Abstract
The purpose of this review was to evaluate the current role of multiparametric magnetic resonance imaging (mp-MRI) in the management of prostate cancer (PC). The diagnosis of PC remains controversial owing to overdetection of indolent disease, which leads to overtreatment and subsequent patient harm. mp-MRI has the potential to equilibrate the imbalance between detection and treatment. The limitation of the data for analysis with this new technology is problematic, however. This issue has been compounded by a paradigm shift in clinical practice aimed at utilizing this modality, which has been rolled out in an ad hoc fashion often with commercial motivation. Despite a growing body of literature, pertinent clinical questions remain. For example, can mp-MRI be calibrated to reliably detect biologically significant disease? As with any new technology, objective evaluation of the clinical applications of mp-MRI is essential. The focus of this review was on the evaluation of mp-MRI of the prostate with respect to clinical utility.
Keywords: Diagnosis, Magnetic resonance imaging, Pathology, Prostatic neoplasms, Urology
INTRODUCTION
Screening for prostate cancer (PC) using prostate-specific antigen (PSA) testing remains controversial. According to the European Randomised Study of Screening for Prostate Cancer, PSA-based screening reduces PC-specific mortality by 21% [1] and yet the findings from this study are insufficient to justify population-based screening. Overdiagnosis by PSA screening of disease that is more appropriately managed with active surveillance is estimated at 50% [1]. Subsequent treatment of men with insignificant disease leads to unnecessary harm, which makes population screening unacceptable [2], even to the vast majority of urologists. For any screening program to maximize benefit and minimize harm, men with clinically low-risk PC need to be identified and managed with active surveillance rather than being indiscriminately radically treated. Low-risk PC as defined by the National Comprehensive Cancer Network is clinical stage T1 to 2a, Gleason score 6 of less, and PSA<10 ng/mL [3]. Overdetection of indolent disease is not the issue but rather overtreatment, which carries an economic burden and risks of urinary incontinence, erectile dysfunction, and urethral stricture [4,5].
The lack of a validated population-based screening protocol for such a ubiquitous disease underlies the dilemma of PC management. In many cases, only well-informed men who meet certain criteria (age, comorbidities) and understand the issues surrounding screening undergo PSA testing, digital rectal exam, and biopsy. Both transrectal and transperineal prostate biopsy are "blind" techniques that randomly detect both high- and low-grade disease with a significant false-negative rate [6]. The detection is random or nontargeted because ultrasound does not reliably demonstrate PC tissue. A better strategy to prevent harm stemming from overdiagnosis of indolent disease is required. The advent of active surveillance protocols based on individual risk stratification of patients has somewhat equilibrated the imbalance between diagnosis and treatment. The complicating factor has been that the biopsy pathology (Gleason score) on which risk stratification is based underestimates PC aggressiveness by up to 30% [1]. This adds the confounding and arguably more serious issue of under-treatment of high-risk disease.
The advent of multiparametric magnetic resonance imaging (mp-MRI) of the prostate and more recently targeted MRI-ultrasound fusion biopsy has provided a targeted and more accurate means of diagnosing PC in men with an elevated PSA level. In the best hands, mp-MRI of the prostate has a specificity approaching 90% and a negative predictive value of around 85% [7,8]. Therefore, a negative mp-MRI result justifies a nonbiopsy approach at least in the first instance. Interval PSA monitoring is a sensible clinical strategy in this setting. The value of mp-MRI is in decreasing the rate of unnecessary biopsy in men with elevated PSA. Emerging data also suggest that mp-MRI of the prostate may prove useful in determining which patients are managed with active surveillance [9]. Pathological diagnosis is still necessary before stratifying patients into high- and low-risk categories. The authors see the role of mp-MRI as a means of clarifying an abnormal PSA result, not replacing PSA as the initial diagnostic test. The downside is that mp-MRI of the prostate is expensive and not readily available in all centers.
Yet questions remain: can mp-MRI be calibrated to detect biologically significant disease?
In the prediagnosis setting, mp-MRI may be useful for clarifying elevated PSA levels. In the setting of active surveillance, it may lead to the detection of PC progression. As with any new technology, objective evaluation of the clinical applications of mp-MRI is essential. How should we judge mp-MRI of the prostate and its ability to detect PC? This last question in particular is the focus of this review.
BACKGROUND: MULTIPARAMETRIC MRI OF THE PROSTATE
mp-MRI combines anatomical T2-weighted imaging (T2WI) with multiple functional parameters (Table 1) to more accurately assess and characterize PC. A high-resolution T2-weighted image is usually combined with a minimum of two functional parameters to achieve the highest sensitivity and specificity [10]. Diffusion-weighted imaging (DWI) and magnetic resonance spectroscopy (MRS) add specificity to lesion characterization. Dynamic contrast enhancement (DCE) with gadolinium adds sensitivity for cancer detection [10].
Table 1. Magnetic resonance sequences and their implications for prostate cancer imaging.
| MR Sequence | Specifics | Implications for prostate cancer imaging |
|---|---|---|
| T1-weighted | Gradient echo sequence with short echo time and short repetition time. Can be used with contrast agents. | Detects hemorrhage secondary to prostate biopsy as hyperintense regions. Used to detect bone metastases and enlarged lymph nodes. A fast pulse sequenced version is used for dynamic contrast-enhanced imaging (see below). |
| T2-weighted | Fast spin echo sequence with long echo time and long repetition time. Tissues with higher free water content are brighter. Fat tissue is also bright. | Differentiates zonal anatomy. Cancers are low in signal. Glandular peripheral zone cancers appear as round or ill-defined low-intensity foci. Central gland cancers have similar signal characteristics to the normal and hypertrophic central gland and can be identified by poorly defined borders and lenticular shape. Extracapsular extension can be directly observed. Prostatitis, hemorrhage, atrophy, benign prostatic hyperplasia, and changes after treatment (e.g., radiation induced arteritis) can be mistaken for cancer. |
| MRS | The MR signal produces a spectrum of resonances that correspond to different molecular arrangements of the isotope being excited. MRS reflects tumour metabolism. | In cancer tissue the production of citrate is reduced, whereas choline is increased, leading to an increased choline to citrate ratio. 3 Tesla MRI allows for better spectral separation and overall signal when used in combination with MRS. |
| DWI-MRI | Water molecules naturally move randomly according to Brownian motion. In tissues with high cellularity (cancer in particular) diffusion is restricted, which results in an increase in MRI signal with this sequence. The degree of sensitivity to water diffusion is reflected in the "b value". The higher the "b value" the more sensitive the tissue to restricted diffusion. Apparent diffusion coefficient maps can be calculated from the MRI images. | Prostate cancer exhibits restricted diffusion (dark on ADC maps, high signal on source MRI images). |
| DCE-MRI | T1-weighted sequence. Low molecular weight contrast agent diffuses from vascular space to extracellular space and then leaks slowly back into the vascular space. The rate of forward leakage, the rate of backward leakage, and the fractional volume of the extracellular space are calculated using pharmacokinetic modelling. | Tumours show early enhancement and early washout of the contrast agent, which enables detection. The higher the tumour grade, the higher these parameters tend to be. |
MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; DWI MRI, diffusion weighted imaging MRI; ADC, apparent diffusion coefficient; DCE MRI, dynamic contrast enhanced MRI.
Adapted from Raz et al. Nat Rev Urol 2010;7:543-51, with permission of Macmillan Publishers Limited [8].
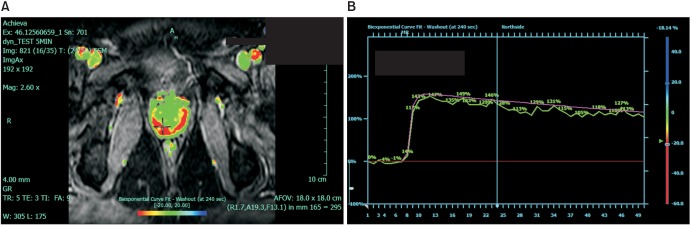
T2WI demonstrates the zonal anatomy and capsule of the prostate. PC in the peripheral zone appears usually appears as a round or poorly defined low signal area (Fig. 1). T2WI alone is sensitive but not specific for PC. In many cases it cannot reliably differentiate PC from prostatic intraepithelial neoplasia, hemorrhage, or prostatitis. Transitional zone tumors are more challenging to detect owing to the similar appearance of benign prostate hypertrophy and malignancy. The addition of at least two functional parameters significantly improves sensitivity and specificity [10]. These parameters include a combination of DWI, DCE, and MRS. DCE evaluates tumor angiogenesis (Fig. 2). It is performed by administration of a gadolinium-based contrast medium. It has been shown to be able to detect significant disease in up to 93% of cases [10]. It has particular clinical utility in patients with a previous negative biopsy result with rising PSA. It is a useful means of detecting recurrence following radical treatment [11].
Fig. 1. T2-weighted image demonstrating an area of low signal intensity in the right peripheral zone consistent with prostate cancer.
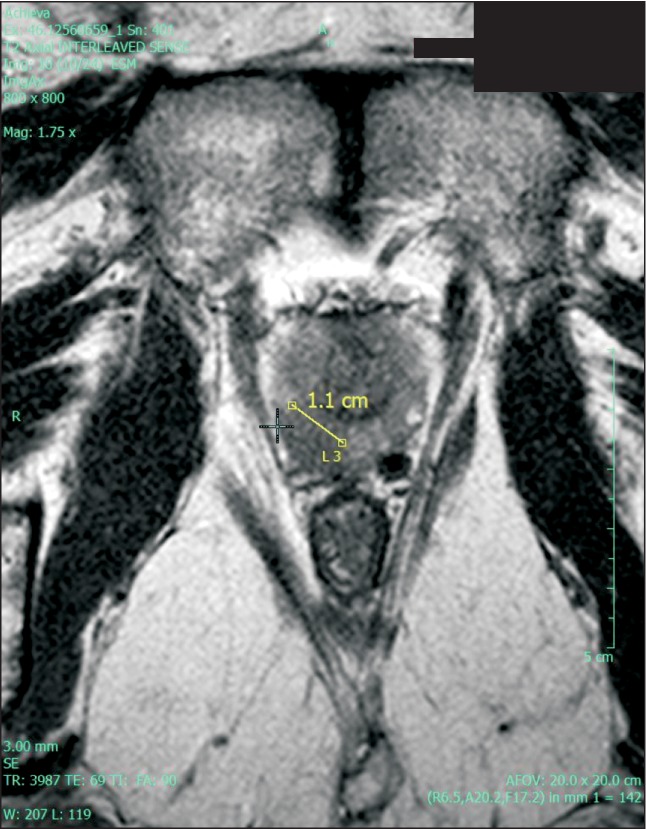
Fig. 2. A dynamic contrast enhancement image (A) and postcontrast washout curve (B) of a right peripheral zone lesion.
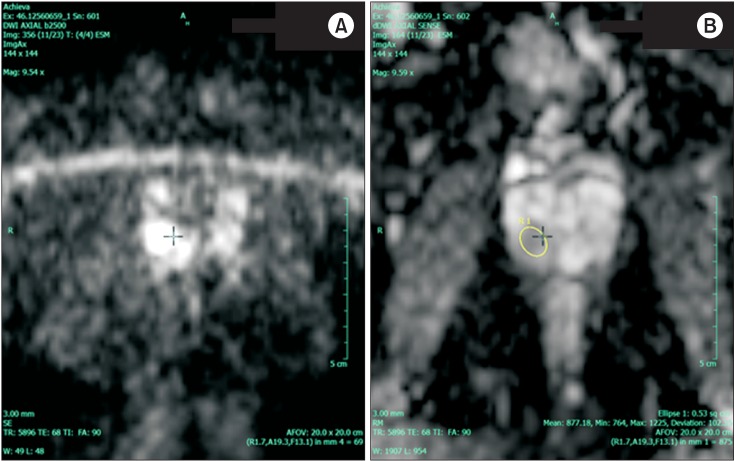
PC restricts the diffusion of water molecules as the result of hypercellularity and destruction of normal glandular tissue. DWI allows an apparent diffusion coefficient, or ADC, map to be calculated, enabling both qualitative and quantitative assessment of PC aggressiveness. Cancer shows a lower ADC value than does normal tissue [12]. The "b value" with respect to diffusion-weighted sequences is a measure of variation in the strength of the magnetic field. At higher b values the sensitivity to water shift or diffusion is increased and anatomical information is filtered out. This enables areas of water restriction (tumor cells) to be more easily identified compared to the normal cells that allow water to diffuse more easily [12]. This is because water movement is restricted in highly cellular tissues. The b value is a topic of growing research interest. Values range from 0 (which is essentially a T2-weighted image) to 2,500 s/mm2. At this higher range, the anatomical features are not distinguishable and sensitivity to diffusion is at its peak. An area of suspicion will show up as a bright spot on a b value map, which corresponds to a darker region on an ADC map (Fig. 3).
Fig. 3. A diffusion-weighted image (b value= 2,500) (A) and the corresponding apparent diffusion coefficient map (B) demonstrating Gleason 4+3 prostate cancer in the right peripheral zone.
Early data suggest that the application of an ultrahigh b value may be beneficial in PC detection. Katahira et al. [13] (n=201) demonstrated by comparison to whole mount pathology that the addition of DWI with a b value of 2,000 s/mm2 to T2WI has the potential to improve the detection of PC. At this early stage, it still important for clinicians to consider the overall risk of significant disease based on a system such as PIRADS (prostate imaging reporting and data system) which incorporates a range of parameters.
Notably, several studies have demonstrated that ADC values correlate with Gleason scores. DWI and ADC can assist in the risk stratification of patients on the basis of tumor aggressiveness [14]. Donati et al. [15] demonstrated that ADC was an independent predictor of tumor aggressiveness, which could significantly differentiate disease with a Gleason score of 6 from disease with a Gleason score of 7 and above. Boesen et al. [16] demonstrated that measuring the ADC of normal tissue and of malignant tissue to develop a ratio leads to a more precise correlation with Gleason score. Furthermore, the authors claimed that the ADC ratio improves accuracy in discriminating tumors with a Gleason score≤7 (3+4) from tumors with a Gleason score≥7 (4+3). While this technology has the potential to reliably differentiate significant disease from indolent disease, it cannot distinguish between individual Gleason scores.
MRS can evaluate tumor metabolism by measuring concentrations of choline, citrate, and creatinine. Benign prostate tissue is rich in citrate. Malignancy is characterized by loss of the citrate peak and corresponding gain in the choline or creatinine peak. This technique is more technically demanding and time consuming and thus not as common as DWI and DCE [12]. Weinreb et al. [17] (n=110) demonstrated that the addition of MRS does not improve the diagnostic accuracy of mp-MRI. Most modern mp-MRI eliminates this phase.
SYSTEMS FOR PREDICTING PC RISK ON IMAGING
A validated process of predicting final pathology from radiological parameters would further enhance risk stratification. A significant percentage of lesions detected by mp-MRI are benign [18]; therefore, it is important that radiologists have a reliable method of assessing the risk of malignancy in all visible lesions. A number of scoring schemes are used by radiologists to predict risk of PC on the basis of mp-MRI findings. The Likert-type scoring system is a subjective 5-point scale that radiologists use to assess risk of malignancy. Being a subjective tool, it relies significantly on the experience of the assessor [19].
PIRADS is a structured radiological reporting scheme for mp-MRI of the prostate (Table 2) that is comparable to breast imaging-reporting and data system (BIRADS) for breast imaging. It has been validated for risk stratification in the setting of repeat biopsies [20]. It is based on three main parameters, namely, the T2 signal, DWI, and DCE with gadolinium. A score is given that correlates with the risk of malignancy on pathological assessment (Table 2). The images presented in Figs. 1 (T2WI), 2 (DCE), and 3 (DWI with corresponding ADC map) demonstrate images from the same patient. On the basis of these parameters, this patient was assigned a PIRADS score of 5 indicating that the lesion in the right peripheral zone was highly suspicious for malignancy. The predicted Gleason score for this lesion was 4+3, largely based on the ADC map (Fig. 3). This prediction was later confirmed by radical prostatectomy pathology. In addition to the PIRADS score, which aims to determine whether a lesion is significant, extraprostatic involvement is usually scored on a 5-point scale [20].
Table 2. Summary of PIRADS classification: a radiological risk stratification system for prostate cancer.
| PIRADS classification | Risk of PC | Multiparametric score with T2, DWI, DCE and (including MRS) |
|---|---|---|
| I | Most probably benign | 3,4 (4,5) |
| II | Probably benign | 5,6 (6-8) |
| III | Indeterminate | 7-9 (9-12) |
| IV | Probably malignant | 10-12 (13-16) |
| V | Highly suspicious of malignancy | 13-15 (17-20) |
PIRADS, prostate imaging reporting and data system; PC, prostate cancer; DWI, diffusion-weighted imaging; DCE, dynamic contrast enhancement; MRS, magnetic resonance spectroscopy.
It can be difficult to assess the risk of a lesion, especially when the various functional parameters are discordant [21]. Vache et al. [21] compared several scoring systems used for mp-MRI of the prostate including the Likert and PIRADS schemes. Those authors argued that experienced radiologists can delineate malignancy from benign change subjectively, even in the absence of a clearly defined score based on the various parameters. Their data were ultimately in favor of a subjective Likert-based system, although Likert was familiar to the radiologists in their center compared with PIRADS.
Rosenkrantz et al. [19] demonstrated that both PIRADS and Likert performed well for tumor localization with the caveat that the more subjective Likert system was preferred in the transition zone, where interpretation is frequently complicated by the presence of benign hypertrophy. According to Junker et al. [22], it may be necessary to amend the PIRADS scoring system for DCE in the transition zone given its limitations in differentiating PC from benign disease in this region. It is important that consensus is reached regarding an objective scoring system in the transition zone so that risk assessment may continue to improve with the use of this technology.
COMPARISON OF PREOPERATIVE mp-MRI WITH RADICAL PROSTATECTOMY HISTOPATHOLOGY
The gold standard of evaluating mp-MRI is comparison of preoperative radiological parameters with the histopathology following analysis of radical prostatectomy specimens. This important comparison has not been comprehensively studied to date, yet is potentially the most valid means of evaluating mp-MRI. The limitations of data with mp-MRI include a lack of easy access owing to cost and limited machines compounded by a correspondingly large-scale paradigm shift to the use of this modality, which has been rolled out in an ad hoc fashion. mp-MRI is currently not subsidized by Medicare in Australia, meaning that patients must pay a substantial out-of-pocket fee. It is largely performed in the private setting. Despite the limitations of the data, however, there are credible studies with promising results.
In terms of comparing preoperative mp-MRI with postoperative histopathology, Styles et al. [23] from Melbourne demonstrated (n=38) that analyzing T2WI and DWI in combination was the best strategy for detecting localized PC with a reported sensitivity of 85% for cancers with a volume >0.5 mL. Kitamura et al. [24] (n=54) examined cancer distribution in men who underwent mp-MRI of the prostate (T2, DWI, MRS) and prostate biopsy followed by radical prostatectomy. The prostate was divided into 12 segments, each of which was examined for malignancy on the basis of T2WI, DWI, and MRS. Notably, DCE with gadolinium was not available. The mp-MRI and biopsy results were compared with the histopathology results from the radical prostatectomy specimens. This comparison demonstrated that T2 and DWI combined had a higher positive rate than any individual sequence when the biopsy result was negative. The authors also raised a practical issue of comparing histopathology with imaging owing to distortion of the radical prostatectomy specimen after fixation.
The data relating to mp-MRI have not all been positive. In a study of men (n=106) who underwent mp-MRI for staging followed by radical prostatectomy, Billing et al. [25] demonstrated that mp-MRI was not able to reliably predict extracapsular extension and seminal vesicle invasion. The authors concluded that mp-MRI has limited value in preoperative staging because of the lack of reliable, predictive data relating to the extent of disease. According to Min et al. [26] (n=126), who retrospectively compared mp-MRI to radical prostatectomy pathology, mp-MRI had high specificity (87.5%) but not sensitivity (65%) for predicting extracapsular extension. Notably, sensitivity improved from 46.4% to 65% with the addition of DWI. The authors concurred that there is a need for a new protocol to reliably predict extracapsular extension; however, they were able to demonstrate that a combination of T1, T2WI, DCE, and DWI resulted in accurate detection of PC. Tanaka et al. [27] (n=67) achieved similar sensitivity (60%) and specificity (86%) for the detection of extracapsular extension as part of staging prior to robotic-assisted radical prostatectomy. Those authors believed that staging mp-MRI has the potential to guide surgical decision-making regarding preservation of the neurovascular bundle.
HISTOPATHOLOGY FROM PROSTATE BIOPSY
When radical prostatectomy is not indicated or not performed, comparison with histopathology from biopsy is an alternative when trying to evaluate the utility of mp-MRI of the prostate to detect biologically significant disease. An indeterminate or suspicious prostatic lesion according to mp-MRI can be biopsied with MRI-ultrasound fusion technology. Comparison with the final histology (Gleason score and volume) gives a good indication as to the reliability of mp-MRI to detect significant disease.
Transrectal or transperineal ultrasound-guided prostate biopsy with a minimum of 12 cores has been the accepted method of confirming PC in men with an elevated PSA level. This method is blind to the location of suspicion within the prostate, and the false-negative rate has been reported to be as high as 50%. Saturation biopsy (≥24 cores) aimed at improving detection rates may not detect more significant cancer and are associated with higher morbidity [28]. This leads to repeated biopsies, especially in men in whom clinical suspicion is high. The key concern is the possibility of having undiagnosed significant disease. The detection rate of significant disease is improved with targeted biopsy with exciting implications for avoiding overdiagnosis [28].
In an era in which prostate biopsy is also evolving, some argue that transperineal biopsy with upwards of 24 cores is a better standard to which MRI should be held. Pepe et al. [29] (n=100) examined the role of mp-MRI in avoiding repeat transperineal saturation biopsies in men with persistently elevated PSA (4.1-10) with a free to total ratio less than 25% and normal results on a digital rectal exam. They demonstrated that mp-MRI did not identify 22% of cancers, however, these were found to be histologically insignificant. At the same time, mp-MRI was shown to improve the diagnosis of significant disease in the anterior zone. The authors argued that 31 of the men would have been spared from repeat saturation biopsy without a corresponding increased risk of aggressive disease.
A pilot study (n=54) examined MRI-ultrasound fusion biopsy for prediction of final pathology. They compared biopsy pathology to final whole-mount pathology. They performed both a mapping biopsy using a 12-point systematic grid and target biopsies of suspicious areas identified by mp-MRI before surgery. The results demonstrated that fusion technology allowed greater accuracy in predicting final pathology compared with conventional methods [30].
COMPARISON TO PROSTATE-SPECIFIC MEMBRANE ANTIGEN POSITRON EMISSION TOMOGRAPHY-COMPUTED TOMOGRAPHY
Regarding primary detection, which is more relevant to mp-MRI or even detecting recurrent disease in the setting of previous radiation, data are scarce. Comparative studies between modalities are important and need to be done. Comparison of mp-MRI of the prostate to other imaging modalities may also yield vital data for assessing effectiveness. One of the key issues in PC management is the early detection of recurrent disease following radical therapy. Early detection avoids or delays systemic therapies and associated side effects. Positron emission tomography-computed tomography (PET-CT) using a choline-based protocol has traditionally been used for this purpose. However, choline uptake in PC cells is poor and therefore this modality is associated with poor sensitivity and specificity, particularly in cases of low PSA levels and high Gleason scores [31]. Prostate-specific membrane antigen (PSMA) is garnering significant interest because it is overexpressed in PC compared with other PSMA-expressing tumors (kidney, small bowel, salivary glands). Almost all adenocarcinomas of the prostate express PSMA. A PSMA-targeted radioligand labeled with gallium 68 (68Ga PSMA PET-CT) has been shown to have a strong affinity to PC cells [32].
In a recent, large retrospective analysis (n=319), Afshar-Oromieh et al. [32] demonstrated that 68Ga PSMA PET-CT scans could detect PC in 82.8% of patients who were referred for investigation of recurrent PC. Pathological analysis demonstrated accumulation of tracer in malignant lesions. Notably, detection of PC was improved at higher PSA levels. Local therapy was suitable for 40% of the patients, thereby delaying systemic therapy and associated complications. There was no correlation with Gleason score or pathological 68Ga PSMA PET-CT scan. Studies comparing PSMA PET-CT with mp-MRI would be useful for evaluating both modalities, particularly if the utility of PSMA PET-CT is to be extended to primary detection rather than just recurrent PC.
mp-MRI IN ACTIVE SURVEILLANCE
Active surveillance is being utilized more frequently in the management of PC. The goal is to minimize the harm caused by overtreatment of low-risk disease while providing a means of identifying men with disease progression who require definitive treatment. A significant number of men on active surveillance protocols have a suspicious lesion that is identifiable on MRI [33]. mp-MRI may prove to be particularly useful in this setting because suspicious lesions can be targeted with fusion biopsy leading to preferential sampling of PC tissue. This means that PC progression can be detected more efficiently and accurately. Growing evidence supports the role of repeat mp-MRI of the prostate and fusion biopsy to improve monitoring of men on active surveillance. In a retrospective analysis, Abdi et al. [34] (n=603) demonstrated that mp-MRI of the prostate with the option of subsequent fusion biopsy improves the detection of PC progression for men under active surveillance. Walton-Diaz et al. [35] (n=152) demonstrated that stable mp-MRI findings were associated with Gleason score stability on biopsy. Importantly, only 2.9 fusion biopsies were needed to detect 1 case of Gleason progression compared with 8.74 saturation biopsies. According to the authors, mp-MRI may be a promising means of reducing the number of biopsies for men on active surveillance.
Siddiqui et al. [36] (n=85) found that mp-MRI could reduce the number of repeat biopsies by up to 68% for men on active surveillance. A tumor that is not detected on mp-MRI is more likely to be low risk [37], and according to Johnson et al. [38], the risk of biologically significant disease in patients with a negative mp-MRI result is low enough to justify deferring definitive treatment without biopsy. The findings in these studies are promising and certainly warrant evaluation in large prospective trials. The PRIAS (Prostate Cancer Research International: Active Surveillance) study, which is the largest prospective study evaluating active surveillance, has commenced recruiting eligible patients to have mp-MRI incorporated into the surveillance data. This will provide reliable information with regards to the feasibility of mp-MRI in the context of active surveillance [39].
CONCLUSIONS
The current literature indicates that mp-MRI of the prostate is a promising technology within PC management. Robust data to confirm many of these findings are still needed. Despite promising data indicating that Gleason score can be predicted without a tissue sample (particularly with DWI), such findings should be interpreted cautiously in the clinical setting, particularly in the scenario of an elevated PSA test and negative mp-MRI of the prostate. The clinical confidence in this aspect of the technology is justifiably more guarded compared with the academic excitement.
mp-MRI of the prostate should not be seen as a future replacement for tissue diagnosis but rather as a useful tool in PC diagnosis and management as well as a reliable means of assessing men in the context of active surveillance. Indeed, the ability for targeted biopsy is a substantial and long-awaited step forward. Owing to financial and practical restrictions alone, it is unlikely that mp-MRI will ever become part of a population-based screening model. Like any new technology, it should be treated judiciously and used in combination with current clinical tools for risk stratification.
More likely than not, the gold standard for evaluating mp-MRI is direct comparison of radiology to histopathology. The development of a more sophisticated, standardized model for correlating radiological parameters with histopathology in addition to higher volumes of good quality data is the logical next research pathway. Ultimately, the ability to reliably predict histological risk of significant PC will determine how we judge mp-MRI.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Toos Sachinwalla for providing mp-MRI images.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Schroder FH. Screening for prostate cancer: current status of ERSPC and screening-related issues. Recent Results Cancer Res. 2014;202:47–51. doi: 10.1007/978-3-642-45195-9_5. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 3.Phillips R. Bladder cancer: Stem cells repopulate tumours. Nat Rev Urol. 2015;12:63. doi: 10.1038/nrurol.2014.352. [DOI] [PubMed] [Google Scholar]
- 4.Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 5.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasoundguided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–29. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Thompson J, Lawrentschuk N, Frydenberg M, Thompson L, Stricker P USANZ. The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int. 2013;112(Suppl 2):6–20. doi: 10.1111/bju.12381. [DOI] [PubMed] [Google Scholar]
- 8.Raz O, Haider M, Trachtenberg J, Leibovici D, Lawrentschuk N. MRI for men undergoing active surveillance or with rising PSA and negative biopsies. Nat Rev Urol. 2010;7:543–551. doi: 10.1038/nrurol.2010.143. [DOI] [PubMed] [Google Scholar]
- 9.Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33:17, e1–e7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013;200:W361–W368. doi: 10.2214/AJR.12.9106. [DOI] [PubMed] [Google Scholar]
- 12.Yacoub JH, Oto A, Miller FH. MR imaging of the prostate. Radiol Clin North Am. 2014;52:811–837. doi: 10.1016/j.rcl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011;21:188–196. doi: 10.1007/s00330-010-1883-7. [DOI] [PubMed] [Google Scholar]
- 14.Luczynska E, Heinze-Paluchowska S, Domalik A, Cwierz A, Kasperkiewicz H, Blecharz P, et al. The utility of diffusion weighted imaging (DWI) using apparent diffusion coefficient (ADC) values in discriminating between prostate cancer and normal tissue. Pol J Radiol. 2014;79:450–455. doi: 10.12659/PJR.890805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donati OF, Afaq A, Vargas HA, Mazaheri Y, Zheng J, Moskowitz CS, et al. Prostate MRI: evaluating tumor volume and apparent diffusion coefficient as surrogate biomarkers for predicting tumor Gleason score. Clin Cancer Res. 2014;20:3705–3711. doi: 10.1158/1078-0432.CCR-14-0044. [DOI] [PubMed] [Google Scholar]
- 16.Boesen L, Chabanova E, Logager V, Balslev I, Thomsen HS. Apparent diffusion coefficient ratio correlates significantly with prostate cancer gleason score at final pathology. J Magn Reson Imaging. 2014 Nov 19; doi: 10.1002/jmri.24801. [Epub] http://dx.doi.org/10.1002/jmri.24801. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb JC, Blume JD, Coakley FV, Wheeler TM, Cormack JB, Sotto CK, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy--results of ACRIN prospective multi-institutional clinicopathologic study. Radiology. 2009;251:122–133. doi: 10.1148/radiol.2511080409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratan F, Niaf E, Melodelima C, Chesnais AL, Souchon R, Mege-Lechevallier F, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol. 2013;23:2019–2029. doi: 10.1007/s00330-013-2795-0. [DOI] [PubMed] [Google Scholar]
- 19.Rosenkrantz AB, Kim S, Lim RP, Hindman N, Deng FM, Babb JS, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology. 2013;269:482–492. doi: 10.1148/radiol.13122233. [DOI] [PubMed] [Google Scholar]
- 20.Portalez D, Mozer P, Cornud F, Renard-Penna R, Misrai V, Thoulouzan M, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol. 2012;62:986–996. doi: 10.1016/j.eururo.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Vache T, Bratan F, Mege-Lechevallier F, Roche S, Rabilloud M, Rouviere O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology. 2014;272:446–455. doi: 10.1148/radiol.14131584. [DOI] [PubMed] [Google Scholar]
- 22.Junker D, Quentin M, Nagele U, Edlinger M, Richenberg J, Schaefer G, et al. Evaluation of the PI-RADS scoring system for mpMRI of the prostate: a whole-mount step-section analysis. World J Urol. 2014 Aug 1; doi: 10.1007/s00345-014-1370-x. [Epub] http://dx.doi.org/10.1007/s00345-014-1370-x. [DOI] [PubMed] [Google Scholar]
- 23.Styles C, Ferris N, Mitchell C, Murphy D, Frydenberg M, Mills J, et al. Multiparametric 3T MRI in the evaluation of intraglandular prostate cancer: correlation with histopathology. J Med Imaging Radiat Oncol. 2014;58:439–448. doi: 10.1111/1754-9485.12189. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura K, Muto S, Yokota I, Hoshimoto K, Kaminaga T, Noguchi T, et al. Feasibility of multiparametric prostate magnetic resonance imaging in the detection of cancer distribution: histopathological correlation with prostatectomy specimens. Prostate Int. 2014;2:188–195. doi: 10.12954/PI.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billing A, Buchner A, Stief C, Roosen A. Preoperative mp-MRI of the prostate provides little information about staging of prostate carcinoma in daily clinical practice. World J Urol. 2014 Nov 29; doi: 10.1007/s00345-014-1448-5. [Epub] http://dx.doi.org/10.1007/s00345-014-1448-5. [DOI] [PubMed] [Google Scholar]
- 26.Min BD, Kim WT, Cho BS, Kim YJ, Yun SJ, Lee SC, et al. Usefulness of a combined approach of t1-weighted, t2-weighted, dynamic contrast-enhanced, and diffusion-weighted imaging in prostate cancer. Korean J Urol. 2012;53:830–835. doi: 10.4111/kju.2012.53.12.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka K, Shigemura K, Muramaki M, Takahashi S, Miyake H, Fujisawa M. Efficacy of using three-tesla magnetic resonance imaging diagnosis of capsule invasion for decision-making about neurovascular bundle preservation in robotic-assisted radical prostatectomy. Korean J Urol. 2013;54:437–441. doi: 10.4111/kju.2013.54.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le JD, Huang J, Marks LS. Targeted prostate biopsy: value of multiparametric magnetic resonance imaging in detection of localized cancer. Asian J Androl. 2014;16:522–529. doi: 10.4103/1008-682X.122864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepe P, Garufi A, Priolo G, Pennisi M. Can 3-Tesla pelvic phased-array multiparametric MRI avoid unnecessary repeat prostate biopsy in patients with PSA < 10 ng/mL? Clin Genitourin Cancer. 2015;13:e27–e30. doi: 10.1016/j.clgc.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Le JD, Stephenson S, Brugger M, Lu DY, Lieu P, Sonn GA, et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol. 2014;192:1367–1373. doi: 10.1016/j.juro.2014.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]galliumlabelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 32.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, et al. Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: A Systematic Review. Eur Urol. 2015;67:627–636. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 34.Abdi H, Pourmalek F, Zargar H, Walshe T, Harris AC, Chang SD, et al. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology. 2015;85:423–428. doi: 10.1016/j.urology.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 35.WaltonDiaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015 Mar 05; doi: 10.1016/j.urolonc.2015.01.023. [Epub] http://dx.doi.org/10.1016/j.urolonc.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, et al. Clinical Implications of a Multiparametric Magnetic Resonance Imaging Based Nomogram Applied to Prostate Cancer Active Surveillance. J Urol. 2015 Jan 26; doi: 10.1016/j.juro.2015.01.088. [Epub] http://dx.doi.org/10.1016/j.juro.2015.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha JY, Kim BH, Park CH, Kim CI. Early experience with active surveillance in low-risk prostate cancer treated. Korean J Urol. 2014;55:167–171. doi: 10.4111/kju.2014.55.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LM, Choyke PL, Figg WD, Turkbey B. The role of MRI in prostate cancer active surveillance. Biomed Res Int. 2014;2014:203906. doi: 10.1155/2014/203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]