing other spinal cord pathologies, there remains a need for systematic evaluation of DTI metrics and potential neurological correlates in order to move DTI from the benchtop into clinical practice.
In the current study we performed axial DTI through the upper cervical spinal cord in nine healthy, neurologically intact control subjects and forty-eight patients with cervical spondylosis, with or without mild to moderate myelopathy, in order to determine whether DTI provides information about spinal cord integrity and neurological impairment beyond conventional MR examination.
Materials and Methods
Patients
A prospective study was carried out to describe the DTI characteristics observed throughout the cervical spinal cord. A series of forty-eight patients (n=48; age range 38–78 years; mean=60 years) diagnosed with cervical spondylosis, with (n=32) or without (n=16) neurological symptomatology, with homogeneous DTI acquisition parameters were examined as part of a prospective NIH-funded study. (Note that this study was funded by the NIH and there are no perceived biases associated with this funding source.) Fifteen patients underwent two or more scans during the period of observation. Nine healthy volunteers underwent the same cervical imaging and served a control group. All procedures complied with the principles of the Declaration of Helsinki and were approved by the Institutional Review Board at our institution.
Conventional MRI
Imaging procedures consisted of both routine conventional MRI scans and DTI scans performed on a 3.0T MR imaging scanner (3T TrioTim; Siemens Healthcare, Erlangen, Germany) using a standard whole body coil array for radiofrequency reception (with only two neck coil elements covering the cervical spinal cord activated). Routine clinical MRI scans consisted of T1-weighted and T2-weighted sequences in the sagittal plane and T2-weighted images in the axial plane. The presence of T2 weighted signal change as well as spinal cord morphometry defined by the anterior-posterior diameter of the cord at the level of highest compression was documented and used for subsequent comparisons with DTI metrics.
Diffusion tensor imaging (DTI)
Axial diffusion-weighted images were collected through the level of most significant canal narrowing. Excitation consisted of a custom two-dimensional, spatially selective radiofrequency excitation pulse (2D–RF) and resulting MR signals were acquired using a reduced FOV EPI readout with ramp sampling (Zoomed-EPI). TE/TR was set to 67msec/5sec, slice thickness was set to 4mm with no gap, NEX=15, and 6 diffusion sensitizing directions were collected at b=500 s/mm2, along with a single T2-weighted (b=o s/mm2) image.
After acquisition of DWIs, eddy-current and motion correction was performed using FSL (FMRIB; Oxford, UK; http://www.fmrib.ox.ac.uk/fsl/). The diffusion tensor was then constructed in AFNI (available at http://afni.nimh.nih.gov). The eigenvalues and eigenvectors were extracted from the diffusion tensor and FA (referenced in [23]), axial diffusivity, AD (corresponding to the largest eigenvalue), radial diffusivity, RD (corresponding to the average of the two smallest eigenvalues), MD (average of all three eigenvalues), and axial diffusion anisotropy (ψ, corresponding to AD – MD, referenced in [24, 25]) were calculated. The measures of AD and RD were chosen because they are believed to reflect the degree of axonal and myelin damage, respectively [26–29]. Additionally, the standard deviation of the primary eigenvector orientation, std(θ), defined as the angle between the primary eigenvector, υ⃑1, and the z-axis or , within the level of highest compression was calculated as a measure of white matter disorganization and tract disruption. For instance, if white matter tracts are tightly packed and organized in a single direction, the standard deviation will be low as the eigenvectors for all voxels will be oriented in the same general direction. Alternatively, if white matter tract orientation is disrupted due to compression or damage, the eigenvector orientation for voxels within the cord at the site of compression will likely be less coherent, resulting in a larger standard deviation of primary eigenvector orientation.
Regions of Interest
Manual segmentation of spinal cord ROIs was performed for the whole cord (no gray/white matter distinction) at each axial image slice location using the T2-weighted anatomical images, similar to previous techniques [30, 31]. ROIs were placed within the spinal cord at each image location such that at least 2 voxels around the edge of the cord were excluded to assure no partial volume contamination from surrounding cerebrospinal fluid. Data from all voxels in the ROI were pooled and averaged across each vertebral level (2–3 slices) and the space between each level (i.e. space commonly exhibiting compression in CSM patients, usually 2–3 slices). DTI measurements at the site of compression were used for subsequent analysis.
Functional Assessment
The current study used the modified Japanese Orthopaedic Association (mJOA) score, the most commonly used functional outcome assessment instrument in CSM patients [32], which has been demonstrated to be a valid and reliable outcome measurement in this population [33]. Moderate symptomatology was defined as having an mJOA score between 11–14, and mild symptomatology was a score between 15–17.
Statistics
Conventional MRI measures of antero-posterior diamter and T2 hyperintensity were explored as predictors of functional impairment. A student’s t-test was used to determine whether there was a significant difference in spinal cord compression between patients with spondylosis and healthy volunteers. Linear regression was used to determine whether the anterior-posterior spinal cord diameter was correlated with mJOA score. An analysis of variance (ANOVA) was used to test whether there was a relationship between spinal cord diameter at the site of compression between patients with no neurological impairment (mJOA=18), mild impairment (mJOA=15–17), and moderate impairment (mJOA=10–14).
Next, the SNR of the spinal cord at various spinal cord segments in healthy controls were compared to historic data [31] using a two-way ANOVA. The repeatability of DTI measurements within the spinal cord were examined in a subset of 15 patients with minimal neurological impairment (mJOA > 17) by calculating the median coefficient of variation for both FA and MD.
A one-way ANOVA was used to determine whether there was a significant difference in FA at the site of compression between patients with no neurological impairment, mild impairment, or moderate impairment. Tukey’s test for multiple comparisons was used to test difference between these individual groups. Linear regression was then used to test whether FA, MD, AD, RD, ψ, or std(θ) at the level of compression correlated with mJOA score. A Bonferroni-corrected P-value < 0.0083 was considered statistically significant for linear regression analysis (Corrected P-value=0.05/6).
Additionally, receiver-operator characteristic (ROC) analysis was used to determine the sensitivity and specificity for DTI indices to differentiate spondylosis patients with neurological symptoms (mJOA < 18) from spondylosis patients without symptoms (mJOA=18), as well as distinguish between moderately affected patients (mJOA < 15) and those with mild or no impairment. The area under the ROC curve (AUC) was used as a measure of DTI metric performance. A two-way ANOVA was used to test whether the ROC performance differed across the various DTI metrics and between detecting any impairment (mJOA=18 vs. mJOA<18) or moderate impairment (mJOA<15 vs. mJOA≥15).
Results
Clinical Symptoms
The majority of the cervical spondylosis cohort with neurological symptoms presented with gait and/or hand coordination difficulty. The mean mJOA score in this cohort was 15.1, with a range from 11–17. Neck pain was the most common clinical presentation in the cohort of cervical spondylosis patients without neurological symptomatology. All sixteen of these patients without symptoms had a mJOA score of 18.
Conventional MRI
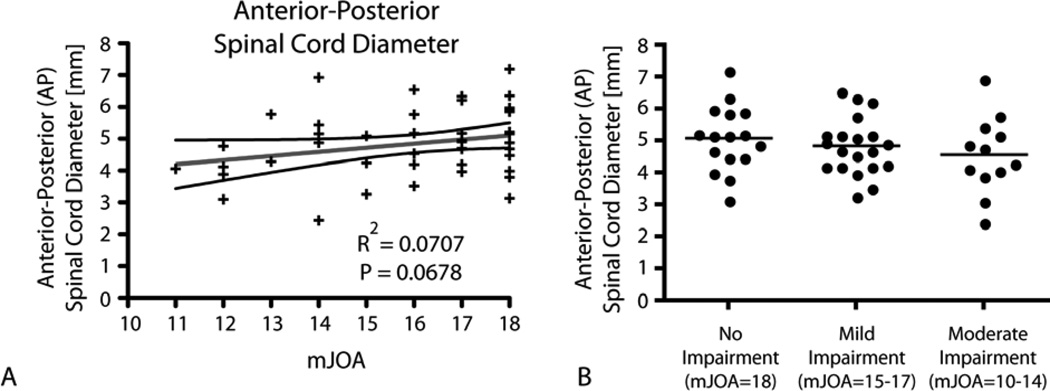
The mean antero-posterior spinal cord diameter in cervical spondylosis patients was significantly narrower in cervical spondylosis patients than healthy controls (4.56 ± 1.02 s.d. millimeters vs. 7.00 ± 0.75 s.d. millimeters; t-test, P < .0001). Within the cervical spondylosis cohort, there was a slight trend between spinal cord diameter and neurological impairment. In particular, we observed a trend between anterior-posterior diameter and mJOA (Fig.1A; R2=0.0707, P=0.0678; Power=0.53%); however, there was no relationship observed between neurological impairment and presence of intraspinal T2 hyperintensity. Additionally, no relationship between spinal cord diameter and severity of neurological impairment (none, mild, and moderate) within the cervical spondylosis cohort was observed (Fig. 1B; ANOVA, P=0.4146).
Figure 1. Conventional MRI Measures of Spinal Cord Compression.
A) Linear regression results comparing mJOA with anterior-posterior spinal cord diameter (R2=0.0707, P=0.0678). B) Comparison of anterior-posterior spinal cord diameter between patients without neurological impairment (mJOA=18), patients with mild impairment (mJOA=15–17), and patients with moderate impairment (mJOA=10–14). Results show no significant difference in spinal cord diameter between these groups (ANVOA, P=0.4146).
Reproducibility and quality of DTI data
High quality b=0 s/mm2 and b=500 s/mm2 images, reaching a maximum SNR of nearly 20 and a SNR full-width, half-max (FWHM) of approximately 80 mm for b=0 s/mm2 images, were acquired using the 2D–RF+Zoomed-EPI sequence. When compared with historic controls [31], axial FA measurements from C2 through the C6–7 level in the nine neurologically-intact volunteers in the current study showed no significant differences, suggesting the pulse sequence used in the current study may provide comparable results with previous studies (two-way ANOVA, P = 0.129 between studies, P = 0.128 across spinal levels, P = 0.410 interaction). To test the repeatability of spinal cord DTI measurements we examined a subset of 15 patients with spinal stenosis, minimal neurological impairment (mJOA ≥ 17), no change in impairment during observation, and more than two scans over an average duration of 200 days (± 13 days standard error of the mean, S.E.M.). Evaluations of MD and FA at C2 in these patients (average distance from site of compression=37.0±3.5mm) demonstrated a median coefficient of variation (CV) for MD and FA of 8.7% and 4.6%, respectively.
Fractional Anisotropy Changes at the Site of Compression
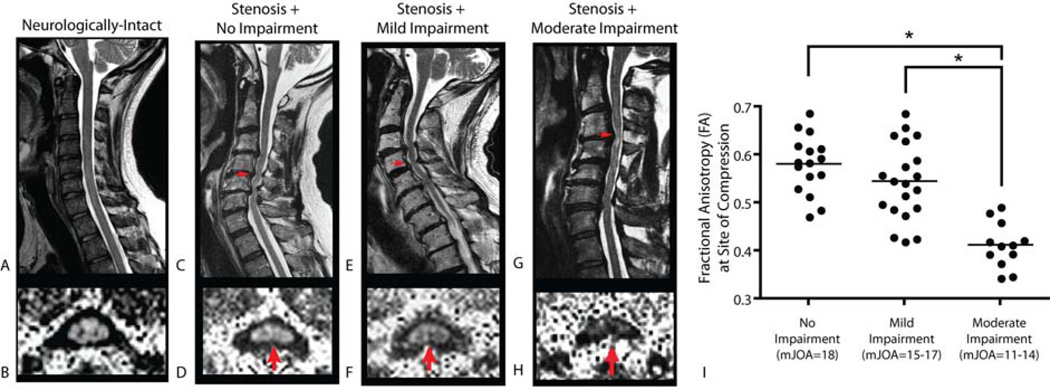
Fractional anisotropy (FA) images demonstrated high diffusion anisotropy within regions of white matter in neurologically-intact control subjects, consistent with healthy, densely packed axon tracts (Fig.2A–B). In contrast with the high diffusion anisotropy in neurologically-intact volunteers, patients with symptomatic spondylosis showed significant narrowing of the spinal canal and a reduction in FA (Fig.2C–D). A closer examination of our patient cohort revealed a pattern of decreased FA at the site of compression with increasing neurological impairment (decreasing mJOA). FA measurements at the site of compression could significantly stratify patients based on categorical severity of neurological impairment (Fig.2E; ANOVA, P<0.0001). For example, patients showing spondylosis without neurological symptoms demonstrated a relatively high FA measurement at the site of compression compared to adjacent levels. In patients with relatively mild neurological impairment, e.g. mJOA=15–17, FA values at the site of compression were slightly decreased relative to adjacent levels, although not significantly different from patients without neurological impairment (Tukey’s test for multiple comparisons, P>0.05 for mild vs. no impairment). In patients with moderate myelopathy, e.g. mJOA=11–14, FA values were markedly reduced compared to adjacent levels and compared to patients with mild or no neurological impairment (Tukey’s test, P<0.05 for moderate vs. mild & moderate vs. no impairment).
Figure 2. Fractional Anisotropy at the Site of Spinal Cord Compression.
Sagittal T2-weighted images (A,C, E, G) and fractional anisotropy (FA) images (B,D,F,H) of a neurologically-intact control subject (A,B), showing high FA in spinal cord white matter (B), a patient with cervical spondylosis without neurological impairment (C,D; mJOA=18), a patient with cervical spondylosis with mild impairment (E,F; mJOA=15), and a patient with cervical spondylosis with moderate impairment (G,H; mJOA=12). Note patients demonstrate a decreasing FA at the site of most severe compression with increasing neurological impairment, suggestive of increasing microstructural damage. Note on axial images posterior = top, anterior = bottom. I) Comparison of FA at the site of compression between patients without neurological impairment (mJOA=18), patients with mild impairment (mJOA=15–17), and patients with moderate impairment (mJOA=10–14). Results show a significant difference between patients with moderate impairment and those with mild or no impairment (ANOVA, P<0.0001; Tukey’s test, P<0.05 for moderate vs. mild or no impairment). * = P < 0.05.
DTI Correlates of Neurological Impairment
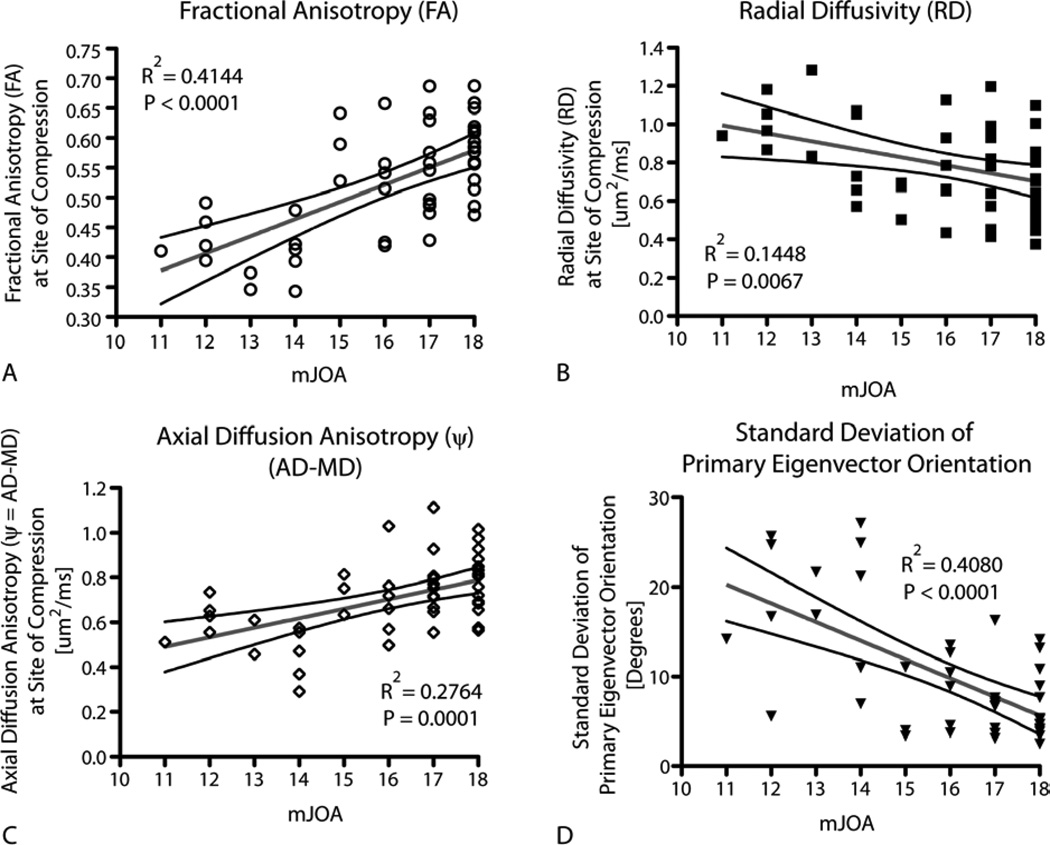
Consistent with more qualitative observations, a significant positive linear correlation was found between FA measurements at the site of compression and mJOA score (Fig.3A; Pearson, R2=0.4144, P<0.0001; Power=93%). No significant linear correlation was observed between MD or AD and mJOA score (R2< 0.03, P>0.25, Power>60% for both MD and AD). A significant, although rather weak, negative correlation was observed between RD and mJOA (Fig.3B; R2=0.1448, P=0.0067, Power = 51%). Although neither MD nor AD were considered statistically significant predictors of mJOA score, ψ, or the difference between AD and MD, showed a positive linear correlation with mJOA (Fig.3C; R2=0.2764, P=0.0001, Power = 52%). To test whether displacement in the orientation of white matter tracts at the site of compression is a significant predictor of neurological impairment, we examined the variance in the primary eigenvector orientation, which should be relatively small in the intact spinal cord, suggesting all white matter tracts are oriented in the same direction (i.e. rostral-caudal). Our results demonstrate a strong negative correlation between the standard deviation of primary eigenvector orientation and mJOA (Fig.3D; R2=0.4080, P<0.0001, Power > 90%), suggesting disorganization in white matter tract orientation at the site of compression is higher in patients with more neurological impairment.
Figure 3. Correlation between DTI and Neurological Impairment.
Linear regression results comparing mJOA with A) FA (R2=0.4144, P<0.0001), B) RD (R2=0.1448, P=0.0067), C) y (R2=0.2764, P=0.0001), and D) standard deviation of primary eigenvector orientation, or std(θ) (R2=0.4080, P<0.0001). All measurements were made at the level of highest spinal cord compression.
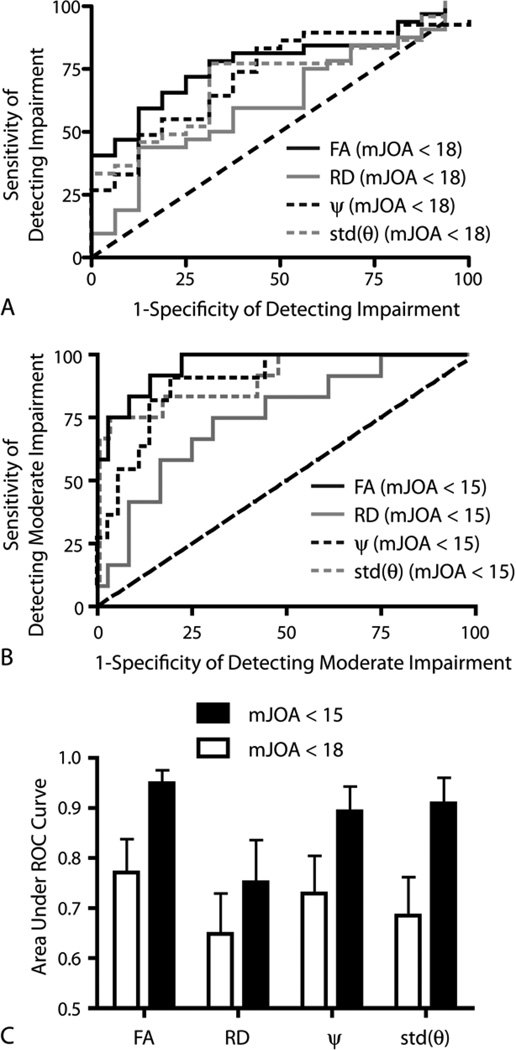
ROC analysis aimed at differentiating spondylosis patents with and without neurological impairment suggested that FA, ψ, and standard deviation in primary eigenvector orientation could identify symptomatic patients with significant sensitivity and specificity. Specifically, an average FA value measured at the site of highest compression less than 0.55 resulted in a 72% sensitivity and 75% specificity of detecting patients with mJOA < 18 (Fig.4A,C; AUC=0.77±0.07 S.E.M., P=0.0024), as well as a 81% sensitivity and 92% specificity of detecting patients with moderate impairment (Fig. 4B,C; mJOA < 15; AUC=0.95±0.03 S.E.M., P<0.0001). Average ψ, RD, and standard deviation of primary eigenvector orientation at the site of compression were not significant predictors of symptomatic patients (mJOA<18) after Bonferroni correction (Fig.4A,C; P>0.0083), but were all significant predictors of moderate impairment (mJOA<15) (Fig.4B; P<0.0095). A side-by-side comparison of the AUC for all DTI metrics suggested no significant difference in metrics performance (ANOVA; DTI Metric, P=0.1064); however, there was a significant increase in ROC performance when identifying stenosis patients with moderate neurological impairment (mJOA<15) compared with identification of patients with only slight neurological impairment (mJOA<18; Fig.4C; ANOVA, Severity of Impairment, P=0.0004).
Figure 4. ROC results for DTI metrics.
A) ROC curves showing the sensitivity and specificity of different DTI metrics to spondylosis patients with mJOA < 18. B) ROC curves showing the sensitivity and specificity of different DTI metrics to stenosis patients with moderate impairment.) Area under the ROC curve (AUC) as a measure of performance for DTI metrics for mJOA < 15 (solid black bars) and mJOA < 18 (white bars).
Discussion
Cervical spondylosis and stenosis are pathological conditions of the spinal column that affect the vast majority of older individuals with increasing age. The diagnosis of CSM typically includes static or dynamic x-rays, CT, myelography, or conventional MRI. Despite the significant advancement of conventional MRI technology since its inception, the value of traditional imaging technologies in diagnosing and predicting early response to treatment remains controversial. Results from the current study suggest a trend may exist between anterio-posterior spinal cord diameter and mJOA; however, the study did not have adequate statistical power (53%) to make a definitive conclusion. However, the low correlation coefficient (R2=0.0707) and slope associated with this relationship suggests that traditional MRI measures are not likely to be predictive of neurological impairment.
Chronic compression of the spinal cord produces pathological changes in the tissue microstructure that can be detected using DTI. For example, results from the current study clearly demonstrates an increase in MD and decrease in FA at the site of chronic compression in patients with chronic stenosis relative to neurologically-intact control subjects, consistent with previous studies [10, 19, 20, 22]. Additionally, we observed a decrease in FA in CSM patients proportional to the degree of neurological impairment as measured with mJOA score, consistent with a recent study by Jones et al. [34]. Our results also suggest FA measurements at the site of compression are a strong biomarker for identifying symptomatic stenosis patients (mJOA < 18 or mJOA < 15), which is a trend also observed by other investigators [35, 36].
Inconsistent with our current findings is the hypothesis that MD is a significant predictor of myelopathy. Data from a study published by Demir et al.[19] suggested MD measurements at the site of compression had a relatively high sensitivity and specificity for identifying symptomatic patients. Additionally, data from Uda et al.[37] suggested MD was the best predictor of myelopathy when compared with FA; however, this study compared healthy control subjects directly with symptomatic patients and did not compare symptomatic and non-symptomatic patients with cervical stenosis. Our results clearly demonstrated no significant correlation between MD and mJOA score when examining a cohort of cervical spondylosis composed of symptomatic and asymptomatic individuals.
Results support the hypothesis that AD and RD may be sensitive biomarkers to axonal dysfunction and demyelination, respectively [26–29]. Previous studies by Song et al. [27, 28] have eloquently demonstrated that dysmyelination results in an increased RD, without a change in AD. Conversely, the studies by Ellingson et al. [26] and Budde et al. [29] have demonstrated a strong correlation between the decrease in AD and a decrease in axonal function. Further supporting this hypothesis is the observation that changes in AD and RD follow distinctly different temporal profiles [30], where the decrease in AD occurs much quicker than the increase in RD. In the current study we observed a negative linear correlation between RD and mJOA, suggesting a decrease in neurological function may accompany an increase in demyelination in patients with chronic spinal cord compression. Although we did not observe a significant correlation between AD or MD and mJOA, axial diffusion anisotropy (ψ), defined as the difference between AD and MD, was significantly correlated with functional impairment. In the context of the current study, we hypothesized that ψ would allow for assessment of axial diffusivity independent of MD, since MD was thought to fluctuate as a result of spinal cord compression. Taken together, these results may imply that some degree of both axonal dysfunction (myelopathy) and demyelination may be present in patients with spinal cord compression.
Results from the current study demonstrated a strong correlation between the standard deviation in primary eigenvector orientation and mJOA score. Consistent with our findings, a recent study by Song et al.[38] showed abnormal deflection in primary eigenvector orientation, as illustrated using FA color maps, in approximately ¾ of patients with myelopathy. In another recent study, Cui et al.[39] documented a high degree of “entropy”, or disorganization, in primary eigenvector orientation at the compression site in patients with CSM compared to healthy controls. These results appear to be consistent with the hypothesis that primary eigenvector orientation, which is thought to be a surrogate of white matter tract orientation, dendritic orientation, or interneuron orientation, becomes more disorganized with increasing neurological impairment. Future studies aimed at exploring the role of axon and cell body morphological changes in mild to moderate CSM may provide more insight into the influence of these mechanisms on DTI measurements.
Clinical Relevance
One of the strengths of this study is that in contrast to some of the other published DTI studies, both neurologically symptomatic and asymptomatic patients with severe cervical spondylosis were analyzed, rather than just symptomatic CSM patients. The inclusion of a spectrum of patients with advanced cervical stenosis and spondylosis mirrors what is encountered in practice by many spine surgeons, and represents an area of clinical management controversy and challenge. As demonstrated in Figure 2, it can be very difficult to discern neurological status based on standard MRI, as each of the study patients has significant stenosis, yet very different neurological status. Although none of the standard MRI parameters (spinal cord diameter, signal change) were able to discern clinical condition between patients with similar appearing MRI scans, DTI was clearly able to discriminate neurological condition. As such, this novel imaging technology may be able to serve as a potential noninvasive biomarker, and help play a role in the treatment algorithm of patients with advanced cervical spondylosis and myelopathy.
Study Limitations
A potential limitation to the current study was the use of only six diffusion sensitizing directions to estimate the diffusion tensor. Although previous spinal cord DTI studies have suggested that white matter integrity in the spinal cord does not require a full tensor, only longitudinal (axial) and transverse (radial) diffusion sensitivity [40], DTI studies in the brain suggest an increase in accuracy of diffusion tensor estimation with increasing number of diffusion directions [41, 42]. Despite this potential limitation, our results in neurologically-intact control subjects were consistent with historical data performed with many more diffusion sensitizing directions. Further, since we performed DTI with a large number of averages, it is likely this signal-to-noise advantage was sufficient to maintain a highly accurate estimate of the diffusion tensor.
Conclusions
Results from the current study support the use of DTI as an imaging biomarker for predicting neurological impairment in patients with mild to moderate CSM.
Acknowledgments
Disclosures/ Grant Support:
NIH/NINDS 1R21NS065419-01A1 (LTH; NS)
NIH/NINDS 1R01NS078494-01A1 (LTH; NS; BME)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. 6. Vol. 11. Spine: Phila Pa 1976; 1986. pp. 521–524. [DOI] [PubMed] [Google Scholar]
- 2.Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. 3. Vol. 32. Spine: Phila Pa 1976; 2007. pp. 342–347. [DOI] [PubMed] [Google Scholar]
- 3.Irwin ZN, Hilibrand A, Gustavel M, et al. Part II: cervical spine. 19. Vol. 30. Spine: Phila Pa 1976; 2005. Variation in surgical decision making for degenerative spinal disorders; pp. 2214–2219. [DOI] [PubMed] [Google Scholar]
- 4.Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112–118. doi: 10.3171/2009.1.SPINE08718. [DOI] [PubMed] [Google Scholar]
- 5.Torg JS, Pavlov H, Genuario SE, et al. Neurapraxia of the cervical spinal cord with transient quadriplegia. J Bone Joint Surg Am. 1986;68(9):1354–1370. [PubMed] [Google Scholar]
- 6.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164(3):771–775. doi: 10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 7.Yue WM, Tan SB, Tan MH, Koh DC, Tan CT. The Torg--Pavlov ratio in cervical spondylotic myelopathy: a comparative study between patients with cervical spondylotic myelopathy and a nonspondylotic, nonmyelopathic population. 16. Vol. 26. Spine: Phila Pa 1976; 2001. pp. 1760–1764. [DOI] [PubMed] [Google Scholar]
- 8.Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY. Reevaluation of the pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg. 2009;1(1):6–10. doi: 10.4055/cios.2009.1.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez de Rota JJ, Meschian S, Fernandez de Rota A, Urbano V, Baron M. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007;6(1):17–22. doi: 10.3171/spi.2007.6.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22(1):38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical myelopathy Analysis of 29 surgical cases. J Neurosurg. 1991;74(6):887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 12.Mastronardi L, Elsawaf A, Roperto R, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7(6):615–622. doi: 10.3171/SPI-07/12/615. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y. Does it predict the outcome of conservative treatment? 6. Vol. 25. Spine: Phila Pa 1976; 2000. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy; pp. 677–682. [DOI] [PubMed] [Google Scholar]
- 14.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26(2):217–226. doi: 10.1097/00006123-199002000-00006. discussion 26–7. [DOI] [PubMed] [Google Scholar]
- 15.Morio Y, Yamamoto K, Kuranobu K, Murata M, Tuda K. Does increased signal intensity of the spinal cord on MR images due to cervical myelopathy predict prognosis? Arch Orthop Trauma Surg. 1994;113(5):254–259. doi: 10.1007/BF00443813. [DOI] [PubMed] [Google Scholar]
- 16.Puzzilli F, Mastronardi L, Ruggeri A, Lunardi P. Intramedullary increased MR signal intensity and its relation to clinical features in cervical myelopathy. J Neurosurg Sci. 1999;43(2):135–139. discussion 9. [PubMed] [Google Scholar]
- 17.Fouyas IP, Statham PF, Sandercock PA. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy. 7. Vol. 27. Spine: Phila Pa 1976; 2002. pp. 736–747. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz ED, Cooper ET, Fan Y, et al. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport. 2005;16(1):73–76. doi: 10.1097/00001756-200501190-00017. [DOI] [PubMed] [Google Scholar]
- 19.Demir A, Ries M, Moonen CT, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229(1):37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 20.Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26(6):1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 21.Ellingson BM, Schmit BD, Kurpad SN. Lesion growth and degeneration patterns measured using diffusion tensor 9.4-T magnetic resonance imaging in rat spinal cord injury. J Neurosurg Spine. 2010;13(2):181–192. doi: 10.3171/2010.3.SPINE09523. [DOI] [PubMed] [Google Scholar]
- 22.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21(2):426–433. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 24.Ellingson BM, Ulmer JL, Schmit BD. Gray and white matter delineation in the human spinal cord using diffusion tensor imaging and fuzzy logic. Acad Radiol. 2007;14(7):847–858. doi: 10.1016/j.acra.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Ellingson BM, Ulmer JL, Schmit BD. Morphology and morphometry of human chronic spinal cord injury using diffusion tensor imaging and fuzzy logic. Ann Biomed Eng. 2008;36(2):224–236. doi: 10.1007/s10439-007-9415-6. [DOI] [PubMed] [Google Scholar]
- 26.Ellingson BM, Kurpad SN, Schmit BD. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed Sci Instrum. 2008;44:28–33. [PubMed] [Google Scholar]
- 27.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 29.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellingson BM, Kurpad SN, Schmit BD. Ex vivo diffusion tensor imaging and quantitative tractography of the rat spinal cord during long-term recovery from moderate spinal contusion. J Magn Reson Imaging. 2008;28(5):1068–1079. doi: 10.1002/jmri.21578. [DOI] [PubMed] [Google Scholar]
- 31.Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging of the neurologically intact human spinal cord. AJNR Am J Neuroradiol. 2008;29(7):1279–1284. doi: 10.3174/ajnr.A1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4(3):286–295. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. 17. Vol. 26. Spine: Phila Pa 1976; 2001. pp. 1890–1894. discussion 5. [DOI] [PubMed] [Google Scholar]
- 34.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion Tensor Imaging Correlates with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy and Predicts Outcome following Surgery. AJNR Am J Neuroradiol. 2012 doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerkovsky M, Bednarik J, Dusek L, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. 1. Vol. 37. Spine: Phila Pa 1976; 2012. pp. 48–56. [DOI] [PubMed] [Google Scholar]
- 36.Hori M, Okubo T, Aoki S, Kumagai H, Araki T. Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging. 2006;23(2):183–188. doi: 10.1002/jmri.20488. [DOI] [PubMed] [Google Scholar]
- 37.Uda T, Takami T, Tsuyuguchi N, et al. Assessment of Cervical Spondylotic Myelopathy using Diffusion Tensor MRI Parameter at 3.0 Tesla. Spine: Phila Pa 1976; 2012. [DOI] [PubMed] [Google Scholar]
- 38.Song T, Chen WJ, Yang B, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20(3):422–428. doi: 10.1007/s00586-010-1587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui JL, Wen CY, Hu Y, Mak KC, Mak KH, Luk KD. Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. Neuroimage. 2011;58(4):1028–1033. doi: 10.1016/j.neuroimage.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 40.Tu TW, Kim JH, Wang J, Song SK. Full tensor diffusion imaging is not required to assess the white-matter integrity in mouse contusion spinal cord injury. J Neurotrauma. 2010;27(1):253–262. doi: 10.1089/neu.2009.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa T, Aoki S, Abe O, et al. Diffusion tensor imaging of the brain: effects of distortion correction with correspondence to numbers of encoding directions. Radiat Med. 2008;26(8):481–487. doi: 10.1007/s11604-008-0262-7. [DOI] [PubMed] [Google Scholar]
- 42.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]