Abstract
Purpose
To investigate the effects of lymph node metastasis, skip metastasis, and other factors related to lymph node status on survival in patients who underwent radical cystectomy (RC) and extended lymph node dissection (eLND).
Materials and Methods
RC and eLND were performed in 85 patients with a diagnosis of bladder cancer. Disease-free survival (DFS) and overall survival (OS) were determined by using a Cox proportional hazards model that included the number of excised lymph nodes, the presence of pathological lymph node metastasis, the anatomical level of positive nodes, the number of positive lymph nodes, lymph node density, and the presence of skip metastasis.
Results
The mean number of lymph nodes removed per patient was 29.4±9.3. Lymph node positivity was detected in 85 patients (34.1%). The mean follow-up duration was 44.9±27.4 months (2-93 months). Five-year estimated OS and DFS for the 85 patients were 62.6% and 57%, respectively. Three of 29 lymph node-positive patients (10.3%) had skip metastasis. Only lymph node positivity had a significant effect on 5-year OS and DFS (p<0.001). No difference in OS and DFS was found between the three patients with skip metastasis and other lymph node-positive patients. Other factors related to lymph node status had no significant effect on 5-year OS and DFS.
Conclusions
No factors related to lymph node status predict DFS and OS, except for lymph node positivity. OS and DFS were comparable between patients with skip metastasis and other lymph node-positive patients.
Keywords: Lymph node excision, Treatment outcome, Urinary bladder neoplasms
INTRODUCTION
The standard treatment for patients with muscle-invasive bladder cancer is radical cystectomy (RC) and bilateral lymph node dissection (LND). In recent years, increasing evidence indicates that extended LND (eLND) improves survival and the accuracy of cancer staging [1,2,3,4,5]. However, the ideal proximal limit of LND is still controversial. Prospective randomized trials on the effectiveness of eLND have recently been undertaken, and the results of these studies (United States Intergroup S1011 and German phase III trial) are forthcoming. In several studies of RC and pelvic LND, lymph node metastasis was reported in nearly one of three or four patients [3,4,6,7,8,9,10]. One of the most important indicators of poor prognosis in patients with bladder cancer is lymph node positivity [4,9,11]. Five-year overall survival (OS) in patients with lymph node-positive bladder cancer is 15% to 30% [1,9,12,13]. Nodal metastasis above a limited or standard template is not uncommon, with up to 16% of all nodal metastasis detected proximal to the aortic bifurcation [14]. However, skip metastasis is less common. Studies, including ours, have reported the incidence of skip metastasis to be between 4.65% and 8.9% [6,8,15,16]. To our knowledge, no study on the survival of patients with skip metastasis has been reported. In the current study, we aimed to investigate the effects of different factors associated with lymph node metastasis-including the presence of skip metastasis, the total number of excised lymph nodes, the number of positive lymph nodes, the anatomical level of positive lymph nodes, and lymph node density-on disease-free survival (DFS) and OS in patients who underwent RC and eLND for bladder cancer.
MATERIALS AND METHODS
In our previous prospective study, conducted between August 2005 and August 2009, 118 RCs and eLNDs were performed; follow-up information was available for 85 of these procedures, which formed the basis of this study [8]. All operations were performed to cure the patients, who had muscle-invasive tumors, poorly differentiated T1 tumors, carcinoma in situ or bladder cancer that had not invaded the muscle, or cancer that was recurrent, multifocal, or refractory to repeated transurethral resection and intravesical treatment. Patients who had received radiotherapy to the pelvis, had undergone pelvic LND for other reasons, or who had received neoadjuvant chemotherapy for bladder cancer were excluded from the study.
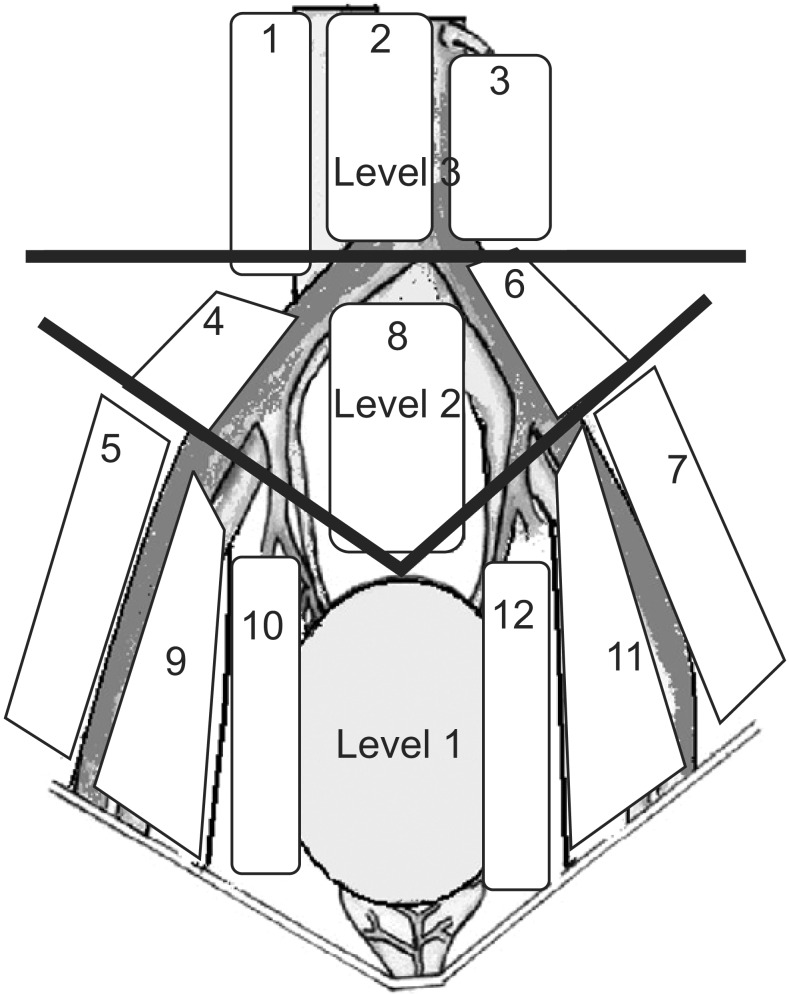
The preoperative evaluation involved a medical history, a routine clinical examination, biochemical and hematologic tests, a bimanual examination under anesthesia, transurethral resection of tumor, abdominal ultrasonography, computerized tomography, and chest x-ray. The 1998 World Health Organization classification was used to determine histopathological type and grade. Pathological staging was based on the 2002 TNM classification. All patients underwent eLND, and the margins were determined on the basis of a previously described protocol [8]. The proximal limit of LND was at the level of origin of the inferior mesenteric artery. LND was continued distal to the femoral canal to include the Cloquet node. The lateral limit on each side of the dissection area was the genitofemoral nerve. Tissue was removed from 12 distinct lymph node regions and from three lymph node levels (Figs. 1, 2). All lymph nodes were registered as metastatic or nonmetastatic. For all patients, the number of lymph nodes in each anatomical location was registered. Lymph node density was calculated as the number of positive lymph nodes divided by the total number of lymph nodes removed. Skip metastasis was defined as lymph node metastasis at levels 2 and/or 3 (not at level 1).
Fig. 1. Anatomical regions of extended lymphadenectomy.
Fig. 2. Lymphadenectomy levels.
The patients were monitored in accordance with a predetermined protocol. Patients were followed up postoperatively once every 4 months in the first year, once every 6 months in the second year, and once a year thereafter. During the follow-up visits, a physical examination was performed and serum biochemical levels were measured. Upper urinary tract and chest imaging were performed at least once a year. Other radiological evaluations were performed as necessary.
The primary outcomes were DFS and OS. Pelvic soft tissue 2 cm or greater located inferior to the aortic bifurcation was defined as local recurrence; tissue from all other sites was defined as systemic recurrence. DFS was defined from the time of RC to the time of radiologically, histologically, and/or clinically confirmed recurrent disease or until the most recent follow-up with no suspicion of recurrence.
DFS and OS were determined based on an analysis of the number of excised lymph nodes, the presence of pathological lymph node metastasis, the anatomical level of positive lymph nodes, the number of positive lymph nodes, lymph node density, and the presence of skip metastasis. The study was approved by the local ethics committee.
The Kaplan-Meier method was used to estimate survival, and the log-rank test was used for comparisons between groups. A multivariate analysis was conducted by using a Cox proportional hazards model to determine the influence of lymph node-related risk factors on survival after adjustment for other explanatory variables. A p-value of <0.05 was considered significant.
RESULTS
Eighty-five patients underwent RC and eLND for the treatment of bladder cancer. The mean number of lymph nodes removed per patient was 29.2±9.3 (median, 27; range, 14-51). Lymph node positivity was detected in 29 of 85 patients (34.1%). The clinical and pathological characteristics of patients are shown in Table 1. Intraoperative arterial or venous injury occurred and was repaired in 9 of 85 patients. Five of these nine patients required a blood transfusion. Postoperative complications-including prolonged lymphatic drainage, deep vein thrombosis, and prolonged ileus-occurred in 15 (17.6%), 2 (2.4%), and 3 patients (3.5%), respectively. The mean duration of follow-up was 44.9±27.4 months (2-93 months). The estimated 5-year OS and DFS rates for the 85 patients were 62.6% and 57%, respectively. The effects of lymph node positivity, number of excised lymph nodes, lymph node density, and number of positive lymph nodes (single or multiple) on 5-year OS and DFS are shown in Table 2.
Table 1. Clinical and pathological characteristics of the patients according to lymph node status.
| Characteristic | Total | Lymph node positive | Lymph node negative |
|---|---|---|---|
| Age (y), median (range) | 61 (27-77) | 63 (39-74) | 60 (27-77) |
| Sex | |||
| Male | 75 (88.2) | 28 (96.6) | 47 (83.9) |
| Female | 10 (11.8) | 1 (3.4) | 9 (16.1) |
| Pathologic T stage | |||
| ≤pT2 | 42 (49.5) | 6 (20.7) | 36 (64.3) |
| pT3 | 32 (37.6) | 17 (58.6) | 15 (26.8) |
| pT4 | 11(12.9) | 6 (20.7) | 5 (8.9) |
| Tumor grade | |||
| PUNLMP | 0 (0) | 0 (0) | 0 (0) |
| Low grade papillary urethelial carcinoma | 11 (12.9) | 0 (0) | 11 (19.3) |
| High grade papillary urethelial carcinoma | 74 (87.1) | 29 (100) | 45 (80.4) |
| Adjuvant treatment | |||
| None | 64 (75.3) | 13 (44.8) | 51 (91.1) |
| Systemic chemotherapy | 21 (24.7) | 16 (55.2) | 5 (8.9) |
| Recurrence | |||
| Yes | 27 (31.8) | 17 (58.6) | 10 (17.9) |
| No | 58 (68.2) | 12 (41.4) | 46 (82.1) |
Values are presented as number (%) unless otherwise indicated.
PUNLMP, papillary urethelial neoplasia with low malignant potential.
Table 2. Effect of lymph node parameters on OS and DFS.
| Characteristic | No. | 5-Year OS (%) | p-value | 5-Year DFS (%) | p-value |
|---|---|---|---|---|---|
| LN status | <0.001 | <0.001 | |||
| LN (-) | 56 | 81.9 | 76.7 | ||
| LN (+) | 29 | 25.5 | 19.2 | ||
| No. of total LNs identified | 0.48 | 0.13 | |||
| Patients with <20 | 16 | 68.8 | 73.9 | ||
| Patients with ≥20 | 69 | 61.3 | 52.9 | ||
| Lymph node density | 0.22 | 0.13 | |||
| <10% | 15 | 20.0 | 13.3 | ||
| ≥10% | 14 | 32.1 | 24.5 | ||
| No. of positive LN | 0.65 | 0.94 | |||
| 1 | 9 | 22.2 | 22.2 | ||
| >1 | 20 | 28.0 | 17.1 |
OS, overall survival; DFS, disease-free survival; LN, lymph node.
Only lymph node positivity had a significant effect on 5-year OS (Fig. 3) and DFS (Fig. 4). Other factors-including the number of excised lymph nodes (<20 vs. ≥20), lymph node density (<10% vs. ≥10%), and number of positive lymph nodes (single or multiple)-had no significant effect on 5-year OS and DFS (Table 2). Evaluation based on a cutoff for lymph node density of 20% also showed no significant effect on 5-year OS (p=0.405) and DFS (p=0.694).
Fig. 3. Overall survival of patients with and without lymph node positivity.
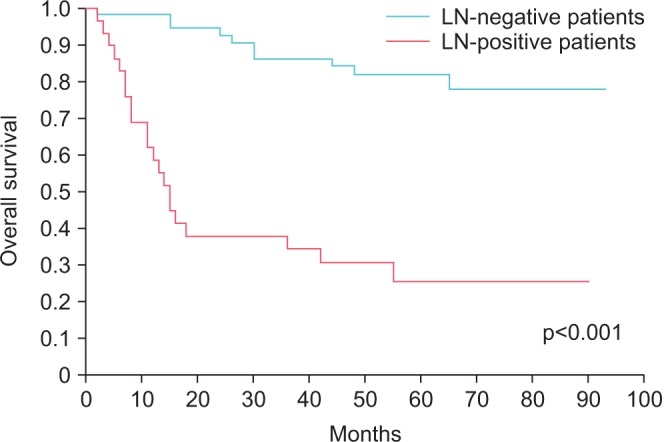
Fig. 4. Disease-free survival of patients with and without lymph node positivity.

Whereas lymph node metastasis was seen only at level 1 in 17 of 29 patients with lymph node positivity, lymph node metastasis was seen at levels 2 and/or 3 (with or without lymph node positivity at level 1) in 12 patients. Only three patients had lymph node metastases at levels 2 and/or 3 in the absence of lymph node positivity at level 1 (skip metastasis). Thirty-nine month DFS was achieved with eLND in one of these three patients (33.3%) with skip metastasis. No significant differences were found between patients with lymph node metastasis at level 1 and patients with lymph node metastases above level 1 (with or without lymph node positivity at level 1), respectively, in OS (28.2% vs. 25%, p=0.556) or DFS (20.6% vs. 16.7%, p=0.731). No significant differences were found between the patients with skip metastasis (n=3) and the other lymph node-positive patients (n=26), respectively, in OS (33.3% vs. 25.6%, p=0.964) or DFS (0% vs. 21.6%, p=0.892).
The multivariate analysis showed that none of the factors related to lymph node status (presence of skip metastases, number of excised lymph nodes, number of positive lymph nodes, lymph node density, anatomical level of positive lymph nodes), except for lymph node positivity, were found to be an independent predictor of 5-year estimated OS or DFS (p<0.001 for both).
Age, sex, tumor stage, grade, adjuvant treatment, lymph node positivity, and presence of skip metastasis were included in a multiple Cox proportional hazards model. Only lymph node positivity was found to be significantly associated with OS (p<0.001; hazard ratio [HR], 13.93; 95% confidence interval [CI] for HR, 4.44-43.77) and DFS (p<0.001; HR, 8.36; 95% CI for HR, 3.12-22.41) (Table 3).
Table 3. Results of multiple Cox proportional hazard model for OS and DFS.
| Variable | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 100 (0.97-1,04) | 1.04 | 0.97 (0.94-1.01) | 0.161 |
| Sex | 1.75 (0.49-6.22) | 0.49 | 0.80 (0.21-3.02) | 0.740 |
| Pathologic T stage | 1.01 (0.45-2.28) | 0.45 | 1.87 (0.84-4.17) | 0.127 |
| LN positivity | 13.93 (4.44-43.77) | 4.44 | 8.36 (3.12-22.41) | <0.001 |
| Presence of skip metastasis | 0.71 (0.13-3.80) | 0.13 | 1.15 (0.30-4.40) | 0.836 |
| Adjuvant treatment | 0.63 (0.24-1.67) | 0.24 | 0.58 (0.24-1.42) | 0.235 |
| Tumor grade | 0.40 (0.11-1.47) | 0.11 | 0.78 (0.21-2.96) | 0.714 |
OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; LN, lymph node.
DISCUSSION
The standard treatment for patients with muscle-invasive bladder cancer is RC and eLND, and many patients are cured with these procedures [1,2,3,4,5]. eLND has been reported to provide long-term survival, even in patients with grossly positive lymph nodes [17]. Zehnder et al. [13] showed that RC and eLND resulted in long-term recurrence-free survival in 25% of patients with positive lymph nodes. Abol-Enein et al. [4] reported that 5-year DFS was achieved in 28% of lymph node-positive patients with standard LND and in 48% of patients with eLND. In that study, eLND was shown to be an independent prognostic indicator of DFS in a multivariate analysis [4]. In a series of RC and eLND procedures, 10-year recurrence-free survival was achieved in 78% to 80% of patients with organ-confined, pathologically negative lymph nodes and in approximately 15% to 30% of patients with positive lymph nodes [1,9,11,12,13,18,19,20]. In our study, 5-year OS and DFS rates were 25.5% and 19.2%, respectively, in patients who underwent eLND.
Results in the literature regarding the relationship between the total number of lymph nodes removed and prognosis are conflicting [5,10,20,21]. In many studies, the threshold for total number of lymph nodes removed during RC was 20 [3]. Dorin et al. [22] showed that the median number of lymph nodes removed was significantly different between two centers (40 nodes vs. 72 nodes) using the same anatomical templates of LND. In addition, the lack of difference in survival between the two centers indicates that the LND template is more important than the number of lymph nodes removed. Similarly, in a cadaver study conducted by Davies et al. [23], the number of lymph nodes removed varied between 10 and 53; this variation resulted because of differences in the patients' anatomical characteristics and because of difficulties in determining the minimum number of lymph nodes to be removed. The limits of LND and the average number of lymph nodes removed are highly variable between studies. In our study, consistent with the literature, the number of lymph nodes removed per patient was between 12 and 51, despite the use of the same template. No significant difference between the number of lymph nodes removed (<20 or ≥20) and OS or DFS was found.
Lymph node density is defined as the ratio of the number of positive lymph nodes to the number of total lymph nodes removed [24,25]. A significant survival advantage was shown in patients with a lymph node density ≤20% compared with those with a lymph node density of >20% [25]. Stein et al. [9] also reported a threshold of prognostic lymph node density of 20%. Subsequently, numerous studies have been performed and different cutoffs for lymph node density have been evaluated [26,27,28]. In our study, cutoffs for lymph node density of 10% and 20% were evaluated, and it was determined that neither cutoff predicted survival. Many factors-such as individual variability, LND template, tissue submission technique, pathological evaluation, and lymph node identification-might affect the number of lymph nodes removed. Therefore, we believe that the number of lymph nodes removed and lymph node density are not good predictors of the quality of eLND or survival. We believe that accurate identification of the lymph node regions and removal of as much of the lymph node and adipose tissue as possible in each lymph node region are important in eLND.
Abol-Enein et al. [29] reported in 2004 that extrapelvic lymph node metastasis always occurred in the obturator and/or internal iliac lymph nodes, i.e., there was no skip metastasis. In 2011, the same group published a study supporting these previous results. In their recent publication, they argue that LND should be extended up to the aortic bifurcation if there is a suspicious or positive (frozen section) endopelvic lymph node; otherwise, eLND is unnecessary [4]. However, Leissner et al. [6] reported that the rate of skip metastasis in their study was 6.8%; Steven and Poulsen [16] reported a rate of 6%. Jensen et al. [15] reported that 2 of 43 lymph node-positive patients (4.65%) in their study had lymph node positivity above the common iliac bifurcation, with no lymph node positivity more distally located within the pelvic region. In our prospective study, conducted as part of the Turkish Urooncology group, the sensitivity, specificity, and positive and negative predictive values-in frozen section examination (FSE) of samples from the obturator region-were 94.7%, 100%, 100%, and 98.1%, respectively. These data indicate the reliability of FSE. However, in the same study, lymph node metastasis in other areas without lymph node metastasis in the obturator region was detected in 16.7% of the patients. Thus, in that study, eLND would not have been performed in the 16.7% of patients with a negative result for lymph nodes in the obturator region (based on FSE), and positive lymph nodes would not have been removed in these patients [8]. In that study, positive lymph nodes would not have been removed in 8.9% of patients if LND had been performed only at level 1 [8]. This result was similar to the results of Leissner et al. [6] and Steven and Poulsen [16]. In the current study, skip metastasis was observed in 3 of 29 patients (10.3%) with positive lymph nodes. This result indicates the possibility of lymph node metastasis at levels 2 and 3 even when lymph nodes at level 1 are negative. We compared lymph node-positive patients with and without skip metastasis and found no differences in OS and DFS. Furthermore, OS and DFS were similar between patients with lymph node metastasis only at level 1 and patients with lymph node metastasis at levels 2 and/or 3 (with or without lymph node positivity at level 1). In other studies, the location of the positive lymph nodes was reported to be unimportant [10,16,27,30]. In our study, survival was adversely affected by lymph node positivity; however, the location of the positive lymph nodes did not significantly affect survival. Indeed, 39-month DFS was achieved in one of three patients (33.3%) with skip metastasis. Therefore, we believe that eLND should be performed in all patients, especially in patients with skip metastasis, to achieve accurate staging and to predict prognosis and long-term DFS after surgery.
In our study, lymph node positivity was found to be the single most important predictor of poor prognosis, similar to the finding of Vazina et al. [7]. Jensen et al. [10] reported that the presence of at least one positive lymph is an indicator of poor prognosis and that lymph node positivity is the most important prognostic indicator. In our study, the finding of at least one positive lymph node was the single most important prognostic indicator in terms of OS and DFS; the number of excised lymph nodes, the number of positive lymph nodes, lymph node density, and the anatomical level of positive lymph nodes were not significant prognostic factors. Because DFS in patients with skip metastasis was similar to that in other lymph node-positive patients, we believe that eLND should be performed in all patients undergoing RC.
Our study had many limitations. First, the number of patients was relatively small. Second, more than one surgeon performed the operations. However, because the template was defined before the study began, the same template for LND was used for all of the patients. In addition, 16 of 29 patients with lymph node positivity had received adjuvant systemic chemotherapy, which might have had an effect on the survival of lymph node-positive patients. However, no prospective randomized studies with a relatively large number of patients have been conducted to determine whether adjuvant or salvage chemotherapy has a significant effect on survival. Thus, in daily practice, many clinics provide systemic chemotherapy-either adjuvant or salvage-to their patients with lymph node positivity or with positive surgical margins after cystectomy.
CONCLUSIONS
The results of this study indicate that the single most important prognostic indicator of survival for patients who underwent RC and eLND was lymph node positivity, with or without skip metastasis. The total number of excised lymph nodes, the location of the lymph nodes, and the number and density of positive lymph nodes did not affect survival. OS and DFS were comparable between patients with skip metastasis and other lymph node-positive patients. Our results need to be confirmed in studies with a larger number of patients, a larger number of patients with skip metastasis, and an extensive follow-up period.
ACKNOWLEDGMENTS
This study was supported by the Turkish Society of Urooncology. The authors thank Recep Büyükalpelli, Haluk Özen, Uğur Altuğ, and Uğur Kuyumcuoğlu allowing the participation of their patients in this study.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Karl A, Carroll PR, Gschwend JE, Knuchel R, Montorsi F, Stief CG, et al. The impact of lymphadenectomy and lymph node metastasis on the outcomes of radical cystectomy for bladder cancer. Eur Urol. 2009;55:826–835. doi: 10.1016/j.eururo.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. 2008;179:873–878. doi: 10.1016/j.juro.2007.10.076. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. 2011;186:1261–1268. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Abol-Enein H, Tilki D, Mosbah A, El-Baz M, Shokeir A, Nabeeh A, et al. Does the extent of lymphadenectomy in radical cystectomy for bladder cancer influence disease-free survival? A prospective single-center study. Eur Urol. 2011;60:572–577. doi: 10.1016/j.eururo.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295–1298. [PubMed] [Google Scholar]
- 6.Leissner J, Ghoneim MA, Abol-Enein H, Thuroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004;171:139–144. doi: 10.1097/01.ju.0000102302.26806.fb. [DOI] [PubMed] [Google Scholar]
- 7.Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol. 2004;171:1830–1834. doi: 10.1097/01.ju.0000121604.58067.95. [DOI] [PubMed] [Google Scholar]
- 8.Baltaci S, Adsan O, Ugurlu O, Aslan G, Can C, Gunaydin G, et al. Reliability of frozen section examination of obturator lymph nodes and impact on lymph node dissection borders during radical cystectomy: results of a prospective multicentre study by the Turkish Society of Urooncology. BJU Int. 2011;107:547–553. doi: 10.1111/j.1464-410X.2010.09504.x. [DOI] [PubMed] [Google Scholar]
- 9.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 10.Jensen JB, Ulhoi BP, Jensen KM. Evaluation of different lymph node (LN) variables as prognostic markers in patients undergoing radical cystectomy and extended LN dissection to the level of the inferior mesenteric artery. BJU Int. 2012;109:388–393. doi: 10.1111/j.1464-410X.2011.10369.x. [DOI] [PubMed] [Google Scholar]
- 11.Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486–492. doi: 10.1016/j.juro.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Bassi P, Ferrante GD, Piazza N, Spinadin R, Carando R, Pappagallo G, et al. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol. 1999;161:1494–1497. [PubMed] [Google Scholar]
- 13.Zehnder P, Studer UE, Daneshmand S, Birkhauser FD, Skinner EC, Roth B, et al. Outcomes of radical cystectomy with extended lymphadenectomy alone in patients with lymph node-positive bladder cancer who are unfit for or who decline adjuvant chemotherapy. BJU Int. 2014;113:554–560. doi: 10.1111/bju.12520. [DOI] [PubMed] [Google Scholar]
- 14.Svatek R, Zehnder P. Role and extent of lymphadenectomy during radical cystectomy for invasive bladder cancer. Curr Urol Rep. 2012;13:115–121. doi: 10.1007/s11934-012-0235-3. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JB, Ulhoi BP, Jensen KM. Lymph node mapping in patients with bladder cancer undergoing radical cystectomy and lymph node dissection to the level of the inferior mesenteric artery. BJU Int. 2010;106:199–205. doi: 10.1111/j.1464-410X.2009.09118.x. [DOI] [PubMed] [Google Scholar]
- 16.Steven K, Poulsen AL. Radical cystectomy and extended pelvic lymphadenectomy: survival of patients with lymph node metastasis above the bifurcation of the common iliac vessels treated with surgery only. J Urol. 2007;178(4 Pt 1):1218–1223. doi: 10.1016/j.juro.2007.05.160. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol. 2001;165:62–64. doi: 10.1097/00005392-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Zehnder P, Studer UE, Skinner EC, Thalmann GN, Miranda G, Roth B, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112:E51–E58. doi: 10.1111/bju.12215. [DOI] [PubMed] [Google Scholar]
- 19.Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–696. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 20.Fleischmann A, Thalmann GN, Markwalder R, Studer UE. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J Clin Oncol. 2005;23:2358–2365. doi: 10.1200/JCO.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 21.Leissner J, Hohenfellner R, Thuroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int. 2000;85:817–823. doi: 10.1046/j.1464-410x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 22.Dorin RP, Daneshmand S, Eisenberg MS, Chandrasoma S, Cai J, Miranda G, et al. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: a comparative mapping study. Eur Urol. 2011;60:946–952. doi: 10.1016/j.eururo.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Davies JD, Simons CM, Ruhotina N, Barocas DA, Clark PE, Morgan TM. Anatomic basis for lymph node counts as measure of lymph node dissection extent: a cadaveric study. Urology. 2013;81:358–363. doi: 10.1016/j.urology.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Herr HW. The concept of lymph node density--is it ready for clinical practice? J Urol. 2007;177:1273–1275. doi: 10.1016/j.juro.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 25.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943–945. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 26.Kassouf W, Agarwal PK, Herr HW, Munsell MF, Spiess PE, Brown GA, et al. Lymph node density is superior to TNM nodal status in predicting disease-specific survival after radical cystectomy for bladder cancer: analysis of pooled data from MDACC and MSKCC. J Clin Oncol. 2008;26:121–126. doi: 10.1200/JCO.2007.12.9247. [DOI] [PubMed] [Google Scholar]
- 27.Wiesner C, Salzer A, Thomas C, Gellermann-Schultes C, Gillitzer R, Hampel C, et al. Cancer-specific survival after radical cystectomy and standardized extended lymphadenectomy for node-positive bladder cancer: prediction by lymph node positivity and density. BJU Int. 2009;104:331–335. doi: 10.1111/j.1464-410X.2009.08403.x. [DOI] [PubMed] [Google Scholar]
- 28.May M, Herrmann E, Bolenz C, Tiemann A, Brookman-May S, Fritsche HM, et al. Lymph node density affects cancer-specific survival in patients with lymph node-positive urothelial bladder cancer following radical cystectomy. Eur Urol. 2011;59:712–718. doi: 10.1016/j.eururo.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Abol-Enein H, El-Baz M, Abd El-Hameed MA, Abdel-Latif M, Ghoneim MA. Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study--a single center experience. J Urol. 2004;172(5 Pt 1):1818–1821. doi: 10.1097/01.ju.0000140457.83695.a7. [DOI] [PubMed] [Google Scholar]
- 30.Mills RD, Turner WH, Fleischmann A, Markwalder R, Thalmann GN, Studer UE. Pelvic lymph node metastases from bladder cancer: outcome in 83 patients after radical cystectomy and pelvic lymphadenectomy. J Urol. 2001;166:19–23. [PubMed] [Google Scholar]