Abstract
Infectious complications after transrectal ultrasound-guided prostate biopsy (TRUS-Bx) appear to be increasing, which reflects the high prevalence of antibiotic-resistant strains of Enterobacteriaceae. Identifying patients at high risk for antibiotic resistance with history taking is an important initial step. Targeted prophylaxis with a prebiopsy rectal swab culture or augmented antibiotic prophylaxis can be considered for patients at high risk of antibiotic resistance. If infectious complications are suspected, the presence of urosepsis should be evaluated and adequate antibiotic treatment should be started immediately.
Keywords: Biopsy, Infection, Prostate
INTRODUCTION
Transrectal ultrasound-guided prostate biopsy (TRUS-Bx) is one of the most commonly performed urologic procedures in the United States and Europe, with approximately one million biopsies performed annually on each continent. TRUS-Bx is a relatively safe procedure and the chances of severe complications are low, but the incidence of infectious complications has recently been rising, along with the potential for more severe complications such as sepsis [1,2]. Escherichia coli is the most common pathogen found in infections after TRUS-Bx [3,4,5,6]. Randomized controlled trials have shown that antibiotic prophylaxis is effective in preventing infectious complications following TRUS-Bx [7]. Fluoroquinolone is the most commonly used antibiotic agent for prophylaxis [7,8]. However, worldwide antibiotic resistance is rising [9,10,11], and as such, infectious complications after TRUS-Bx by fluoroquinolone-resistant E. coli are rising as well [9,12,13,14].
INCIDENCE OF INFECTIOUS COMPLICATIONS AFTER TRUS-Bx
The incidence of infectious complications after TRUS-Bx is reported to range from 0.1% to 7%; the incidence of infections requiring admission is from 0.6% to 4.1% [15]. In Korea, the reported incidence of infectious complications after TRUS-Bx is from 0.65% to 3.1% [16,17,18]. In another Asia-Europe multicenter study that included Korea, the reported incidence of febrile urinary tract infection (UTI) was 3.5% and the incidence of infections requiring admission was 3.1% [19]. In Japan, the reported incidence of febrile UTI was from 0.5% to 0.76% [20,21], and in Taiwan, the incidence was 5.4% before fluoroquinolone prophylaxis and 0.9% after fluoroquinolone prophylaxis [22]. A Turkish study reported an incidence of infections requiring admission of 2% [23].
The incidence of infectious complications after TRUS-Bx has been rising in recent years. In a study that analyzed complications after prostate biopsy from SEER (Surveillance, Epidemiology and End Results)-Medicare data from 1991 to 2007, infectious complications after prostate biopsy increased in recent years [1]. In a Canadian report, the incidence of infectious complications that required admission was 1.0% in 1996 but increased to 4.1% in 2005, 72% of which was sepsis [2]. Another recent Canadian report also stated that the incidence of infectious complications was 0.52% from 2002 to 2009 but increased to 2.15% from 2010 to 2011 [24]. The main reason for this increase in infectious complications is the rise in fluoroquinolone resistance [9].
PREVALENCE OF FLUOROQUINOLONE RESISTANCE IN INFECTIOUS COMPLICATIONS
In one Korean study, acute bacterial prostatitis after TRUS-Bx occurred in 1.36% of patients and the prevalence of fluoroquinolone-resistant strains was 23.8% [25]. In a Japanese study, acute bacterial prostatitis developed in 1.3% of patients and all urine and blood cultures yielded levofloxacin-resistant E. coli [26]. In another Japanese study, the rate of genitourinary tract infection was 0.76% and E. coli was the most frequently isolated strain, of which 77.8% showed levofloxacin resistance [21]. In a North American cohort, 2.77% of cases developed infection after biopsy, of which 55% had fluoroquinolone-resistant infection [6]. In a French prospective study, 0.67% of cases had acute bacterial prostatitis, of which 95% showed fluoroquinolone resistance [27]. In an Australian study that analyzed E. coli bacteremia after TRUS-Bx, 62% were fluoroquinolone-resistant [28]. Overall, the reported prevalence of fluoroquinolone resistance in infectious complications after TRUS-Bx ranges from 24% to 100%, and considering the recent trend in increasing antibiotic resistance, fluoroquinolone resistance is expected to rise rapidly. Along with the problem of fluoroquinolone resistance, one should also be wary of the emergence of extended-spectrum beta-lactamase (ESBL)-producing bacteria. One study reported an incidence of ESBL-producing bacteria of 43% in acute prostatitis after TRUS-Bx [29].
RECTAL SWAB CULTURE BEFORE TRUS-Bx
Investigators from the United States [12] and Japan [30] monitored the rates of fluoroquinolone-resistant E. coli before TRUS-Bx, with results of 23% and 13%, respectively. The purpose of monitoring for fluoroquinolone-resistant E. coli before TRUS-Bx would be to select appropriate prophylactic antibiotics that can specifically target antibiotic-resistant organisms. Targeted prophylaxis may not only prevent infectious complications and sepsis after TRUS-Bx but also suppress the rise of antibiotic-resistant bacteria. In a study conducted to evaluate targeted antibiotic prophylaxis in men undergoing TRUS-Bx in the United States, there were no infectious complications in the 112 men who received targeted antibiotic prophylaxis, whereas there were 9 cases (including 1 of sepsis) among the 345 men on empirical therapy [31]. That study also evaluated the cost-effectiveness of targeted prophylaxis and showed that targeted prophylaxis yielded a cost savings of US $4,499 per TRUS-Bx infectious complication averted. However, debate remains as to whether rectal swab cultures should be routinely performed before TRUS-Bx. In a Canadian study, despite a significant correlation between patients who developed infections and the detection of ciprofloxacinresistant organisms, only 9.0% of the total group with ciprofloxacin resistance developed an infectious complication [32]. Future studies will need to evaluate the cost effectiveness and clinical utility of a prebiopsy rectal culture in targeted antibiotic prophylaxis [32].
SOLUTIONS FOR FLUOROQUINOLONE RESISTANCE
1. Identifying high-risk patients with history taking
Fluoroquinolone use in the previous 3 to 6 months prior to TRUS-Bx was a common risk factor for fluoroquinolone resistance in several studies [5,33,34,35]. The longer the period of fluoroquinolone use, the higher the incidence of fluoroquinolone resistance [35]. Therefore, thorough history taking is of paramount importance for identifying recent fluoroquinolone usage for other conditions such as UTI, chronic prostatitis, heart valve surgery, and artificial instrument insertion surgery (Fig. 1).
Fig. 1. Flow chart to choose antibiotic prophylaxis for transrectal prostate biopsy. *Alternative antibiotics may be recommended in regions where the rate of fluoroquinolone resistance is high, but levels of evidence from clinical studies are not high.
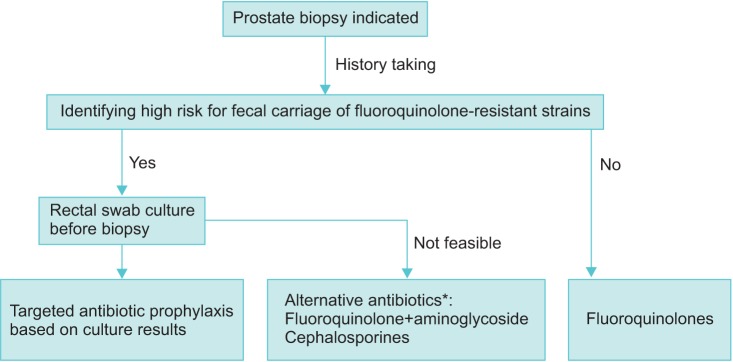
2. Targeted antibiotic prophylaxis
The evidence for routine rectal swab culture before all TRUS-Bx is still indeterminate. However, in cases of a high risk of fluoroquinolone resistance, performing prebiopsy rectal swab culture to identify rectal bacterial flora would be of great assistance in preventing or treating infectious complications. Prebiopsy rectal swab culture should be done 1 to 2 weeks before TRUS-Bx. If the patient has a history of recent antibiotic use, however, prebiopsy rectal swab culture should be postponed or its results should be interpreted cautiously. Targeted antibiotic prophylaxis based on rectal swab culture results showed a notable decrease in the incidence of infectious complications after TRUS-Bx caused by fluoroquinolone-resistant organisms as well as a decrease in the overall cost of care [31].
3. Changing prophylactic antibiotics for TRUS-Bx
Currently, fluoroquinolone is the recommended antibiotic for TRUS-Bx in US and European guidelines. It is also the most widely used antibiotic agent for antibiotic prophylaxis for TRUS-Bx in practice. However, in regions such as Korea where the rate of fluoroquinolone resistance is high, following the US and European guidelines may be less effective for antibiotic prophylaxis. If prebiopsy rectal swab culture is done, susceptible antibiotic agents should be used, and if it is not done, prophylactic antibiotic agents should be changed in patients suspected of fluoroquinolone resistance. For this, attempts have been made to add an aminoglycoside such as amikacin to fluoroquinolone or to use third-generation cephalosporins for prophylaxis. However, this may cause another problem in addition to fluoroquinolone resistance: emergence of ESBL-producing bacteria. In a Korean report, 20% of patients with infectious complications were found to have ESBL-producing bacteria [25], and in a Canadian report, the incidence of ESBLproducing bacteria was 4.6% [32]. ESBL-producing bacteria are usually resistant to most antibiotics with the exception of carbapenems (imipenem, meropenem). However, it is not recommended to use potent antibiotics such as imipenem or piperacillin/tazobactam for general prophylaxis because this may eventually cause emergence of carbapenem-resistant Enterobacteriaceae that could make us vulnerable to fatal infections.
4. Treating fluoroquinolone-resistant E. coli
Because infectious complications af ter TRUS-Bx could be fatal, immediate admission and implementation of antibiotics is warranted in cases suspected of sepsis. If a prebiopsy rectal swab culture is done, a susceptible antibiotic agent targeting the suspected causative bacteria should be used. If not, third-generation cephalosporins and aminoglycoside may be the optimal choice, at least in Korea [25]. Because resistance to gentamicin and tobramycin is already high in Korea, amikacin is the recommended aminoglycoside. If ESBL-producing bacteria are suspected or cephalosporins are ineffective, use of carbapenems such as imipenem or meropenem should not be delayed. Once the results of the antibiotic susceptibility test are confirmed, de-escalation therapy is recommended, which consists of switching from a broad-spectrum empiric antibiotic therapy to a narrower spectrum. After the patient is discharged, continued treatment for a sufficient period of time is necessary to cure prostatitis.
CONCLUSIONS
Fluoroquinolones have been the most common choice for prophylactic antibiotics preceding TRUS-Bx. Infectious complications after TRUS-Bx are increasing, and this appears to be due to an increasing prevalence of floroquinolone-resistant strains in the rectal flora. Therefore, identifying the risk for fecal carriage of floroquinolone-resistant strains by history taking should be the initial step in the TRUS-Bx procedure. If a risk of fluoroquinolone resistance is present, targeted antimicrobial prophylaxis using rectal swab cultures or alternative antibiotics may be recommended for prophylaxis. In patients with infectious complications after TRUS-Bx, it is essential to administer appropriate antibiotics immediately.
Footnotes
CONFLICTS OF INTEREST: The author has nothing to disclose.
References
- 1.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 Suppl):S12–S17. doi: 10.1016/j.juro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Hadway P, Barrett LK, Waghorn DJ, Hasan K, Bdesha A, Haldar N, et al. Urosepsis and bacteraemia caused by antibioticresistant organisms after transrectal ultrasonography-guided prostate biopsy. BJU Int. 2009;104:1556–1558. doi: 10.1111/j.1464-410X.2009.08959.x. [DOI] [PubMed] [Google Scholar]
- 4.Hedelin H, Claesson BE, Wilpart A. Febrile reactions after transrectal ultrasound-guided prostatic biopsy: a retrospective study. Scand J Urol Nephrol. 2011;45:393–396. doi: 10.3109/00365599.2011.590996. [DOI] [PubMed] [Google Scholar]
- 5.Mosharafa AA, Torky MH, El Said WM, Meshref A. Rising incidence of acute prostatitis following prostate biopsy: fluoroquinolone resistance and exposure is a significant risk factor. Urology. 2011;78:511–514. doi: 10.1016/j.urology.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 6.Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77:1035–1041. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 7.Zani EL, Clark OA, Rodrigues Netto N., Jr Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011;(5):CD006576. doi: 10.1002/14651858.CD006576.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Wolf JS, Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379–1390. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Feliciano J, Teper E, Ferrandino M, Macchia RJ, Blank W, Grunberger I, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy--are fluoroquinolones still effective prophylaxis? J Urol. 2008;179:952–955. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 10.Johnson L, Sabel A, Burman WJ, Everhart RM, Rome M, MacKenzie TD, et al. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am J Med. 2008;121:876–884. doi: 10.1016/j.amjmed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, Kim ME, et al. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J Infect Chemother. 2011;17:440–446. doi: 10.1007/s10156-010-0178-x. [DOI] [PubMed] [Google Scholar]
- 12.Liss MA, Chang A, Santos R, Nakama-Peeples A, Peterson EM, Osann K, et al. Prevalence and significance of fluoroquinolone resistant Escherichia coli in patients undergoing transrectal ultrasound guided prostate needle biopsy. J Urol. 2011;185:1283–1288. doi: 10.1016/j.juro.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–968. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Young JL, Liss MA, Szabo RJ. Sepsis due to fluoroquinoloneresistant Escherichia coli after transrectal ultrasound-guided prostate needle biopsy. Urology. 2009;74:332–338. doi: 10.1016/j.urology.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 15.American Urological Association. AUA/SUNA white paper on the incidence, prevention and treatment of complications related to prostate needle biopsy [Internet] Linthicum, MD: American Urological Association; [cited 2013 Feb 22]. Available from: https://www.auanet.org/resources/white-paper-onthe-incidence-prevention-and-treatment-of-complicationsrelated-to-prostate-needle-biopsy.cfm. [Google Scholar]
- 16.Choi JW, Kim TH, Chang IH, Kim KD, Moon YT, Myung SC, et al. Febrile urinary tract infection after prostate biopsy and quinolone resistance. Korean J Urol. 2014;55:660–664. doi: 10.4111/kju.2014.55.10.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Kim SI, Ahn HS, Choi JB, Kim YS, Kim SJ. Risk factors for acute prostatitis after transrectal biopsy of the prostate. Korean J Urol. 2010;51:426–430. doi: 10.4111/kju.2010.51.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park DS, Hwang JH, Choi DK, Gong IH, Hong YK, Park S, et al. Control of infective complications of transrectal prostate biopsy. Surg Infect (Larchmt) 2014;15:431–436. doi: 10.1089/sur.2013.138. [DOI] [PubMed] [Google Scholar]
- 19.Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Cek M, Grabe M, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013;63:521–527. doi: 10.1016/j.eururo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Shigemura K, Matsumoto M, Tanaka K, Yamashita M, Arakawa S, Fujisawa M. Efficacy of combination use of Betalactamase inhibitor with penicillin and fluoroquinolones for antibiotic prophylaxis in transrectal prostate biopsy. Korean J Urol. 2011;52:289–292. doi: 10.4111/kju.2011.52.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togo Y, Kubo T, Taoka R, Hiyama Y, Uehara T, Hashimoto J, et al. Occurrence of infection following prostate biopsy procedures in Japan: Japanese Research Group for Urinary Tract Infection (JRGU) - a multi-center retrospective study. J Infect Chemother. 2014;20:232–237. doi: 10.1016/j.jiac.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Tsai YS, Chen CH, Jou YC, Yang WH, Chang CC, Tzai TS. Febrile infection in post-prostate biopsy: results of a ten-year single-institution study in South Taiwan. Surg Infect (Larchmt) 2014;15:24–28. doi: 10.1089/sur.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cam K, Kayikci A, Akman Y, Erol A. Prospective assessment of the efficacy of single dose versus traditional 3-day antimicrobial prophylaxis in 12-core transrectal prostate biopsy. Int J Urol. 2008;15:997–1001. doi: 10.1111/j.1442-2042.2008.02147.x. [DOI] [PubMed] [Google Scholar]
- 24.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pepin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62:453–459. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Bang JH, Choe HS, Lee DS, Lee SJ, Cho YH. Microbiological characteristics of acute prostatitis after transrectal prostate biopsy. Korean J Urol. 2013;54:117–122. doi: 10.4111/kju.2013.54.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigehara K, Miyagi T, Nakashima T, Shimamura M. Acute bacterial prostatitis after transrectal prostate needle biopsy: clinical analysis. J Infect Chemother. 2008;14:40–43. doi: 10.1007/s10156-007-0570-3. [DOI] [PubMed] [Google Scholar]
- 27.Campeggi A, Ouzaid I, Xylinas E, Lesprit P, Hoznek A, Vordos D, et al. Acute bacterial prostatitis after transrectal ultrasoundguided prostate biopsy: epidemiological, bacteria and treatment patterns from a 4-year prospective study. Int J Urol. 2014;21:152–155. doi: 10.1111/iju.12207. [DOI] [PubMed] [Google Scholar]
- 28.Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, et al. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis. 2012;54:1406–1412. doi: 10.1093/cid/cis194. [DOI] [PubMed] [Google Scholar]
- 29.Ozden E, Bostanci Y, Yakupoglu KY, Akdeniz E, Yilmaz AF, Tulek N, et al. Incidence of acute prostatitis caused by extended-spectrum beta-lactamase-producing Escherichia coli after transrectal prostate biopsy. Urology. 2009;74:119–123. doi: 10.1016/j.urology.2008.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Minamida S, Satoh T, Tabata K, Kimura M, Tsumura H, Kurosaka S, et al. Prevalence of fluoroquinolone-resistant Escherichia coli before and incidence of acute bacterial prostatitis after prostate biopsy. Urology. 2011;78:1235–1239. doi: 10.1016/j.urology.2011.07.1392. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–1279. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 32.Taylor S, Margolick J, Abughosh Z, Goldenberg SL, Lange D, Bowie WR, et al. Ciprofloxacin resistance in the faecal carriage of patients undergoing transrectal ultrasound guided prostate biopsy. BJU Int. 2013;111:946–953. doi: 10.1111/j.1464-410X.2012.11637.x. [DOI] [PubMed] [Google Scholar]
- 33.Akduman B, Akduman D, Tokgoz H, Erol B, Turker T, Ayoglu F, et al. Long-term fluoroquinolone use before the prostate biopsy may increase the risk of sepsis caused by resistant microorganisms. Urology. 2011;78:250–255. doi: 10.1016/j.urology.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 34.Durham LK, Ge M, Cuccia AJ, Quinn JP. Modeling antibiotic resistance to project future rates: quinolone resistance in Escherichia coli. Eur J Clin Microbiol Infect Dis. 2010;29:353–356. doi: 10.1007/s10096-009-0862-x. [DOI] [PubMed] [Google Scholar]
- 35.Yagci D, Yoruk F, Azap A, Memikoglu O. Prevalence and risk factors for selection of quinolone-resistant Escherichia coli strains in fecal flora of patients receiving quinolone therapy. Antimicrob Agents Chemother. 2009;53:1287–1289. doi: 10.1128/AAC.01228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


