Abstract
In Kenya, more than 10 million episodes of acute febrile illness are treated annually among children under 5 years. Most are clinically managed as malaria without parasitological confirmation. There is an unmet need to describe pathogen-specific etiologies of fever. We enrolled 370 febrile children and 184 healthy controls. We report demographic and clinical characteristics of patients with Plasmodium falciparum, group A streptococcal (GAS) pharyngitis, and respiratory viruses (influenza A and B, respiratory syncytial virus [RSV], parainfluenza [PIV] types 1–3, adenovirus, human metapneumovirus [hMPV]), as well as those with undifferentiated fever. Of febrile children, 79.7% were treated for malaria. However, P. falciparum was detected infrequently in both cases and controls (14/268 [5.2%] versus 3/133 [2.3%], P = 0.165), whereas 41% (117/282) of febrile children had a respiratory viral infection, compared with 24.8% (29/117) of controls (P = 0.002). Only 9/515 (1.7%) children had streptococcal infection. Of febrile children, 22/269 (8.2%) were infected with > 1 pathogen, and 102/275 (37.1%) had fevers of unknown etiology. Respiratory viruses were common in both groups, but only influenza or parainfluenza was more likely to be associated with symptomatic disease (attributable fraction [AF] 67.5% and 59%, respectively). Malaria was overdiagnosed and overtreated. Few children presented to the hospital with GAS pharyngitis. An enhanced understanding of carriage of common pathogens, improved diagnostic capacity, and better-informed clinical algorithms for febrile illness are needed.
Introduction
In the developing world, acute febrile illness is the most common reason for presenting to a health-care provider, and infections leading to these acute febrile illnesses are responsible for the large majority of childhood deaths after the neonatal period.1 For many years, health-care providers assumed that malaria was the major etiology of childhood febrile illness, and empiric treatment guidelines for fever in malaria-endemic areas have emphasized antimalarial administration. Recently, the burden of malaria has declined in several sub-Saharan African countries2,3 including Kenya.4 These data, along with the availability of rapid malaria diagnostics, have prompted guideline revisions to advocate parasitological confirmation of malaria infection prior to treatment. However, even in areas in which malaria is historically low and other pathogens are prevalent, it remains the clinical diagnosis in the majority of patients.5,6 This practice persists in part due to incomplete understanding of the burden of other etiologies of fever.
Of non-malaria etiologies of fever, multiple studies implicate respiratory viruses as important causes of childhood fever in east Africa.7–14 In addition, Kenya has a high burden of rheumatic heart disease,15–17 and it is likely that streptococcal pharyngitis and acute rheumatic fever are underdiagnosed in febrile patients, though it is unclear whether these patients are presenting to a provider for care.
We conducted a case–control study of the etiologies of pediatric fevers in a single outpatient clinic in western Kenya. We enrolled consecutive febrile children and afebrile controls and used enhanced diagnostic testing to estimate the relative contributions to pediatric fevers of Plasmodium falciparum malaria, respiratory viruses (including influenza A and B, respiratory syncytial virus [RSV], parainfluenza virus [PIV] types 1–3, human metapneumovirus [hMPV], and adenovirus), and group A streptococcal (GAS) pharyngitis. Comparison with asymptomatic controls allowed estimation of the fraction of fevers attributable to each pathogen.
Methods
Study site.
Bungoma East district lies 70 km east of the Kenya–Uganda border. The district is rural, and 52% households fall below the poverty line.18 Most families engage in subsistence farming and small-scale animal husbandry. Very few homes have access to electricity or municipal water, although latrine coverage is high. Farming and animal husbandry are interspersed closely with homes. Malaria transmission is perennial with a seasonal peak following the rains in May–June. More than 95% households own an insecticide-treated net.19 Approximately one-third of the district is under surveillance through the Webuye Health and Demographic Surveillance System (WHDSS). A database of > 80,000 residents and their vital statistics is maintained through biannual household visits.
The study was conducted in the outpatient department of Webuye District Hospital (WDH), which is located in the single small peri-urban center of Bungoma East district in the center of the WHDSS. WDH is a public hospital in which 300–350 children are admitted per month and approximately 1,200 sick children under 5 years of age are cared for on an outpatient basis (not including immunization and specialty clinics).
Patient enrollment.
Febrile children and afebrile controls in a 2:1 ratio were enrolled continuously over a 13-month period beginning in November 2011, with approximately equal numbers of children between 1–5 and 6–12 years enrolled each month to capture pathogen seasonality. Each day, the study nurse identified all potentially eligible febrile children from the queue at the outpatient department. Children were eligible if they had a temperature > 37.5°C and resided within the WHDSS. We enrolled consecutive eligible children until the quota was met for that month (17 febrile children between 1 year and 5 years and 17 febrile children between 6 and 12 years). Afebrile participants were enrolled to achieve a 1:2 ratio in each age category. Children with apparent skin or soft-tissue infections were excluded owing to the lack of diagnostic uncertainty (N = 2). Only one child from any household was eligible for enrollment. The parent or guardian of those meeting the enrollment criteria provided consent for participation. Children older than 8 years of age provided assent. Afebrile control children were enrolled from eye clinic, follow-up visits for fractures, postoperative visits, or children accompanying parents for other reasons. We excluded children accompanying sick family members and those who had objective fever or history of fever within the past 7 days.
Data and sample collection.
A study nurse recorded vital signs, performed a clinical exam, and administered a standardized questionnaire. Immunization status was reported by the parent or guardian (86%) or recorded from the child's record (14%). The laboratory technician drew venous blood sample for malaria smear, hemoglobin level, and serum samples to be stored for later analysis. The laboratory technician also performed a throat swab, for rapid diagnosis of GAS pharyngitis, and a nasopharyngeal swab, which was transferred to viral transport media and frozen at −80°C.
Results of the physical exam, hemoglobin test, malaria smear, and rapid streptococcal test were available immediately for review by the clinical officer, who evaluated the patient, assigned a diagnosis, and recommended treatment according to usual clinical practice. Final diagnosis and prescribed treatment were recorded for all children.
Sample analysis.
Group A Streptococcus was detected in throat swabs using Clearview Strep A Exact II Dipstick (Alere, Orlando, FL) according to manufacturer's instructions. Hemoglobin concentration was measured using Mission Plus Hb strips (ACON Laboratories Inc., San Diego, CA). Nylon flocked swabs were used to collect nasopharyngeal samples, which were then vortexed in a tube of viral transport media (VTM). The VTM was transferred to cryovials and frozen at −80°C within 48 hours. A total volume of 100 μL of VTM was used for the total nucleic acid extraction with the MagNa Pure 96 DNA and Viral NA large volume kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. The samples were then tested via real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) using the AgPath-ID One-Step RT-PCR Kit (Applied Biosystems, Carlsbad, CA) for eight viral pathogens (adenovirus, influenza A and B viruses, hMPV, PIV 1–3, and RSV) as previously described.20
For purposes of immediate patient care, thick and thin blood smears were prepared and stained with 5% Giemsa and read by a laboratory technician. PCR was subsequently performed on whole blood from all patients who provided a whole blood sample (N = 401) using a real-time PCR assay targeting the P. falciparum lactate dehydrogenase (pfldh) gene on genomic DNA (gDNA) extracted from whole blood using Chelex-100 (Sigma-Aldrich, St. Louis, MI).21 Cycle threshold lines were set manually by personnel masked to other clinical data; all plates included water as a negative control, reaction plates were assembled in a PCR hood, and a randomly selected subset of 20% samples were also tested in a real-time PCR assay targeting human RNAse P to ensure adequate gDNA extraction.
Sample size and data analysis.
We calculated that 200 patients would be required in each strata (febrile under 5, febrile 5 and older, and afebrile) to estimate the strata-specific prevalence of a pathogen expected to be present in 5% (±3%) patients with 95% confidence.
Data were entered into a Microsoft Access database and exported to Stata 11.2 (Statacorp, College Station, TX) for analysis. χ2 tests were used to compare categorical variables across groups of patients and t tests were used for continuous variables. A P value ≤ 0.05 was considered significant. Attributable fraction (AF) was calculated by estimating the odds of fever among patients with and without each pathogen. A separate logistic regression model was used to estimate odds of fever for each pathogen and only children who were tested for that pathogen were included in the model. Odds ratios (ORs) were adjusted for age and gender. AF was calculated as AF = 1 − 1/OR.
Ethical clearance.
The study protocol was approved by the Moi University Research and Ethics Committee, Webuye District Hospital, and the Duke University Institutional Review Board.
Results
Enrollment.
From November 2011 to December 2012, we enrolled 554 participants, of which 276 (49.8%) were male, and 344 (62.1%) were under 5 years of age; 370 (66.8%) were febrile cases and 184 (33.2%) were afebrile controls (Table 1). The majority of children (92.0%) over the age of 5 years were in school. Of febrile children under age 5 years, 26.1% were wasted (weight-for-age Z-score [WAZ] < −2), compared with 23.3% among afebrile children (P = 0.62). Very few children reported chronic comorbid conditions, and these were equally divided between febrile and afebrile children (human immunodeficiency virus [HIV] N = 2, sickle-cell disease N = 8, asthma N = 4); almost all (99.0%) were fully immunized as per Kenya Ministry of Health guidelines.
Table 1.
Clinical characteristics of febrile and afebrile children
| Febrile | Afebrile | P value | |
|---|---|---|---|
| Total (N = 370) | Total (N = 184) | ||
| n or mean (% or SD) | n or mean (% or SD) | ||
| Patient demographics | |||
| Male | 179 (48.4%) | 97 (52.7%) | 0.37 |
| Age (years) | 4.4 (2.82) | 4.8 (3.14) | 0.08 |
| Under 5 | 241 (65.1%) | 81 (44%) | 0.04 |
| In school | 163 (44.8%) | 84 (53.2%) | 0.09 |
| Anthropometrics (mean, SD) | |||
| MUAC (cm) | 17.25 (8.48) | 16.6 (2.31) | 0.31 |
| Severe or moderate malnutrition | 3 (0.8%) | 3 (1.6%) | 0.38 |
| Weight for age | |||
| Wasted (WAZ < −2)† | 82/343 (23.9%) | 32/161 (19.9%) | 0.31 |
| Received all immunizations | 365 (98.9%) | 183 (99.5%) | 0.07 |
| Vital signs | |||
| Temperature | 38.16 (0.64) | 36.7 (0.6) | N/A |
| Duration of illness (days) | 2.13 (2.1) | − | − |
| Respiratory rate | 33.06 (9.41) | − | − |
| Tachypnea* | 96 (26%) | − | − |
| Pulse (beats/min) | 100.92 (22.59) | − | − |
| Symptoms | |||
| Cough | 179 (48.4%) | 19 (10.3%) | < 0.001 |
| Vomiting | 59 (15.9%) | 10 (5.4%) | < 0.001 |
| Diarrhea | 39 (10.5%) | 13 (7.1%) | 0.19 |
| Unable to drink/breast-feed | 23 (6.2%) | 0 (0%) | < 0.001 |
| Nasal congestion | 37 (10.0%) | 7 (3.8%) | 0.01 |
| Difficulty breathing | 5 (1.4%) | 0 (0%) | 0.11 |
| Chest pain | 1 (0.3%) | 0 (0%) | 0.48 |
| Headache | 8 (2.2%) | 2 (1.1%) | 0.37 |
| Convulsions | 3 (0.8%) | 0 (0%) | 0.22 |
| Sore throat | 0 (0%) | 0 (0%) | − |
| Rash | 6 (1.6%) | 3 (1.6%) | 1.0 |
| Treatment prior to visit | |||
| Any | 135 (36.5%) | − | − |
| Antimalarial | 52 (14.1%) | − | − |
| Antibiotic | 41 (11.1%) | − | − |
| Physical examination | |||
| Spleen enlarged | 2 (0.5%) | 0 (0%) | 0.32 |
| Liver enlarged | 2 (0.5%) | 0 (0%) | 0.32 |
| Palmar pallor | 2 (0.5%) | 2 (1.1%) | 0.47 |
| Conjuctival pallor | 1 (0.3%) | 1 (0.5%) | 0.61 |
| Wasting | 3 (1.1%) | 3 (1.63%) | 0.38 |
| Rash | 10 (2.7%) | 3 (1.63%) | 0.56 |
| Throat | |||
| Tonsillar exudates | 1 (0.3%) | 2 (1.1%) | 0.22 |
| Mouth ulcer | 4 (1.1%) | 1 (0.5%) | 0.53 |
| Ears | |||
| Discharge | 6 (1.6%) | 1 (0.5%) | 0.29 |
| Signs of infection | 8 (2.2%) | 0 (0%) | 0.05 |
| Neck | |||
| Adenopathy | 2 (0.5%) | 0 (0%) | 0.32 |
| Neck stiffness | 2 (0.05%) | 0 (0%) | 0.32 |
| Heart murmur | 2 (0.5%) | 1 (0.5%) | 0.99 |
| Lungs | |||
| Crepitations | 2 (0.5%) | 0 (0%) | 0.32 |
| Chest indrawing/stridor | 2 (0.6%) | 0 (0%) | 0.32 |
| Wheezing | 4 (1.1%) | 12 (6.5%) | 0.001 |
| Dehydration | 4 (1.4%) | 0 (0%) | 0.16 |
MUAC = mid upper arm circumference; SD = standard deviation; WAZ = wasted-for-age Z-score.
According to WHO criteria: > 40 BPM for under 5 and > 30 for over 5.
WAZ up to 10 years only.
Clinical presentations and management.
Among 370 febrile cases, the most common presenting symptom was cough (48.4%) (Table 1). Vomiting, diarrhea, inability to drink or breast-feed, and nasal congestion were other common presenting complaints (each > 10%). Median duration of illness was 2 days. Some patients had received treatment before presentation, including antimalarials (52/370 [14.1%]), antibacterials (32/370 [11.1%], most commonly amoxicillin, trimethoprim/sulfamethoxazole, or penicillin), or both (18/370 [4.8%]). Severe anemia (Hb < 7.0 g/dL) was rare, but moderate anemia (7–11 g/dL) was relatively common in both febrile (28.4%) and afebrile children (27.7%). Abnormal physical examination findings were rare. Malaria smear was interpreted as positive for P. falciparum in 35.1% of all febrile patients and 26.7% controls. Overall, 24% febrile children were admitted to the hospital; none died during their subsequent hospital course.
Over 90% febrile children were given a clinical diagnosis of malaria, including all nine with GAS pharyngitis. Of febrile children, 41% were diagnosed with acute respiratory tract infection (ARI) and 39% were diagnosed with both malaria and ARI. Treatment with antimalarials was common; 80% of all children and 72% children with a negative routine smear were prescribed antimalarials. 62% of all children were prescribed an antibiotic, and 45% received both an antibiotic and an antimalarial. Only four of eight febrile children with GAS infection received an antibiotic, and seven of eight received an antimalarial agent.
Among control children 85% reported no fever in the last 2 weeks, and 81% had no complaint on the day they were enrolled. Of those who reported a complaint, none were visiting the clinic for their complaints. A small number of controls reported cough (10%) or congestion (3.8%).
Microbiologic diagnoses.
Despite the high frequency of malaria clinical diagnoses, only 14/268 (5.2%) febrile patients and 3/133 (2.3%) controls tested positive for P. falciparum in a real-time PCR assay (Table 2).
Table 2.
Pathogens in febrile and afebrile subjects
| Pathogens present | Febrile | Afebrile | ||
|---|---|---|---|---|
| Total (N = 370) | Total (N = 184) | |||
| Number/tested | % | Number/tested | % | |
| Malaria† | 14/268 | 5.2% | 3/133 | 2.3% |
| GAS | 8/354 | 2.3% | 1/161 | 0.6% |
| Influenza A/B | 58/286 | 20.3%* | 9/118 | 7.6%* |
| RSV | 15/284 | 5.3% | 8/118 | 6.8% |
| PIV1/2/3 | 29/286 | 10.1%* | 5/118 | 4.2%* |
| Adenovirus | 30/286 | 10.5% | 10/118 | 8.5% |
| hMPV | 9/286 | 3.2% | 2/118 | 1.7% |
| Coinfected‡ | 22/269 | 8.2% | 5/115 | 4.4% |
| Other/unknown§ | 102/275 | 37.1%* | 59/116 | 50.9%* |
hMPV = human metapneumovirus; GAS = group A streptococcal; PIV = parainfluenza virus; RSV = respiratory syncytial virus.
P < 0.05 for comparison between Febrile and Afebrile.
As detected by a real-time polymerase chain reaction (PCR) assay.
Denominator for each pathogen includes all children tested for that pathogen. Children are only included in denominator of “Coinfected” if they were tested for both malaria and respiratory viruses or if they were GAS positive and had another infection (N = 1).
Children only included in denominator of other/unknown if tested for all three pathogens.
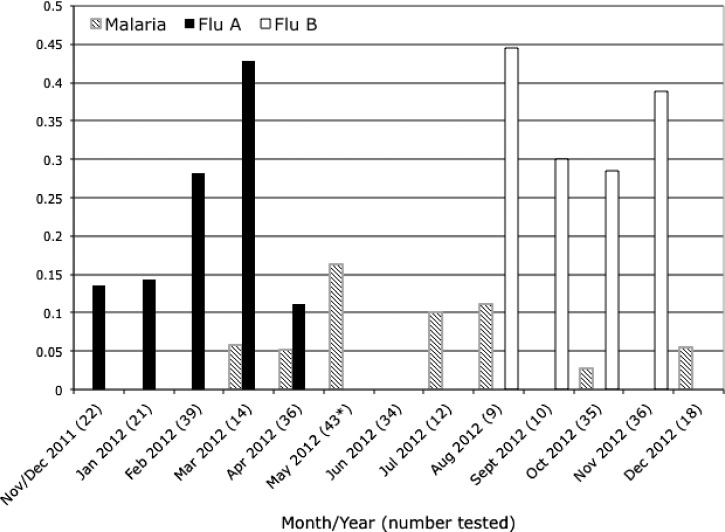
Among febrile children, influenza (20.3%) was the most common respiratory virus detected by PCR assays of nasopharyngeal specimens. Influenza appeared seasonal, with all influenza A cases presenting between November 2011 and April 2012 and a subsequent series of influenza B cases between August 2012 and December 2012 (Figure 1). Other common viruses detected were adenovirus (10.5%) and PIV1/2/3 (10.1%). All tested viruses were also detected in the afebrile controls. Influenza and PIV were found more commonly in febrile children than among controls (Table 2).
Figure 1.
Seasonality of malaria, influenza A, and influenza B in febrile children. * No patients were tested for influenza in May.
Rapid streptococcal tests were positive in only eight (2.3%) febrile children and one (0.6%) afebrile child. Of the eight febrile children with GAS pharyngitis, one reported sore throat and one met Jones criteria for acute rheumatic fever.22
Overall, 8.2% febrile patients were infected with more than one pathogen. Children with influenza were significantly more likely to report a family member ill with similar symptoms than children with malaria, adenovirus, or RSV (P < 0.05).
When comparing microbiological diagnoses to clinical management, we find that 93.1% children infected with influenza were diagnosed with malaria, 82.7% received an antimalarial agent, 67.2% received an antibacterial agent, and 51.7% received both. Of children, 50% with influenza and 24.1% with PIV were diagnosed with acute respiratory tract infection. Of those with positive malaria PCR, 85.7% received an antimalarial agent, 57.1% received an antibiotic, and 42.9% received both.
Clinical and microbiologic correlations.
On the basis of microbiologic results, we assigned febrile children to diagnostic groups for malaria (5.2%), GAS (2.3%), respiratory virus (41.5%), or other/unknown (37.1%) and used these assignments to evaluate associations with clinical data (Table 3). Most notably, compared with children in whom no respiratory virus was detected, children with any respiratory virus more frequently reported that other family members were ill (21.4%), complained of cough (65%), and were tachypneic (35%; all P < 0.05). Among children with a respiratory virus detected, there was a trend toward more diarrhea (6/30 [20%]) in children infected with adenovirus (Table 4) (P = 0.11).
Table 3.
Clinical characteristics of febrile patients by pathogen type
| Malaria | GAS | Respiratory virus | Other/unknown† | |
|---|---|---|---|---|
| Number/tested (%) | 14/268 (5.2%) | 8/354 (2.3%) | 117/282 (41.5%) | 102/275 (37.1%) |
| Children | ||||
| Age (years) | ||||
| Under 5 (%) | 8 (57.1%) | 3 (37.5%) | 77 (65.8%) | 64 (62.8%) |
| Over 5 (%) | 6 (42.9%) | 5 (62.5%) | 40 (34.2%) | 38 (37.3%) |
| Male | 7 (50%) | 1 (12.5%) | 57 (48.7%) | 51 (50%) |
| Temperature (mean, SD) | 38.8 (1.4) | 38.5 (0.49) | 38.3 (0.67) | 38.0 (0.50) |
| Other family members ill | 2 (14.3%) | 0 (0%) | 25 (21.4%)* | 7 (6.9%) |
| Clinical | ||||
| Pulse | 112.9 (27.9)* | 102.8 (13.5) | 103.1 (21.3) | 104.2 (25.0) |
| Respiratory rate | 34.7 (9.9) | 33.5 (9.95) | 34.5 (9.18) | 32.3 (11.4) |
| Tachypnea | 4 (28.6%) | 2 (25%) | 41 (35.0%)* | 15 (14.7%) |
| Hemoglobin | 10.3 (2.4) | 12.1 (2.6) | 11.7 (1.6) | 11.5 (1.59) |
| Anemia | ||||
| Severe | 2 (14.3%) | 1 (12.5%) | 2 (1.7%) | 1 (1.0%) |
| Moderate | 5 (35.7%) | 0 (0%) | 29 (24.8%) | 34 (33.3%) |
| Normal | 7 (50%) | 7 (87.5%) | 86 (73.5%) | 67 (65.7%) |
| Symptoms | ||||
| Diarrhea | 0 (0%) | 0 (0%) | 13 (11.1%) | 8 (7.8%) |
| Cough | 3 (21.3%) | 1 (12.5%) | 76 (65.0%)* | 40 (39.2%) |
| Unable to drink/breast-feed | 2 (14.3%)* | 0 (0%) | 9 (7.7%) | 7 (6.9%) |
| Nasal congestion | 0 (0%) | 2 (25.0%) | 10 (8.6%) | 11 (10.8%) |
| Vomiting | 4 (28.6%) | 2 (25.0%) | 23 (19.7%) | 9 (8.8%) |
| Hospital diagnosis | ||||
| Malaria | 13 (92.9%) | 8 (100%) | 112 (95.7%) | 86 (84.3%) |
| ARI/URTI | 4 (28.6%) | 2 (25%) | 50 (42.7%) | 41 (40.2%) |
| Treatment | ||||
| Antimalarial | 12 (85.7%) | 7 (87.5%) | 93 (79.5%) | 76 (74.5%) |
| Antibiotic | 8 (50%) | 4 (50%) | 79 (61.5%) | 63 (55.9%) |
| Both | 6 (42.9%) | 3 (37.5%) | 57 (43.6%) | 44 (37.3%) |
| Hospitalization | ||||
| Admitted | 9 (64.3%)* | 3 (37.5%) | 26 (22.2%) | 14 (13.7%) |
| Days in hospital | 3.44 (1.51) | 3 (1.41) | 3.11 (0.90) | 3.7 (1.27) |
GAS = group A streptococcus.
P < 0.05.
Children only included in denominator of other/unknown if tested for all three pathogens.
Table 4.
Respiratory viruses in febrile children and clinical characteristics
| Influenza | RSV | PIV1/2/3 | Adenovirus | hMPV | Coinfected† | |
|---|---|---|---|---|---|---|
| Number/tested (%) | 58/286 (20.3%) | 15/284 (5.3%) | 29/286 (10.1%) | 30/286 (10.5%) | 9/286 (3.15%) | 21/278 (7.6%) |
| As a percent of all respiratory viruses | 50.60% | 10.50% | 21.70% | 21% | 6.30% | − |
| Percent occurring with other viruses | 12.50% | 46.20% | 50.00% | 39.30% | 55.60% | − |
| Children | ||||||
| Age (years) | ||||||
| Under 5 (%) | 36 (62.1%) | 11 (73.3%) | 20 (69.0%) | 21 (70.0%) | 6 (66.7%) | 14 (66.7%) |
| Over 5 (%) | 22 (37.9%) | 4 (26.7%) | 9 (31.0%) | 9 (30.0%) | 3 (33.3%) | 7 (33.3%) |
| Male | 29 (50%) | 9 (60.0%) | 13 (44.8%) | 14 (46.7%) | 5 (55.6%) | 12 (57.1%) |
| Temperature (mean, SD) | 38.4 (0.70) | 38.2 (0.66) | 38.2 (0.698) | 38.3 (0.54) | 38.1 (0.72) | 38.3 (0.66) |
| Other family members ill | 18 (31.6%)* | 1 (6.67%) | 6 (20.7%) | 3 (10%) | 1 (12.5%) | 5 (23.8%) |
| Clinical | ||||||
| Pulse | 108.3 (24.4)* | 104.5 (16.8) | 100.9 (16.0) | 97.6 (16.9) | 108.7 (24.6) | 113.1 (16.4) |
| Respiratory rate | 34.9 (7.3) | 32.3 (9.16) | 33.7 (11.8) | 35.9 (11.2) | 36.9 (13.8) | 36.8 (13.4) |
| Tachypnea | 26 (44.8%) | 4 (26.7%) | 9 (31.0%) | 9 (30.0%) | 4 (44.4%) | 10 (47.6%) |
| Hemoglobin | 12.0 (1.76) | 11.53 (1.70) | 11.3 (1.15) | 11.72 (1.40) | 11.58 (1.20) | 11.7 (1.13) |
| Anemia | ||||||
| Severe | 1 (1.75%) | 1 (6.67%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Moderate | 11 (19.3%) | 2 (13.3%) | 11 (37.9%) | 8 (26.7%) | 3 (33.3%) | 6 (28.6%) |
| Normal | 45 (79%) | 12 (80.0%) | 18 (62.1%) | 22 (73.3%) | 6 (66.7%) | 15 (71.4%) |
| Symptoms | ||||||
| Diarrhea | 6 (10.3%) | 1 (6.67%) | 2 (6.90%) | 6 (20.0%) | 1 (11.1%) | 3 (14.3%) |
| Cough | 39 (67.2%) | 11 (73.3%) | 18 (62.1%) | 17 (56.7%) | 5 (55.6%) | 13 (61.9%) |
| Unable to drink/breast-feed | 7 (12.1%) | 0 (0%) | 2 (6.90%) | 1 (3.33%) | 1 (11.1%) | 2 (9.5%) |
| Nasal congestion | 3 (5.2%) | 4 (26.7%) | 5 (17.2%) | 2 (6.67%) | 1 (11.1%) | 4 (19.1%) |
| Vomiting | 8 (13.8%) | 3 (20.0%) | 5 (17.2%) | 10 (33.3%) | 2 (22.2%) | 6 (28.6%) |
| Treatment | ||||||
| Antimalarial | 48 (82.8%) | 10 (66.7%) | 19 (65.5%) | 27 (90%) | 6 (66.7%) | 15 (71.4%) |
| Antibiotic | 39 (67.2%) | 11 (73.3%) | 20 (68.9%) | 19 (63.3%) | 6 (66.7%) | 16 (80%) |
| Both | 30 (51.7%) | 7 (46.7%) | 11 (37.9%) | 16 (53.3%) | 3 (33.3%) | 10 (50%) |
| Hospitalization | ||||||
| Admitted | 15 (25.9%) | 2 (13.3%) | 6 (20.7%) | 6 (20.0%) | 2 (22.2%) | 4 (19.1%) |
| Days in hospital | 3.17 (1.03) | 4 | 3 (0) | 3.67 (1.15) | 3 (1.41) | 3.33 (0.58) |
hMPV = human metapneumovirus; PIV = parainfluenza virus; RSV = respiratory syncytial virus; SD = standard deviation.
P < 0.05.
Defined as ≥ 2 respiratory viruses. Only one child was coinfected with malaria plus a respiratory virus, and one child was coinfected with group A streptococcus (GAS) plus a respiratory virus; these two are not included in the coinfected group here.
Though 10% afebrile controls reported cough, there was no significant association between infection and cough in afebrile children.
Population attributable fraction of microbiologic etiologies.
Asymptomatic carriage of pathogens is common, and the proportion of fevers or illness caused by a detected pathogen can be estimated by comparing carriage in healthy children. Pathogens equally prevalent in cases and controls are considered infrequent contributors to febrile illness, and pathogens detected only in febrile children would be considered likely to be causing fever. In our study population, P. falciparum, influenza, PIV, RSV, hMPV, and adenovirus were each detected in both febrile cases and afebrile controls, but only influenza and PIV were detected significantly more frequently in febrile than afebrile children. We thus estimated the odds of fever given infection for influenza and PIV and calculated, among children with each pathogen, the fraction of fevers attributable to each (Table 5). In this analysis, of 58 febrile children with influenza, 67.5% could attribute their fevers to the virus while the remaining 32.5% have afebrile influenza infection. The percentage is slightly higher in younger children. There was a trend toward greater odds of being febrile with PIV (OR = 2.47, P = 0.069), with an estimated AF of 59%. There were no afebrile children under 5 years with P. falciparum; therefore, the AF of fever could not be calculated in this age group.
Table 5.
AF estimates
| OR | P | AF | |
|---|---|---|---|
| Malaria | 2.420 | 0.172 | − |
| Under 5* | − | − | − |
| Over 5 | 1.161 | 0.848 | − |
| Flu | 3.080 | 0.003 | 0.675 |
| Under 5 | 2.920 | 0.034 | 0.658 |
| Over 5 | 3.500 | 0.030 | 0.714 |
| RSV | 0.750 | 0.525 | − |
| Under 5 | 0.960 | 0.946 | − |
| Over 5 | 0.510 | 0.352 | − |
| PIV1/2/3 | 2.474 | 0.069 | 0.596 |
| Under 5 | 1.860 | 0.281 | − |
| Over 5 | 5.510 | 0.111 | − |
| Adenovirus | 1.210 | 0.625 | − |
| Under 5 | 1.290 | 0.601 | − |
| Over 5 | 1.300 | 0.679 | − |
AF = attributable fraction; OR = odds ratio; PIV = parainfluenza virus; RSV = respiratory syncytial virus.
There were no afebrile children under 5 years with parasitemia so the odds of fever could not be estimated.
Discussion
We demonstrate that among febrile children presenting in 2011–2012 to a rural Kenyan district hospital, malaria was both uncommon and overdiagnosed, respiratory viruses including influenza and PIV were common, and GAS pharyngitis was rare.
Respiratory pathogens were common: we detected at least one virus in nearly half of all febrile patients and, importantly, one-third of afebrile children. Comparing these groups, only influenza and PIV were more common in febrile than afebrile children. We draw two important conclusions from this observation. First, respiratory viruses were overall more commonly associated with febrile illness than malaria. Second, a substantial proportion of respiratory viral infections did not cause an overt febrile illness, at least at the time of presentation. Therefore, efforts to estimate the burden of illness of these pathogens should include surveillance of asymptomatic control patients.
In malaria-endemic areas, it is not possible to clinically distinguish falciparum malaria from other etiologies of fever.23–25 Indeed, in our study, the clinical presentations of respiratory viruses and malaria were not sufficiently distinct to allow them to be distinguished without pathogen-specific testing. For example, while cough was more common in those with influenza than malaria (67.2% versus 21.3%), nearly one-third of patients with influenza did not report cough, and nearly one-quarter of patients with malaria did have cough. Similarly, tachypnea was common in both groups, and abnormal findings on physical exam were uncommon in both groups. Improved point-of-care diagnostics are needed to discriminate treatable etiologies of fever in these children.
GAS pharyngitis was rare in our study, despite the high prevalence of rheumatic heart disease in Kenya.15,16 Several explanations may account for this observation. First, streptococcal pharyngitis is primarily a disease of children between the ages of 5 and 15 years26 and the majority of children enrolled in this study were under the age of 5 years; however, even among 129 febrile patients of ages 5 years and over, as well as 81 afebrile patients in this age group, only 5 tested positive for GAS throat infection. A second possibility is that, within populations, streptococcal infections are epidemic, and we sampled in a period between outbreaks. However, in low-income countries, there are an estimated 0.4 cases of symptomatic GAS pharyngitis per person per year,16 so it seems unlikely that this evidently high risk population would be almost entirely free of infection. Third, the sensitivity of our rapid antigen detection test may not have been sufficient to detect asymptomatic throat carriage. To evaluate this possibility, we performed culture on sheep blood agar of 58 throat swabs obtained concurrently with the sample used for the rapid strep test and noted no increase in prevalence in this subset (data not shown). A fourth possibility is that pre-enrollment antibiotic treatment (most commonly amoxicillin, trimethoprim/sulfamethoxazole, or penicillin) may have eradicated streptococcal infection. However, less than 10% patients reported receipt of an antibacterial agent prior to presentation. Finally, a fifth and perhaps most likely explanation is that symptomatic group A pharyngitis does not prompt treatment seeking in our study population. This has been observed elsewhere.27,28 The same may be true for acute rheumatic fever, which can cause severe acute illness but commonly presents with subtle manifestations, especially with recurrent episodes.29 Our study supports the notion that, to prevent rheumatic heart disease, efforts to treat streptococcal pharyngitis and acute rheumatic fever will need to be implemented in community settings.
Malaria prevalence in febrile and afebrile patients as measured by P. falciparum PCR was low, in stark contrast with the microscopic diagnosis performed in the hospital. However, three-quarters of children with a documented negative smear still received an antimalarial, indicating that overtreatment with antimalarials is not simply related to lack of access to diagnosis.
Our study had several limitations. First, information on comorbid illnesses was reported by the parent or guardian and may therefore be inaccurate. We did not test for HIV infection; however, HIV prevalence in adolescents in the study catchment area is known to be less than 1%,30 and prevalence in younger children is similarly low (Wendy P. O'Meara, personal communication). Second, entrance physical examinations were performed by the study nurse, and sensitivity and specificity of those physical exam findings is unknown. However, this does reflect the realities of care and staffing levels at the district hospital level, and prior literature suggests there is poor predictive value of signs in this setting. Third, we examined a limited set of pathogens in a population that is likely affected by many others.5,31–33 Further testing of banked samples is ongoing. Finally, our control group was not randomly selected, instead being drawn from patients presenting to the hospital for other reasons. Therefore, bias may have been introduced if this group is not representative of either the pediatric population as a whole or the population from which the cases were drawn (if, for example, treatment-seeking behaviors are different for patients with febrile illness compared with other diagnoses). Nevertheless, our afebrile controls were similar demographically to the febrile cases.
Despite these limitations, this study has several notable strengths. First, by looking at a broader spectrum of potential pathogens, including parasites, viruses, and a bacterial pathogen, we were able to describe the relative frequency of these disease-causing agents within the same children. Second, by enrolling both patients who were managed as outpatients as well as those ultimately admitted to the hospital, we capture a more complete spectrum of febrile illness than focusing solely on hospitalized patients. In addition, our study population was not limited to those with respiratory symptoms. Previous work has focused on the etiology of acute respiratory infections or pneumonia in patients presenting with respiratory-specific symptoms.7,12,14 However, we found that 30% children who would not have been classified as an acute respiratory illness were infected with a respiratory virus. Finally, the inclusion of afebrile controls allows us to estimate the background prevalence of malaria, respiratory viruses, and group A Streptococcus during the study period, and thus to calculate attributable fractions for fever. This is especially important given that each of these pathogens can cause symptomatic or asymptomatic infection (i.e., carriage). In our study, carriage of pathogens was common and this is likely true in other contexts. Such information should be held in mind when interpreting the results from fever etiology studies lacking a comparison group.
The costs of malaria overdiagnosis are high, including the morbidity of the missed opportunity for identification and treatment of the actual underlying illness, the financial costs of malaria medications, and the cost of increased selection pressure leading to malaria drug resistance. There is an unmet need for improved diagnostic capacity and improved clinical algorithms for acute febrile illnesses. Access to diagnostic testing must go hand in hand with better understanding of the relationship between carriage and disease, and confidence to use the results to guide treatment decisions; only then will it be possible to improve care of the child with acute febrile illness.
ACKNOWLEDGMENTS
We thank Kyaw Thwai and Steven Meshnick for their assistance with the P. falciparum real-time PCR testing. We are deeply grateful to Webuye District Hospital administration for their support and to all the patients and their guardians who participated.
Footnotes
Financial support: This study was supported by a pilot grant from Duke Global Health Institute.
Authors' addresses: Wendy P. O'Meara, Division of Infectious Diseases and International Health, Duke University Medical Center, Duke Global Health Institute, Durham, NC, and Moi University School of Public Health, College of Health Sciences, Eldoret, Kenya, E-mail: wpo@duke.edu. Joshua A. Mott, Barry Fields, and Kabura Wamburu, Centers for Disease Control and Prevention, Nairobi, Kenya, E-mails: zud9@cdc.gov, bsf2@cdc.gov, and WKabura@kemricdc.org. Jeremiah Laktabai and Janice Armstrong, Moi University School of Medicine, College of Health Sciences, Eldoret, Kenya, E-mails: drlaktabai@yahoo.com and janasimiyu@yahoo.com. Steve M. Taylor and Christopher W. Woods, Division of Infectious Diseases and International Health, Duke University Medical Center, Duke Global Health Institute, Durham Veterans Affairs Medical Center, Durham, NC, E-mails: steve.taylor@duke.edu and chris.woods@duke.edu. Charles MacIntyre, Reeshi Sen, and William Pan, Duke Global Health Institute, Durham, NC, E-mails: cwmacintyre@gmail.com, reeshisen00@gmail.com, and william.pan@duke.edu. Diana Menya, Moi University School of Public Health, College of Health Sciences, Eldoret, Kenya, E-mail: dianamenya@gmail.com. Bradly P. Nicholson, Division of Infectious Diseases and International Health, Duke University Medical Center, Durham Veterans Affairs Medical Center, Durham, NC, E-mail: brad.nicholson@duke.edu. Thomas L. Holland, Division of Infectious Diseases and International Health, Duke University Medical Center, Durham, NC, E-mail: Thomas.Holland@duke.edu.
References
- 1.World Health Organization World Health Statistics. 2013. http://apps.who.int/gho/data/node.main.ChildMortDistRegion?lang=en Available at. Accessed November 19, 2014.
- 2.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, Gatakaa H, Githure J, Borgemeister C, Keating J, Beier JC. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leslie T, Mikhail A, Mayan I, Anwar M, Bakhtash S, Nader M, Chandler C, Whitty CJ, Rowland M. Overdiagnosis and mistreatment of malaria among febrile patients at primary healthcare level in Afghanistan: observational study. BMJ. 2012;345:e4389. doi: 10.1136/bmj.e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz MA, Lebo E, Emukule G, Njuguna HN, Aura B, Cosmas L, Audi A, Junghae M, Waiboci LW, Olack B, Bigogo G, Njenga MK, Feikin DR, Breiman RF. Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis. 2012;206((Suppl 1)):S53–S60. doi: 10.1093/infdis/jis530. [DOI] [PubMed] [Google Scholar]
- 8.Yazdanbakhsh M, Kremsner PG. Influenza in Africa. PLoS Med. 2009;6:e1000182. doi: 10.1371/journal.pmed.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:223–235. doi: 10.1016/S1473-3099(11)70008-1. [DOI] [PubMed] [Google Scholar]
- 10.Majanja J, Njoroge RN, Achilla R, Wurapa EK, Wadegu M, Mukunzi S, Mwangi J, Njiri J, Gachara G, Bulimo W. Impact of influenza A (H1N1) pdm09 virus on circulation dynamics of seasonal influenza strains in Kenya. Am J Trop Med Hyg. 2013;88:940–945. doi: 10.4269/ajtmh.12-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waitumbi JN, Kuypers J, Anyona SB, Koros JN, Polhemus ME, Gerlach J, Steele M, Englund JA, Neuzil KM, Domingo GJ. Outpatient upper respiratory tract viral infections in children with malaria symptoms in western Kenya. Am J Trop Med Hyg. 2010;83:1010–1013. doi: 10.4269/ajtmh.2010.10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, Jagero G, Muluare PO, Gikunju S, Nderitu L, Balish A, Winchell J, Schneider E, Erdman D, Oberste MS, Katz MA, Breiman RF. Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS ONE. 2012;7:e43656. doi: 10.1371/journal.pone.0043656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, Lengeler C, Cherpillod P, Kaiser L, Genton B. Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 14.Berkley JA, Munywoki P, Ngama M, Kazungu S, Abwao J, Bett A, Lassauniére R, Kresfelder T, Cane PA, Venter M, Scott JA, Nokes DJ. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- 16.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 17.Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu449. http://dx.doi.org/10.1093/eurheartj/ehu449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poverty Rates by County Kenya Bureau of Statistics. 2005/6. https://www.opendata.go.ke/Counties/Poverty-Rates-by-County/z6za-e7yb Available at. Accessed November 19, 2014.
- 19.Obala AA, Mangeni JN, Platt A, Aswa D, Abel L, Namae J, OMeara WP. What is threatening the effectiveness of insecticide-treated bednets? A case-control study of environmental, behavioral, and physical factors associated with prevention failure. PLoS One. 2015. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C, Ahmed JA, Eidex RB, Nyoka R, Waiboci LW, Erdman D, Tepo A, Mahamud AS, Kabura W, Nguhi M, Muthoka P, Burton W, Breiman RF, Njenga MK, Katz MA. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS ONE. 2011;6:e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. doi: 10.1186/1475-2875-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidelines for the diagnosis of rheumatic fever. Jones criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. 1992;268:2069–2073. [PubMed] [Google Scholar]
- 23.Bassat Q, Machevo S, O'Callaghan-Gordo C, Sigaúque B, Morais L, Díez-Padrisa N, Ribó JL, Mandomando I, Nhampossa T, Ayala E, Sanz S, Weber M, Roca A, Alonso PL. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am J Trop Med Hyg. 2011;85:626–634. doi: 10.4269/ajtmh.2011.11-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P, Slesak G, Tongyoo N, Chanthongthip A, Panyanouvong P, Sibounheuang B, Phommasone K, Dohnt M, Phonekeo D, Hongvanthong B, Xayadeth S, Ketmayoon P, Blacksell SD, Moore CE, Craig SB, Burns MA, von Sonnenburg F, Corwin A, de Lamballerie X, González IJ, Christophel EM, Cawthorne A, Bell D, Newton PN. Causes of non-malarial fever in Laos: a prospective study. The Lancet Global Health. 2013;1:e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor SM, Molyneux ME, Simel DL, Meshnick SR, Juliano JJ. Does this patient have malaria? JAMA. 2010;304:2048–2056. doi: 10.1001/jama.2010.1578. [DOI] [PubMed] [Google Scholar]
- 26.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:e86–e102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pondei K, Kunle-Olowu OE, Peterside O. The aetiology of non-malarial febrile illness in children in the malaria-endemic Niger Delta Region of Nigeria. Asian Pacific Journal of Tropical Disease. 2013;3:56–60. [Google Scholar]
- 28.Bergmark R, Bergmark B, Blander J, Fataki M, Janabi M. Burden of disease and barriers to the diagnosis and treatment of group a beta-hemolytic streptococcal pharyngitis for the prevention of rheumatic heart disease in Dar Es Salaam, Tanzania. Pediatr Infect Dis J. 2010;29:1135–1137. doi: 10.1097/inf.0b013e3181edf475. [DOI] [PubMed] [Google Scholar]
- 29.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 30.Wachira J, Ndege S, Koech J, Vreeman R, Ayuo P, Braitstein P. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in western Kenya. J Acquir Immune Defic Syndr. 2013;65:e58–e66. doi: 10.1097/QAI.0b013e3182a14f9e. [DOI] [PubMed] [Google Scholar]
- 31.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86:246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertz JT, Munishi OM, Ooi EE, Howe S, Lim WY, Chow A, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Gubler DJ, Crump JA. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland LJ, Cash AA, Huang YJ, Sang RC, Malhotra I, Moormann AM, King CL, Weaver SC, King CH, LaBeaud AD. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg. 2011;85:158–161. doi: 10.4269/ajtmh.2011.10-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]