Abstract
Influenza accounts for a large burden of acute respiratory tract infections in high-income countries; data from lower-income settings are limited due to lack of confirmatory testing. Consecutive outpatients presenting to the largest tertiary care hospital in southern Sri Lanka were surveyed for influenza-like illness (ILI), defined as acute onset of fever ≥ 38.0°C and cough. Patients were administered a questionnaire and nasal/nasopharyngeal sampling for rapid influenza A/B testing. We enrolled 311 patients with ILI from March to November 2013: 170 (54.7%) children and 172 (55.3%) males. Approximately half (147, 47.3%) tested positive for influenza, but 253 (81.4%) were prescribed antibiotics. On bivariable analysis, symptoms associated with influenza included pain with breathing (P < 0.001), headache (P = 0.005), fatigue (P = 0.003), arthralgias (P = 0.003), and myalgias (P = 0.006) in children and pain with breathing (P = 0.01), vomiting (P = 0.03), and arthralgias (P = 0.03) in adults. Our final clinical predictive models had low sensitivity and fair specificity—50.0% (95% CI: 38.6–61.4%) and 83.2% (95% CI: 73.4–90.0%), respectively, in children and 52.2% (95% CI: 39.9–64.2%) and 81.4% (95% CI: 70.0–89.4%), respectively, in adults. Our study confirms the ability of rapid influenza testing to identify an influenza epidemic in a setting in which testing is not routinely available.
Introduction
Each year, acute respiratory infections account for a substantial burden of morbidity and mortality worldwide.1 Viral pathogens such as influenza virus, respiratory syncytial virus, parainfluenza virus, adenovirus, and rhinovirus cause the majority of respiratory infections, although secondary bacterial infections are estimated to occur in 10–50% of these patients.2 Influenza virus, in particular, has the potential to cause pandemics and accounts for significant morbidity, lost productivity, and health-care utilization each year.3
Data from high-income countries indicate that influenza affects 10–20% of the population annually.4 In many lower-income settings in tropical and subtropical climates, the prevalence of influenza is not well characterized due to limited surveillance and laboratory capacity.5 Improving the diagnosis of influenza in such settings is vital for both epidemiologic and clinical purposes, which include measuring disease burden, directing public health measures, reducing unnecessary antibiotic use, and targeting antiviral use.6
In Sri Lanka, a total of 1,560 hospitalizations due to influenza were reported in the country's Annual Health Bulletin in 2012, but the true burden of influenza is likely greater due to limited laboratory testing. In 2003–2004, influenza accounted for 11% of acute respiratory infections in patients presenting to Colombo North Teaching Hospital in Ragama, located in the more urbanized Western Province.7 Data regarding the pattern and prevalence of influenza from more rural settings such as southern Sri Lanka continue to be limited due to the complexities associated with laboratory testing.
The purpose of this study was to assess the cross-sectional prevalence of influenza, as diagnosed using a newer-generation rapid influenza test, among outpatients presenting to the largest tertiary care center in southern Sri Lanka over a 9-month period. We developed a clinical predictive model for rapid test-positive influenza to identify patients who may be targeted for limited rapid testing. Our results suggest that rapid influenza testing could be useful for epidemiologic assessment and clinical care in regions with limited formal laboratory capacity.
Materials and Methods
Study participants.
This was a cross-sectional study performed in the Outpatient Department (OPD) of Teaching Hospital Karapitiya (THK), the largest (1,500 bed) public tertiary care hospital in southern Sri Lanka. The OPD of this hospital serves over 1,000 patients daily between the hours of 8 am and 7 pm.
Adults and children presenting to the OPD from March to November 2013 were screened for the presence of influenza-like illness (ILI) by MBBS-qualified research assistants. Consecutive patients ≥ 1 year of age were enrolled if they met the definition of ILI, as defined by the World Health Organization: tympanic temperature ≥ 38°C/100.4°F and acute onset of cough in the past 7 days without alternative diagnosis.8 Screening was carried out by research assistants between 8 am and 3 pm on Monday–Friday and 8 am to noon on Saturday. All patients who endorsed acute onset of cough in the past 7 days had their tympanic temperature checked for study eligibility; multiple patients from the same household were eligible for enrollment. Consent was obtained from patients ≥ 18 years of age and the guardians of patients 1–17 years, and assent was obtained from patients 12–17 years. Enrolled patients were administered a standardized questionnaire in the local language of Sinhala and a physical examination was conducted. A nasopharyngeal sample was collected from all patients for whom it was possible; patients unable to tolerate nasopharyngeal sample collection had a nasal sample collected instead. Patients received standard clinical assessment and treatment including physical examination, additional diagnostic testing, and prescriptions from their routine care providers in the OPD. Details regarding patients' clinical diagnoses and management were recorded. Research assistants were not involved in clinical decision making or treatment, and OPD clinical personnel were not involved in study screening or enrollment procedures.
Ethical approval for this study was obtained from the Ruhuna University Ethical Review Committee, Duke University Institutional Review Board, and Johns Hopkins Medicine Institutional Review Board.
Rapid influenza testing.
The nasal/nasopharyngeal sample was used immediately for rapid influenza testing using the Veritor Flu A + B system (Becton, Dickinson and Company, Franklin Lakes, NJ). This rapid chromatographic immunoassay detects influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs using a single processed sample. The performance characteristics of the Veritor test were documented by Hassan and others, who showed that in pediatric patients, the sensitivity and specificity of the test when compared with polymerase chain reaction (PCR) were 90.2% and 99.1%, respectively, for influenza A and 87.5% and 100%, respectively, for influenza B.9
In this study, the result of the rapid influenza test was used solely for surveillance and research purposes and not released to clinicians, since the device had not been approved for clinical use in Sri Lanka at the time.
Statistical analysis.
The proportion of patients who tested positive for influenza was calculated. Bivariable logistic regression was first carried out to determine the unadjusted associations (odds ratios [OR] with 95% confidence intervals [CIs]) between rapid influenza test positivity and patients' sociodemographic and clinical characteristics. STATA, version 11 (STATACorp, College Station, TX), was used for all statistical analyses.
Predictive models for influenza were constructed separately for children and adults using multivariable logistic regression and generally following previously described methods.10 In our analyses, any sociodemographic feature or clinical variable that had a P value less than 0.05 on bivariable analysis was included in the predictive model, with the exception of variables related to 1) finances and productivity, 2) the anatomic location of sampling, and 3) diagnoses and treatment received. These variables were not included as they were considered either minimally relevant to a clinical predictive model or unavailable at the time a clinician would be using the model. For children, the variables of myalgias and arthralgias were collinear, and thus arthralgias was excluded. Age in children was included as a categorical variable: 1–4 years, 5–9 years, 10–14 years, and 15–17 years. To create a more parsimonious model, variables were excluded in a stepwise fashion by decreasing P value until all P values were < 0.05 in the multivariable model. With each iteration of the model, the performance of the model was evaluated by creating receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC). The sensitivity, specificity, and likelihood ratios for positive (LR+) and negative (LR−) test results were calculated for the full and parsimonious models using cutoff points that maximized both sensitivity and specificity.
Results
ILI cohort.
From March to November 2013, a total of 32,869 patients were screened for ILI. Of these screened patients, 330 met eligibility criteria and 311 (94.2% of eligible) consented to study participation. Of 311 enrolled patients, the majority were male (172, 55.3%) and 1–17 years in age (170, 54.7%). Most lived close to the hospital, with the median distance traveled being 5.0 km and the largest proportion (71, 22.8%) originating from Galle, the city nearest to THK. Of adults, 100 (70.9%) had a 10th grade education or less. Thirty-one adults (22.0%) were merchants/shopkeepers, 29 (20.6%) were housewives, 22 (15.6%) were laborers, and 10 (7.1%) were farmers. A total of 135 (43.6%) reported a sick contact with similar illness in the past month and 54 (17.4%) reported travel in the past 30 days. The median expenditure on travel for seeking diagnosis and treatment of the illness was 60 Rupees (0.47 USD) and the median days of work or school missed was 1 day.11 No patient reported receiving an influenza vaccination previously.
Influenza positivity.
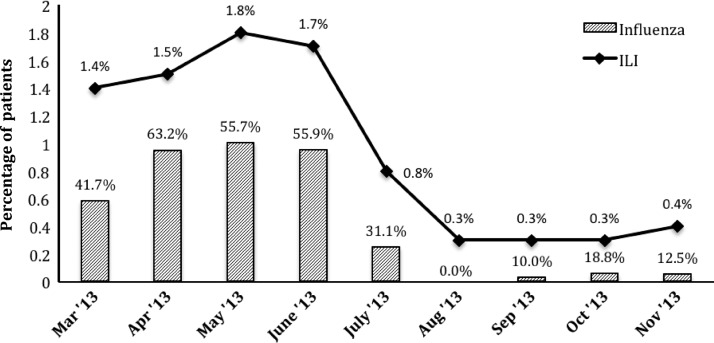
Of all patients, 147 (47.3%) tested positive for influenza using the rapid test: 94 (63.9% of positives) for influenza A and 53 (36.1%) for influenza B. Figure 1 shows the percentage of patients who were enrolled and who tested positive for influenza each month. ILI among outpatients ranged from 0.3% to 1.8% each month, with a peak from April to June 2013. Influenza positivity also peaked during this period, ranging from 55.7% to 63.2% of ILI cases. In Table 1, the sociodemographic characteristics of influenza-negative and influenza-positive patients are compared. Children with influenza tended to be older (median 9.4 years versus 5.5 years, P < 0.001) and miss more days of school (P = 0.005) than influenza-negative children. Adults with influenza were more likely to report a sick contact in the past 30 days (53.6% versus 25.7%, P < 0.001) than influenza-negative adults. No other sociodemographic characteristics were associated with influenza positivity on bivariable analysis.
Figure 1.
Enrolled influenza-like illness (ILI) and influenza-positive cases in southern Sri Lanka, March–November 2013. The percentage of ILI cases among total screened patients is depicted by the line graph. The bars indicate the percentage of ILI cases that were positive for influenza by rapid influenza testing.
Table 1.
Sociodemographic characteristics of patients enrolled with influenza-like illness (ILI) in southern Sri Lanka, March–November 2013*
| Characteristic | Frequency (%) or median (IQR) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Influenza positive (N = 147) | Influenza negative (N = 159) | |||
| Age, children 1–18 years | 9.4 (5.8–12.2) | 5.5 (3.6–7.9) | − | < 0.001 |
| Age, adults ≥ 18 years | 42.4 (29.4–52.4) | 41.9 (24.3–56.7) | − | 0.68 |
| Male | 87 (59.2%) | 82 (51.6%) | 1.36 (0.84–2.20) | 0.18 |
| Hometown | ||||
| Galle | 32 (21.8%) | 37 (23.3%) | 0.92 (0.52–1.63) | 0.75 |
| Poddala | 14 (9.5%) | 25 (15.7%) | 0.56 (0.26–1.19) | 0.10 |
| Wanduramba | 8 (5.4%) | 6 (3.8%) | 1.47 (0.43–5.26) | 0.49 |
| Akmeemana | 12 (8.2%) | 10 (6.3%) | 1.32 (0.51–3.54) | 0.53 |
| Distance to THK (km) | 5 (3–15) | 5 (3–15) | − | 0.72 |
| Time to THK (minutes) | 30 (15–50) | 30 (15–60) | − | 0.81 |
| Occupation (adults) | ||||
| Housewife | 15 (21.7%) | 14 (20.0%) | 1.11 (0.45–2.75) | 0.80 |
| Merchant | 14 (20.3%) | 16 (22.9%) | 0.86 (0.35–2.09) | 0.71 |
| Laborer | 10 (14.5%) | 12 (17.2%) | 0.82 (0.29–2.26) | 0.67 |
| Farmer | 5 (7.3%) | 5 (7.1%) | 1.02 (0.22–4.64) | 0.98 |
| Unemployed | 6 (8.7%) | 9 (12.9%) | 0.65 (0.18–2.18) | 0.43 |
| Education (adults) | ||||
| ≥ 12th Grade | 21 (30.4%) | 19 (27.1%) | 1.17 (0.53–2.62) | 0.67 |
| Sick contact in past month (adults) | 37 (53.6%) | 18 (25.7%) | 3.34 (1.54–7.30) | < 0.001 |
| Travel—yes/no | 21 (14.3%) | 32 (20.1%) | 0.66 (0.34–1.26) | 0.18 |
| Rupees spent for travel related to illness | 70 (36–120) | 60 (32–120) | − | 0.86 |
| Days missed | ||||
| Work (adults) | 0 (0–1) | 0 (0–1) | 0.66 | |
| School (children) | 1 (1–2) | 1 (0–1) | 0.005 | |
CI = confidence intervals; IQR = interquartile range; OR = odds ratio; THK = Teaching Hospital Karapitiya.
Influenza-positive and influenza-negative patients, as determined by rapid influenza testing, were compared. Number (%) and odds ratios (OR) with 95% confidence intervals (CI) and P values are listed for categorical variables, and medians with interquartile range and P values using the Kruskal–Wallis test are listed for continuous variables. P values listed in bold are significant at < 0.05.
Clinical characteristics.
The clinical characteristics of children and adults who were rapid influenza test negative and positive were compared, as shown in Table 2. Children with influenza were more likely to report pain with breathing (16.7% versus 1.1%, P < 0.001), headache (83.3% versus 64.0%, P = 0.005), fatigue (87.2% versus 70.8%, P = 0.01), arthralgias (69.2% versus 46.1%, P = 0.003), and myalgias (69.2% versus 48.3%, P = 0.006). Adults with influenza were more likely to report pain with breathing (37.7% versus 18.6%, P = 0.01), vomiting (17.4% versus 5.7%, P = 0.03), and arthralgias (95.7% versus 84.3%, P = 0.03). Samples obtained from the nasopharynx were more likely to be positive than samples obtained from the nares (90.5% versus 9.5%, P = 0.004). No other clinical characteristics differed significantly between influenza-positive and influenza-negative patients. Overall, 253 (81.4%) patients received a prescription for an antibiotic, including 164 (52.7%) for penicillins and 64 (20.6%) for first-generation cephalosporins. Sixty-four (20.6%) patients were ordered an additional diagnostic test, most commonly a complete blood count. The most common clinical diagnosis was “unspecified viral fever” in 142 (45.7%), followed by “upper respiratory infection” (66, 21.2%) and “lower respiratory infection” (45, 14.5%).
Table 2.
Clinical characteristics of patients enrolled with influenza-like illness (ILI) in southern Sri Lanka, March–November 2013*
| Characteristic | Frequency (%) or median (IQR) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Influenza positive | Influenza negative | |||
| Children < 18 years, n | 78 | 89 | − | − |
| Fever (days) | 2 (1–3) | 2 (1–3) | − | 0.62 |
| Cough (days) | 2 (1–3) | 2 (2–3) | − | 0.39 |
| Rhinitis/congestion | 42 (53.9%) | 59 (66.3%) | 0.59 (0.30–1.16) | 0.10 |
| Sore throat | 36 (46.2%) | 38 (42.7%) | 1.15 (0.60–2.22) | 0.65 |
| Shortness of breath | 19 (24.4%) | 17 (19.1%) | 1.36 (0.61–3.06) | 0.41 |
| Pain with breathing | 13 (16.7%) | 1 (1.1%) | 17.6 (2.49–756.1) | < 0.001 |
| Anorexia | 62 (79.5%) | 61 (68.5%) | 1.78 (0.83–3.88) | 0.11 |
| Vomiting | 24 (30.8%) | 25 (28.1%) | 1.14 (0.55–2.34) | 0.70 |
| Abdominal pain | 9 (11.5%) | 8 (9.0%) | 1.32 (0.43–4.16) | 0.59 |
| Headache | 65 (83.3%) | 57 (64.0%) | 2.81 (1.28–6.38) | 0.005 |
| Fatigue | 68 (87.2%) | 63 (70.8%) | 2.81 (1.19–7.03) | 0.01 |
| Arthralgias | 54 (69.2%) | 41 (46.1%) | 2.63 (1.33–5.24) | 0.003 |
| Myalgias | 54 (69.2%) | 43 (48.3%) | 2.41 (1.22–4.79) | 0.006 |
| Prior antibiotic—yes/unsure | 24 (30.8%) | 21 (23.6%) | 1.44 (0.68–3.03) | 0.30 |
| Clinical diagnosis | ||||
| Upper respiratory illness | 18 (23.1%) | 21 (23.6%) | 0.97 (0.44–2.12) | 0.94 |
| Lower respiratory illness | 10 (12.8%) | 14 (15.7%) | 0.79 (0.29–2.05) | 0.59 |
| Unspecified viral fever | 34 (43.6%) | 40 (44.9%) | 0.095 (0.49–1.83) | 0.86 |
| Antibiotic prescribed | 67 (85.9%) | 75 (84.3%) | 1.14 (0.44–2.97) | 0.77 |
| Additional diagnostic test ordered | 17 (21.8%) | 18 (20.2%) | 1.10 (0.49–2.48) | 0.80 |
| Adults ≥ 18 years, n | 69 | 70 | − | − |
| Fever (days) | 2 (1–3) | 2 (1–3) | − | 0.51 |
| Cough (days) | 2 (1–3) | 2 (2–3) | − | 0.10 |
| Rhinitis/ congestion | 42 (60.9%) | 41 (58.6%) | 1.10 (0.53–2.29) | 0.78 |
| Sore throat | 43 (62.3%) | 37 (52.9%) | 1.48 (0.71–3.07) | 0.26 |
| Shortness of breath | 25 (36.2%) | 21 (30.0%) | 1.33 (0.62–2.87) | 0.44 |
| Pain with breathing | 26 (37.7%) | 13 (18.6%) | 2.65 (1.15–6.28) | 0.01 |
| Anorexia | 60 (87.0%) | 54 (77.1%) | 1.98 (0.75–5.49) | 0.13 |
| Vomiting | 12 (17.4%) | 4 (5.7%) | 3.47 (0.97–15.5) | 0.03 |
| Abdominal pain | 3 (4.4%) | 1 (1.4%) | 3.14 (0.24–167.0) | 0.30 |
| Headache | 60 (87.0%) | 60 (85.7%) | 1.11 (0.38–3.33) | 0.83 |
| Fatigue | 61 (88.4%) | 59 (84.3%) | 1.42 (0.48–4.37) | 0.48 |
| Arthralgias | 66 (95.7%) | 59 (84.3%) | 4.10 (1.01–23.8) | 0.03 |
| Myalgias | 66 (95.7%) | 62 (88.6%) | 2.84 (0.64–17.2) | 0.12 |
| Prior antibiotic–y/unsure | 10 (14.5%) | 13 (18.6%) | 0.74 (0.27–2.01) | 0.52 |
| Clinical diagnosis | ||||
| Upper respiratory illness | 12 (17.4%) | 14 (20.0%) | 0.84 (0.32–2.16) | 0.69 |
| Lower respiratory illness | 10 (14.5%) | 10 (14.3%) | 1.02 (0.35–2.95) | 0.97 |
| Unspecified viral fever | 31 (44.9%) | 36 (51.4%) | 0.77 (0.37–1.58) | 0.44 |
| Antibiotic prescribed | 56 (81.2%) | 51 (72.9%) | 1.60 (0.67–3.91) | 0.25 |
| Additional diagnostic test ordered | 14 (20.3%) | 14 (20.0%) | 1.02 (0.41–2.54) | 0.97 |
CI = confidence intervals; IQR = interquartile range; OR = odds ratio.
Characteristics of Influenza-positive and influenza-negative patients were compared. Number (%) and odds ratios (OR) with 95% confidence intervals (CI) and P values are listed for categorical variables, and medians with IQR and P values using the Kruskal–Wallis test are listed for continuous variables. P values listed in bold are significant at < 0.05.
Clinical predictive modeling.
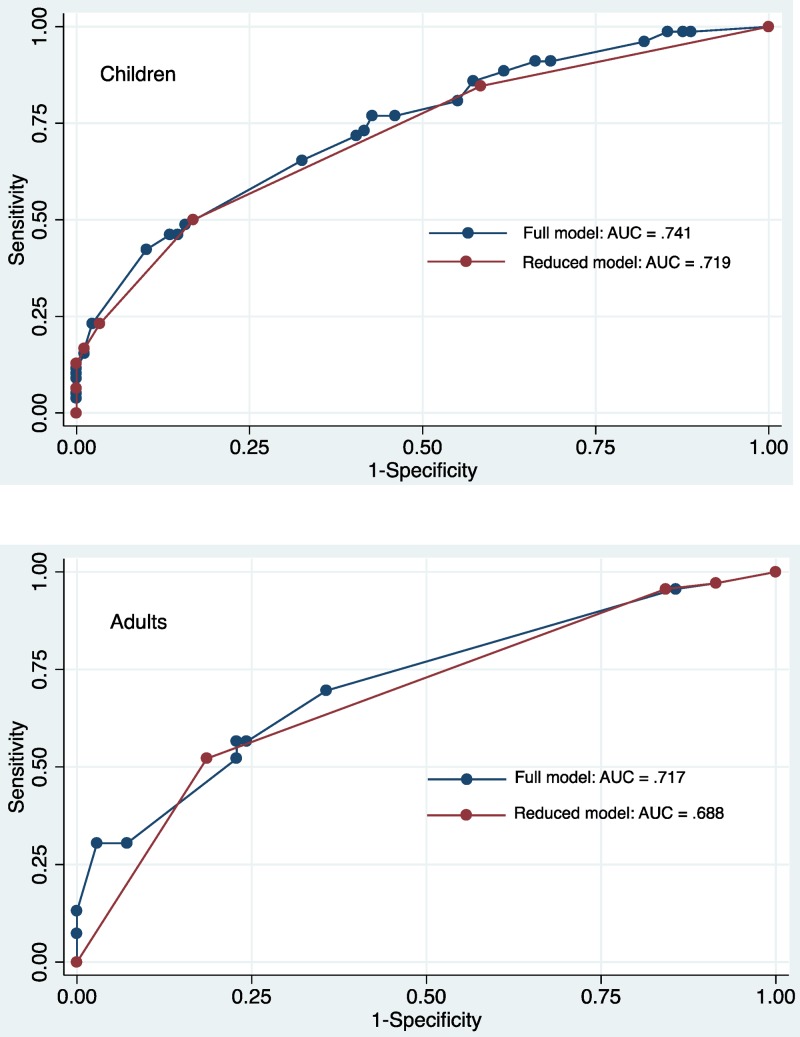
Predictive models for influenza were constructed separately for children and adults using multivariable logistic regression (Table 3). For children, the full model included categorized age, pain with breathing, headache, fatigue, and myalgias and had an AUC of 0.741. The reduced model included categorized age and pain with breathing and had an AUC of 0.719. There was no significant difference in the AUCs of the two models (P = 0.27, Figure 2 ).
Table 3.
Predictive model for influenza constructed using multivariable logistic regression among patients enrolled with influenza-like illness (ILI) in southern Sri Lanka, March–November 2013 (odds ratios [OR] with 95% confidence intervals [CI] and P values)*
| Full model | Reduced model | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Children | ||||
| Age of child, categorized | 2.02 (1.27–3.22) | 0.003 | 2.26 (1.49–3.43) | < 0.001 |
| Pain with breathing | 8.55 (1.04–70.2) | 0.05 | 8.90 (1.08–73.0) | 0.04 |
| Headache | 1.28 (0.52–3.11) | 0.59 | − | − |
| Fatigue | 2.23 (0.89–5.60) | 0.09 | − | − |
| Myalgias | 1.04 (0.47–2.28) | 0.93 | − | − |
| Adults | ||||
| Sick contact | 3.23 (1.51–6.89) | 0.002 | 3.62 (1.73–7.59) | 0.001 |
| Pain with breathing | 1.92 (0.83–4.42) | 0.126 | − | − |
| Vomiting | 2.38 (0.68–8.39) | 0.18 | − | − |
| Arthralgias | 3.96 (0.95–16.4) | 0.06 | 4.93 (1.23–19.7) | 0.02 |
CI = confidence intervals; OR = odds ratio.
P values listed in bold are significant at < 0.05.
Figure 2.
Receiver operating characteristic (ROC) curves of predictive models for influenza among children and adults presenting with influenza-like illness (ILI) in southern Sri Lanka, 2013. ROC curves for the full and reduced models, as described in Table 3, are shown.
For adults, the full predictive model included history of sick contacts, pain with breathing, vomiting, and arthralgias, and had an AUC of 0.717. The reduced model had an AUC of 0.688, and included a history of sick contacts and arthralgias. There was no significant difference in the AUCs between the two models (P = 0.17). Table 4 shows the performance characteristics of the models at the cutoff points, which maximized the sensitivity and specificity.
Table 4.
Sensitivity, specificity, and likelihood ratios of the positive (LR+) and negative (LR−) test result of the full and reduced models for influenza, among children and adults with influenza-like illness (ILI) in southern Sri Lanka, March–November 2013*
| Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | |
|---|---|---|---|---|
| Children | ||||
| Full model | 76.9 (65.8–85.4) | 57.3 (46.4–67.6) | 1.80 (1.38–2.36) | 0.40 (0.26–0.61) |
| Reduced model | 50.0 (38.6–61.4) | 83.2 (73.4–90.0) | 2.97 (1.78–4.95) | 0.60 (0.48–0.75) |
| Adults | ||||
| Full model | 69.6 (57.2–79.8) | 64.3 (51.9–75.1) | 1.95 (1.37–2.77) | 0.47 (0.33–0.69) |
| Reduced model | 52.2 (39.9–64.2) | 81.4 (70.0–89.4) | 2.81 (1.64–4.82) | 0.59 (0.46–0.76) |
CI = confidence intervals; LR = likelihood ratio; OR = odds ratio.
Values are shown for the cutoff value at which sensitivity and specificity were maximized in each model.
For children, the predicted probability, P, was calculated as follows: 10 ln(P/[1–P]) = −11 + 8 (age category) + 22 (pain with breathing), with age categories as follows: 0 (< 5 years), 1 (5–9 years), 2 (10–14 years), 3 (15–17 years). For children, the cutoff point that maximized sensitivity and specificity correlated with a right-sided value of 5, and resulted in a predicted probability of 62.3%. Thus, any child with ILI who was ≥ 10 years old or who had pain with breathing had equal or higher probability of influenza than the cutoff.
For adults, the predictive equation was 10 ln(P/[1–P]) = −20 + 13 (sick contact) + 16 (joint pain). The cutoff point that maximized sensitivity and specificity correlated with a right-sided value of 9, and resulted in a predicted probability of 71.4%. Thus, any adult with ILI who had a history of similar sick contact and arthralgias had equal or higher probability of influenza than the cutoff.
Discussion
We describe an epidemic of influenza in southern Sri Lanka, as identified by means of a newer-generation rapid influenza test. During the study period, influenza virus accounted for almost 50% of ILI cases among outpatients presenting to this tertiary care hospital; however, 81.4% of all patients (including 83.7% of patients who tested positive for influenza) received a prescription for an antibiotic. We developed clinical predictive models for rapid-test-positive influenza. In the setting of an epidemic, rapid testing may provide real-time surveillance information and help direct public health measures. In addition, limited rapid testing could be targeted to ILI patients with a high clinical probability of influenza in whom confirmation would justify withholding antibiotics and/or using antivirals.
In this study, the percentage of outpatients presenting with ILI ranged from 0.3% to 1.8% per month, with almost 50% of ILI cases overall being due to influenza. The percentage of ILI was similar to that in other settings—in the United States, the national baseline among outpatients is 2.0%, with increases up to 4.6% during seasonal peaks.12,13 There are few published data regarding patterns of influenza in Sri Lanka, but surveillance samples tested by the national reference laboratory, Medical Research Institute (MRI), showed that the prevalence of laboratory-confirmed influenza among outpatients presenting with ILI to sentinel hospital sites was 12% in 2011 and 16% in 2012.14 The proportion of influenza in this study was much higher than documented in prior years and was initially largely under recognized, which highlights the need for continued surveillance and testing from all regions of the country. Since 2005, Sri Lanka has taken commendable action in conducting influenza surveillance and now has up to 20 surveillance sites in the OPDs of 20 hospitals located throughout the country. However, laboratory data are not always readily available, and in fact there was an interruption in testing from May to July 2013 (overlapping with the influenza peak in our study), which may have been due to high number of samples received or insufficient laboratory capacity. Samples that were tested by MRI during the period of our study indicate that influenza B, H1N1pdm 2009, and H3N2 were the predominant influenza strains.15,16 In settings where complex laboratory testing may not be easily available, rapid testing may provide real-time information regarding epidemics and seasonal patterns of influenza. The peak in influenza from April to June seen in our study corresponded with one of the rainy seasons in southern Sri Lanka. Studies correlating trends in influenza with temperature, humidity, rainfall, and social behaviors are needed to better understand the regional patterns of influenza in Sri Lanka.
The level of comorbid symptoms in this study was high, as has been documented in other studies of ILI patients.17 Children with influenza were more likely to report pain with breathing, headache, fatigue, arthralgias, and myalgias, whereas adults with influenza were more likely to report pain with breathing, vomiting, and arthralgias. Pleuritic chest pain, although not universally reported in ILI, has previously been described in patients with pandemic H1N1.18 Symptoms including nausea/vomiting, myalgias, and headache have all been previously reported more commonly in patients with influenza than in influenza-negative patients.10,19,20 In our study, children with influenza were older (median age 9.4 years) than children who tested negative for influenza (median age 5.5 years), which may indicate varying patterns of exposure and immunity within age groups. The other etiologies of ILI in this community need to be further explored.
Despite unspecified viral illness being the most common clinical diagnosis, most patients presenting with ILI in this study received a prescription for an antibiotic, with most of these likely being unnecessary. Studies from the United States have revealed that over 50% of outpatient visits for acute respiratory illnesses result in antibiotic prescriptions and that 75% of all antibiotics prescribed by office-based physicians are for respiratory infections.21,22 In developing settings where confirmatory diagnostic testing is limited, antibiotics may be overprescribed for fear of missing bacterial infections—according to the 2010 Global Burden of Disease Study, lower respiratory infections are still the fourth leading cause of mortality worldwide.1 Few studies have investigated antibiotic prescribing patterns for ILI in developing settings, but Bhavnani and others showed that antibiotic prescribing patterns for ILI may be similar in Thailand. Among patients presenting with ILI to five OPDs, 82% received a prescription for an antibiotic.6 The OPD environment may help drive the overutilization of antibiotics, as continuity of care and medical records are lacking, and the implementation of techniques such as delayed antibiotic prescriptions is not feasible.23
We were able to develop simple clinical prediction tools for influenza for both children and adults. For children, this tool included the two variables of age and pain with breathing, such that anyone with pain with breathing or age 10–17 years would have a higher likelihood of testing positive for influenza. For adults, the predictive model included a history of sick contacts and arthralgias, such that any adult with both these characteristics would have a higher likelihood of testing positive for influenza. Although a negative result from these models would not be clinically useful, a positive result could be useful in targeting ILI patients for limited influenza diagnostic testing and subsequently avoiding antibiotic use or targeting antiviral use. The simplicity of these models suggests that they may be usable in the busy OPD setting of this free public hospital, where more than 1,000 patients are typically seen daily and individual patient visits generally last less than 5 minutes. Prior studies have shown that the use of clinical prediction models alone is generally insufficient for identifying influenza-positive patients; however, these rules in combination with rapid diagnostic testing may be useful.24
Our models have to be validated in both epidemic and non-epidemic settings. In addition, our models were developed using rapid influenza test results, which may miss some cases of influenza. However, this was a newer-generation test with high sensitivity, and generating models from these data is reasonable for identifying patients who may benefit from future rapid testing. In resource-limited settings lacking formal laboratory testing capabilities, rapid testing could be useful in identifying epidemics, as was done in our study. However, the price of rapid tests will remain a barrier for the foreseeable future, as these tests generally cost about 20 USD per patient.25 In addition, although access to a rapid influenza test was available in our study using a test device that had been approved in the United States, the test result could not be provided to providers and patients, as the device had not been approved for clinical use in Sri Lanka. This highlights some of the complexities involved in improving access to diagnostics in resource-limited settings.
In conclusion, we were able to document a high proportion of influenza positivity in this population of outpatients presenting with ILI in southern Sri Lanka. However, the majority of patients, whether they later tested positive for influenza or not, received a prescription for an antibiotic. In such settings, rapid testing may be useful for both surveillance and clinical purposes, helping direct public health measures, reduce unnecessary antibiotic prescriptions, and combat the growing threat of antimicrobial resistance.
ACKNOWLEDGMENTS
We would like to acknowledge Larry Park for his help with statistical analysis. In addition, we thank the study team consisting of research assistants, phlebotomists, and laboratory technicians who were involved in this work, and the OPD physicians and patients who participated in this study. Finally, we would like to acknowledge Becton, Dickinson and Company for providing the Veritor rapid influenza test kits.
Footnotes
Financial support: This work was supported by the NIH Research Training Grant #R25 TW009337 funded by the Fogarty International Center and the National Institute of Mental Health.
Authors' addresses: L. Gayani Tillekeratne and Truls Østybe, Global Health Institute, Duke University, Durham, NC, E-mails: gayani.tillekeratne@dm.duke.edu and truls.ostbye@dm.duke.edu. Champica K. Bodinayake, Ajith Nagahawatte, and Vasantha Devasiri, Departments of Medicine, Microbiology and Pediatrics, Ruhuna University, Galle, Sri Lanka, E-mails: bodinayake@yahoo.co.uk, ajithnagahawatte@yahoo.co.uk, and vdevasiri@gmail.com. Dhammika Vidanagama and Wasantha Kodikara Arachchi, Teaching Hospital Karapitiya, Galle, Sri Lanka, E-mails: dhammikasv@yahoo.com and kody@sltnet.lk. Ruvini Kurukulasooriya, Duke-Ruhuna Collaborative Research Center, Ruhuna University, Galle, Sri Lanka, E-mail: ruhunasearch@gmail.com. Aruna Dharshan De Silva, Genetech, Research Institute, Colombo, Sri Lanka, E-mail: dslv90@yahoo.com. Megan E. Reller, Department of Pathology, Johns Hopkins University, Baltimore, MD, E-mail: mreller1@jhmi.edu. Christopher W. Woods, Department of Medicine, Duke University, Durham, NC, and Global Health Institute, Duke University, Durham, NC, E-mail: chris.woods@duke.edu.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peltola V, Ruuskanen O. Editorial commentary: respiratory viral infections in developing countries: common, severe, and unrecognized. Clin Infect Dis. 2008;46:58–60. doi: 10.1086/524020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson MA. Influenza cost and cost-effectiveness studies globally–a review. Vaccine. 2013;31:5339–5348. doi: 10.1016/j.vaccine.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Influenza. 2008. http://www.who.int/immunization/topics/influenza/en/ Available at. Accessed September 23, 2014.
- 5.Azziz Baumgartner E, Dao CN, Nasreen S, Bhuiyan MU, Mah EMS, Al Mamun A, Sharker MA, Zaman RU, Cheng PY, Klimov AI, Widdowson MA, Uyeki TM, Luby SP, Mounts A, Bresee J. Seasonality, timing, and climate drivers of influenza activity worldwide. J Infect Dis. 2012;206:838–846. doi: 10.1093/infdis/jis467. [DOI] [PubMed] [Google Scholar]
- 6.Bhavnani D, Phatinawin L, Chantra S, Olsen SJ, Simmerman JM. The influence of rapid influenza diagnostic testing on antibiotic prescribing patterns in rural Thailand. Int J Infect Dis. 2007;11:355–359. doi: 10.1016/j.ijid.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Perera KV, Chan KH, Ma E, Peiris JS. Influenza virus infections among a sample of hospital attendees in Ragama, Sri Lanka. Ceylon Med J. 2010;55:40–44. doi: 10.4038/cmj.v55i2.1987. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO Interim Global Epidemiological Surveillance Standards for Influenza. Geneva, Switzerland: World Health Organization; 2012. pp. 1–61. [Google Scholar]
- 9.Hassan F, Nguyen A, Formanek A, Bell JJ, Selvarangan R. Comparison of the BD Veritor System for Flu A+B with the Alere BinaxNOW influenza A&B card for detection of influenza A and B viruses in respiratory specimens from pediatric patients. J Clin Microbiol. 2014;52:906–910. doi: 10.1128/JCM.02484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee VJ, Yap J, Cook AR, Tan CH, Loh JP, Koh WH, Lim EA, Liaw JC, Chew JS, Hossain I, Chan KW, Ting PJ, Ng SH, Gao Q, Kelly PM, Chen MI, Tambyah PA, Tan BH. A clinical diagnostic model for predicting influenza among young adult military personnel with febrile respiratory illness in Singapore. PLoS ONE. 2011;6:e17468. doi: 10.1371/journal.pone.0017468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Central Bank of Sri Lanka Current Economic Indicators. 2014. http://www.cbsl.gov.lk/htm/english/_cei/er/e_1.asp Available at. Accessed September 17, 2014.
- 12.Centers for Disease Control and Prevention Overview of Influenza Surveillance in the United States. 2013. http://www.cdc.gov/flu/weekly/overview.htm Available at. Accessed September 10, 2014.
- 13.Fowlkes A, Giorgi A, Erdman D, Temte J, Goodin K, Di Lonardo S, Sun Y, Martin K, Feist M, Linz R, Boulton R, Bancroft E, McHugh L, Lojo J, Filbert K, Finelli L. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the Influenza Incidence Surveillance Project, 2010–2011. J Infect Dis. 2014;209:1715–1725. doi: 10.1093/infdis/jit806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unit E, Sri Lanka Ministry of Health Epidemiological Bulletin-Sri Lanka-First Quarter 2013. 2013. http://www.epid.gov.lk/web/images/pdf/bulletin/2013/1st_qeb_2013.pdf Available at. Accessed January 9, 2015.
- 15.Unit E, Sri Lanka Ministry of Health Epidemiological Bulletin-Sri Lanka-Second Quarter 2013. 2013. http://www.epid.gov.lk/web/images/pdf/bulletin/2013/2nd_qeb_2013.pdf Available at. Accessed January 9, 2015.
- 16.Unit E, Sri Lanka Ministry of Health Epidemiological Bulletin-Sri Lanka-Third Quarter 2013. 2013. http://www.epid.gov.lk/web/images/pdf/bulletin/2013/3rd_qeb_2013.pdf Available at. Accessed January 9, 2015.
- 17.Bilcke J, Coenen S, Beutels P. Influenza-like-illness and clinically diagnosed flu: disease burden, costs and quality of life for patients seeking ambulatory care or no professional care at all. PLoS ONE. 2014;9:e102634. doi: 10.1371/journal.pone.0102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31:676–682. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman MJ, Attia MW. Clinical predictors of influenza in children. Arch Pediatr Adolesc Med. 2004;158:391–394. doi: 10.1001/archpedi.158.4.391. [DOI] [PubMed] [Google Scholar]
- 20.Stein J, Louie J, Flanders S, Maselli J, Hacker JK, Drew WL, Gonzales R. Performance characteristics of clinical diagnosis, a clinical decision rule, and a rapid influenza test in the detection of influenza infection in a community sample of adults. Ann Emerg Med. 2005;46:412–419. doi: 10.1016/j.annemergmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Gill JM, Fleischut P, Haas S, Pellini B, Crawford A, Nash DB. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349–354. [PubMed] [Google Scholar]
- 22.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little P, Moore M, Kelly J, Williamson I, Leydon G, McDermott L, Mullee M, Stuart B. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ. 2014;348:g1606. doi: 10.1136/bmj.g1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michiels B, Thomas I, Van Royen P, Coenen S. Clinical prediction rules combining signs, symptoms and epidemiological context to distinguish influenza from influenza-like illnesses in primary care: a cross sectional study. BMC Fam Pract. 2011;12:4. doi: 10.1186/1471-2296-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montalto NJ. An office-based approach to influenza: clinical diagnosis and laboratory testing. Am Fam Physician. 2003;67:111–118. [PubMed] [Google Scholar]