Abstract
Interventions targeting adult mosquitoes are used to combat transmission of vector-borne diseases, including dengue. Without available vaccines, targeting the primary vector, Aedes aegypti, is essential to prevent transmission. Older mosquitoes (≥ 7 days) are of greatest epidemiological significance due to the 7-day extrinsic incubation period of the virus. Age-grading of female mosquitoes is necessary to identify post-intervention changes in mosquito population age structure. We developed models using near-infrared spectroscopy (NIRS) to age-grade adult female Ae. aegypti. To determine if diet affects the ability of NIRS models to predict age, two identical larval groups were fed either fish food or infant cereal. Adult females were separated and fed sugar water ± blood, resulting in four experimental groups. Females were killed 1, 4, 7, 10, 13, or 16 days postemergence. The head/thorax of each mosquito was scanned using a near-infrared spectrometer. Scans from each group were analyzed, and multiple models were developed using partial least squares regression. The best model included all experimental groups, and positively predicted the age group (< or ≥ 7 days) of 90.2% mosquitoes. These results suggest both larval and adult diets can affect the ability of NIRS models to accurately assign age categories to female Ae. aegypti.
Introduction
Dengue viruses (DENVs) cause more human morbidity and mortality worldwide than any other arthropod-borne virus,1,2 with an estimated 390 million infections per year.3 The principal vector, Aedes aegypti, is highly anthropophilic, feeding almost exclusively on humans during daylight hours, and traveling only short distances to obtain blood meals.4,5 Currently, no vaccines or chemotherapeutic treatments are available for dengue infections, and therefore controlling the vector is the only available method to prevent transmission.
After imbibing an infective blood meal, it takes approximately 7–12 days for a female Ae. aegypti to become infectious,6 while the virus undergoes its extrinsic incubation period. Therefore, older mosquitoes (those ≥ 7 days) are considered to be of the greatest epidemiological importance. When using insecticide to control mosquito populations, it is expected that the age structure of the population will change, with a greater proportion of mosquitoes being younger (< 7 days) following the intervention. As the geographical range of dengue transmission has increased, however, threats to the efficacy of dengue vector-control strategies have increased, with insecticide resistance emerging in many dengue-endemic countries.7–13 To evaluate the efficacy of current control strategies, it is important to assess their impact on the age structure of the population. We currently lack a rapid, easy and cost-effective way to determine the biological age of mosquitoes.
Several strategies have been used in the past for determining the physiological age of both malaria and dengue vectors. Dissection of the ovaries can indicate whether a female has laid eggs (parous) or not (nulliparous),14 as well as estimate the number of gonotrophic cycles the female has undergone.15 Though these procedures give an indication of the number of complete blood meals the female has taken in its lifetime, they do not accurately predict the biological age of the mosquito. These procedures are also slow, labor intensive, and require specially trained technicians.
Another technique involving analysis of the cuticular hydrocarbons of female mosquitoes can provide an estimate of biological age,16–18 although the accurate use of this method requires information about environmental factors that are not always easily obtained. A laboratory assay has also been developed to identify the age of field-collected Ae. aegypti through transcriptional profiling,19–21 but as with many molecular assays, it requires specially trained technicians and can be costly. With the skills, time, and resources required for these techniques, it has not previously been possible to analyze the large number of mosquitoes required to evaluate the effectiveness of an intervention in shifting the age structure of target mosquito populations.
Recently, the use of near-infrared spectroscopy (NIRS) has improved our ability to quickly and accurately age-grade anopheline mosquitoes.22,23 This technique is rapid, nondestructive, and requires little technical training. Using a spectrometer, the absorption of specific wavelengths of light by the head and thorax of the mosquito is measured, indicating changes in the composition of C–H, N–H, and O–H functional groups over the course of the mosquito life span. Recent studies have shown NIRS models can accurately predict young (< 7 days) and old (≥ 7 days) Anopheles spp. correctly 78–89% of the time.23 If applied to Ae. aegypti, NIRS could readily be used in the field to determine the effectiveness of interventions by identifying if a shift in the age structure of a population has occurred.
Herein, we evaluate the ability of NIRS to accurately identify the age group (young versus old) of female Ae. aegypti. The predictive ability of NIRS can decrease in situations where laboratory-reared insects obtain different larval nutrition.24 The natural variations in larval and adult diets that occur in field-collected mosquitoes will likely also affect the ability of NIRS models (calibrated using laboratory-reared mosquitoes) to accurately identify the age group of field-collected mosquitoes. To test the hypothesis that diet contributes to changes in near-infrared spectral scans of female Ae. aegypti, we raised larvae on two distinct diets: fish food and infant cereal plus yeast. Adults from each larval group were then fed either sugar water alone or sugar water plus blood. Our findings highlight some of the challenges that arise when translating the use of NIRS for age-grading Ae. aegypti from the laboratory to the field.
Materials/Methods
Mosquitoes.
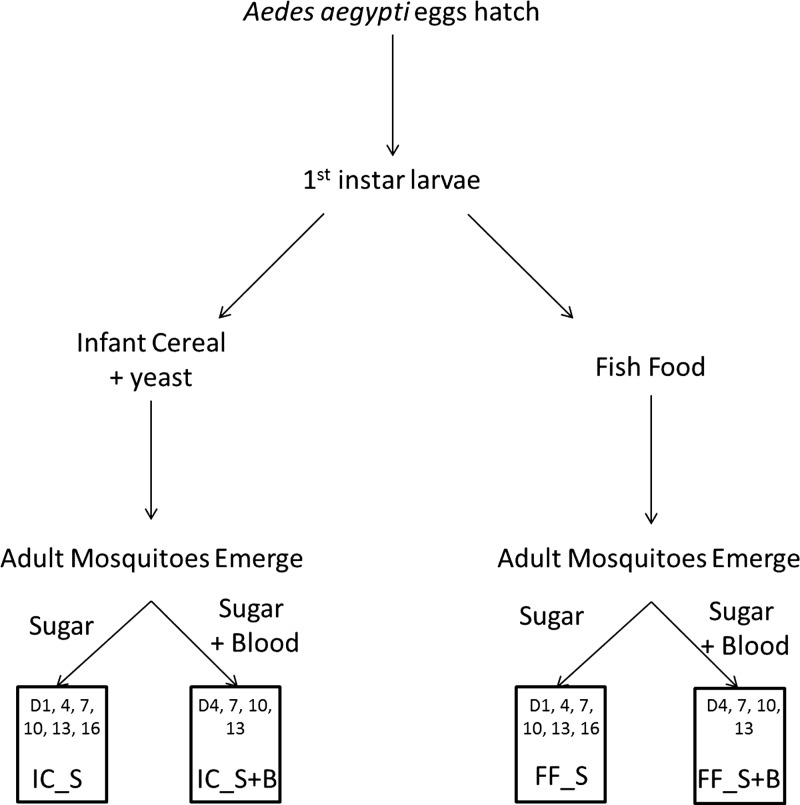
Eggs of the insecticide-susceptible New Orleans laboratory strain of Ae. aegypti were hatched in trays of deionized water and maintained under normal insectary conditions at the Centers for Disease Control and Prevention in Atlanta, GA (26°C, 60% humidity, 12:12 hour day:night lighting with 30-minute dawn/dusk periods). On hatching, first larval instars were separated into pans of approximately 150 larvae per tray (Figure 1). Half of the larval trays were provided nutrition from ground fish food (Staple Diet Koi Food, Doctors Foster and Smith, Rhinelander, WI; 40% minimum crude protein, 10% minimum crude fat, 4% maximum crude fiber), while the remaining pans were fed a combination of infant cereal (Nestum, Nestle Corporation, Glendale, CA; 13 g carbohydrate, 1 g protein, and 0 g fat per serving) and yeast in a 3:1 ratio. Food was provided daily, and water was cleaned every other day until pupation. Pupae were removed from trays each day and placed into cages marked with emergence dates. Every 24 hours, un-emerged pupae were placed in a new cage. Adults were offered sugar water (10% corn syrup solution), and a subset was additionally fed on rabbit blood. Rabbit blood was offered twice per week via direct feeding, and engorged females were placed in new cages and allowed to oviposit in paper-lined cups. These different larval and adult diets resulted in four distinct experimental groups of adult females: infant cereal plus yeast and sugar (IC_S), infant cereal plus yeast, sugar, and blood (IC_S+B), fish food and sugar (FF_S), and fish food, sugar, and blood (FF_S+B).
Figure 1.
Schematic describing how mosquitoes were separated by larval nutrition source and then by adult nutrition source, resulting in four experimental groups: infant cereal plus sugar (IC_S), infant cereal plus sugar and blood (IC_S+B), fish food plus sugar (FF_S), and fish food plus sugar and blood (FF_S+B). Boxes indicate the days post-emergence that female mosquitoes were killed for each experimental group.
Previous studies have indicated that preservation in RNAlater® is an effective means to preserve mosquitoes for later analysis by NIRS.23,25 Therefore, IC_S and FF_S females were knocked down with chloroform and stored in 1.5-mL tubes containing RNAlater® on days 1, 4, 7, 10, 13, and 16 postemergence. Individuals from the FF_S+B and IC_S+B cohorts were knocked down and stored in RNAlater® on days 4, 7, 10, and 13 postemergence. To allow the RNAlater® to fully penetrate the samples, the tubes of mosquitoes containing RNAlater® were initially stored at 4°C overnight and then kept at −20°C for up to 1 month before scanning. A maximum of 20 mosquitoes were stored in each tube.
Scanning.
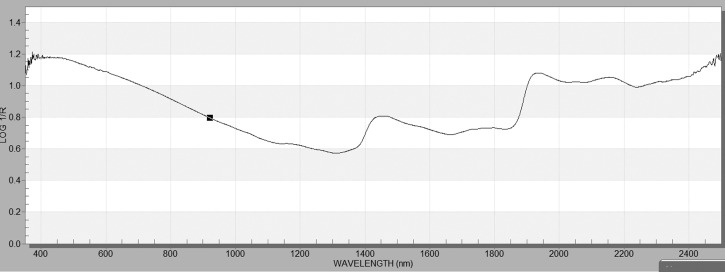
Immediately prior to scanning, mosquitoes were removed from tubes and laid on a paper towel to allow residual RNAlater® to be absorbed. Tubes of mosquitoes representing all experimental groups and age cohorts were scanned in random order, with approximately 20 mosquitoes per tube scanned at a time. Mosquitoes were scanned using a LabSpec 4 near-infrared spectrometer (ASD Inc., Boulder, CO). Mosquitoes were positioned on their side on a white Spectralon base, with the bifurcated fiber-optic probe approximately 2.4 mm above the base. Individual mosquito heads and thoraces were then positioned under the probe, and the instrument measured the absorbance of light, collecting 20 spectra that were averaged, saved, and displayed using the ASD software Indico™ Pro version 6.022 (Figure 2).
Figure 2.
Example of spectra collected from the head and thorax of a female Ae. aegypti.
Statistical analysis.
Data from the resulting spectra were saved as .asd files, which were converted to .spc files for analysis in GRAMS IQ (Thermo Scientific, Waltham, MA) using ASD to SPC Version 6.0, with data format (absorbance) and wavelength as the x-axis. These .spc files were then imported into GRAMS IQ to develop and test several different calibration models.
Models were developed separately for IC_S, IC_S+B, FF_S and FF_S+B (Tables 1 and 2). Each experimental group of mosquitoes was separated into a calibration set and a validation set. The validation set was created by removing a subset of nine individual mosquito scans from each experimental group and age cohort. A model was also developed by combining all experimental groups. Each calibration from each experimental group of mosquitoes was also used to predict all other mosquitoes from other experimental groups. Since each experimental group was reared separately, these were considered as independent test sets.26
Table 1.
Mean predicted age and 95% confidence interval for the cross-validation model, validation set (subset of calibration experimental group(s) removed from model development), and test sets (samples reared and treated different than the calibration set)
| IC_S | |||||
|---|---|---|---|---|---|
| Cross-validation set prediction | Validation set prediction | Test set prediction | |||
| Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) |
| 1 (N = 32) | 1.4 (0.8, 2.0) | 1 (N = 9) | 0.9 (0, 2.1) | 1 (N = 40) | 3.7 (3.3, 4.2) |
| 4 (N = 33) | 6.8 (6.4, 7.3) | 4 (N = 9) | 5.5 (4.2, 6.8) | 4 (N = 122) | 4.7 (4.1, 5.2) |
| 7 (N = 32) | 7.3 (6.6, 8.1) | 7 (N = 9) | 8.7 (6.9, 10.4) | 7 (N = 119) | 7.4 (6.9, 7.9) |
| 10 (N = 32) | 10.1 (9.4, 10.8) | 10 (N = 8) | 10.1 (7.5, 12.6) | 10 (N = 121) | 9.8 (9.2, 10.3) |
| 13 (N = 31) | 14.0 (13.4, 14.6) | 13 (N = 9) | 13.3 (11.8, 14.9) | 13 (N = 120) | 9.4 (9.0, 9.9) |
| 16 (N = 30) | 10.9 (10.4, 11.5) | 16 (N = 9) | 11.7 (10.4, 13.0) | 16 (N = 40) | 11.6 (10.4, 12.9) |
| IC_S+B | |||||
| Cross-validation set prediction | Validation set prediction | Test set prediction | |||
| Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) | Actual age | Mean prediction age (95% CI) |
| 1 (N = 32) | 2.4 (2.0, 2.9) | 1 (N = 9) | 2.3 (1.3, 3.3) | 1 (N = 40) | 5.1 (4.7, 5.5) |
| 4 (N = 64) | 2.2 (5.6, 6.7) | 4 (N = 18) | 5.8 (4.8, 6.7) | 4 (N = 82) | 6.9 (6.5, 7.4) |
| 7 (N = 63) | 7.5 (7.1, 7.9) | 7 (N = 18) | 7.8 (6.8, 8.8) | 7 (N = 79) | 9.1 (8.7, 9.6) |
| 10 (N = 63) | 10.0 (9.5, 10.4) | 10 (N = 17) | 10.1 (9.0, 11.1) | 10 (N = 81) | 10.9 (10.4, 11.39) |
| 13 (N = 62) | 12.1 (11.3, 12.8) | 13 (N = 18) | 11.6 (10.6, 12.6) | 13 (N = 80) | 10.8 (10.4, 11.1) |
| 16 (N = 30) | 10.9 (10.4, 11.4) | 16 (N = 9) | 11.3 (9.9, 12.8) | 16 (N = 40) | 12.5 (11.7, 13.3) |
| FF_S | |||||
| Cross-validation set prediction | Validation set prediction | Test set prediction | |||
| Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) |
| 1 (N = 31) | 2.0 (1.1, 2.9) | 1 (N = 9) | 3.5 (2.0, 5.1) | 1 (N = 41) | 3.9 (1.9, 3.9) |
| 4 (N = 31) | 7.3 (6.6, 8.0) | 4 (N = 9) | 6.6 (4.4, 8.7) | 4 (N = 124) | 4.5 (3.9, 5.1) |
| 7 (N = 31) | 8.5 (7.6, 9.4) | 7 (N = 9) | 8.8 (7.4, 10.1) | 7 (N = 120) | 6.7 (6.2, 7.2) |
| 10 (N = 32) | 10.8 (10.0, 11.7) | 10 (N = 9) | 9.9 (8.3, 11.4) | 10 (N = 120) | 8.2 (7.6, 8.7) |
| 13 (N = 31) | 9.9 (9.3, 10.4) | 13 (N = 9) | 8.2 (7.3, 9.2) | 13 (N = 120) | 9.1 (8.6, 9.6) |
| 16 (N = 31) | 12.5 (11.6, 13.4) | 16 (N = 9) | 12.1 (10.9, 13.4) | 16 (N = 39) | 6.5 (5.7, 6.3) |
| FF_S+B | |||||
| Cross-validation set prediction | Validation set prediction | Test set prediction | |||
| Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) |
| 1 (N = 31) | 4.0 (3.4, 4.5) | 1 (N = 9) | 3.7 (2.8, 4.7) | 1 (N = 41) | 2.4 (1.7, 3.0) |
| 4 (N = 64) | 6.2 (5.5, 6.9) | 4 (N = 18) | 5.9 (4.8, 7.0) | 4 (N = 82) | 5.2 (4.6, 5.7) |
| 7 (N = 61) | 8.4 (7.9, 9.0) | 7 (N = 18) | 8.3 (7.5, 9.1) | 7 (N = 81) | 7.2 (6.7, 7.6) |
| 10 (N = 63) | 10.2 (9.7, 10.7) | 10 (N = 18) | 9.5 (8.7, 10.3) | 10 (N = 80) | 8.9 (8.4, 9.4) |
| 13 (N = 62) | 9.9 (9.5, 10.3) | 13 (N = 18) | 9.5 (8.8, 10.2) | 13 (N = 80) | 10.2 (9.7, 10.7) |
| 16 (N = 31) | 12.3 (11.4, 13.2) | 16 (N = 9) | 11.7 (10.5, 12.9) | 16 (N = 39) | 8.6 (8.9, 9.1) |
| IC_S+B + FF_S+B | |||||
| Cross-validation set prediction | Validation set prediction | Test set prediction | |||
| Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) | Actual age | Mean predicted age (95% CI) |
| 1 (N = 63) | 3.1 (2.7, 3.5) | 1 (N = 18) | 3.1 (2.2, 4.0) | 1 | N/A |
| 4 (N = 128) | 6.0 (5.7, 6.4) | 4 (N = 36) | 5.9 (5.2, 6.6) | 4 | N/A |
| 7 (N = 124) | 8.2 (7.8, 8.6) | 7 (N = 36) | 8.1 (8.4, 8.8) | 7 | N/A |
| 10 (N = 126) | 10.0 (9.7, 10.4) | 10 (N = 35) | 9.8 (9.1, 10.5) | 10 | N/A |
| 13 (N = 124) | 10.9 (10.5, 11.4) | 13 (N = 36) | 10.6 (9.9, 11.2) | 13 | N/A |
| 16 (N = 61) | 11.3 (10.8, 11.9) | 16 (N = 18) | 11.1 (10.1, 12.0) | 16 | N/A |
Table 2.
Comparison of the predictive ability of models using different experimental groups for calibration
| Calibration group | Validation set prediction | Test set prediction | Overall predictive ability | ||||
|---|---|---|---|---|---|---|---|
| % Correct < 7 days | % Correct ≥ 7 days | % Overall correct | % Correct < 7 days | % Correct ≥ 7 days | % Overall correct | ||
| IC_S | 83.3 (N = 15) | 96.2 (N = 25) | 90.1 (N = 40) | 85.8 (N = 139) | 83.8 (N = 243) | 84.5 (N = 382) | 87.3% |
| IC_S+B | 77.8 (N = 21) | 97.8 (N = 43) | 90.1 (N = 64) | 62.3 (N = 76) | 98.0 (N = 197) | 84.5 (N = 273) | 85.5% |
| FF_S | 61.1 (N = 11) | 92.6 (N = 25) | 80.0 (N = 36) | 76.4 (N = 126) | 66.7 (N = 186) | 70.3 (N = 312) | 71.2% |
| FF_S+B | 70.4 (N = 19) | 95.6 (N = 43) | 86.1 (N = 62) | 87.0 (N = 107) | 84.4 (N = 168) | 85.4 (N = 275) | 85.5% |
| IC_S+B + FF_S+B | 79.7 (N = 43) | 96.6 (N = 86) | 90.2 (N = 129) | N/A | N/A | N/A | 90.2% |
The percentage correctly identified as young and old, and the overall percentage of mosquitoes correctly identified are shown for samples predicted from validation set (subset of calibration experimental group(s) removed from model development) and test sets (samples reared and treated different than the calibration set).
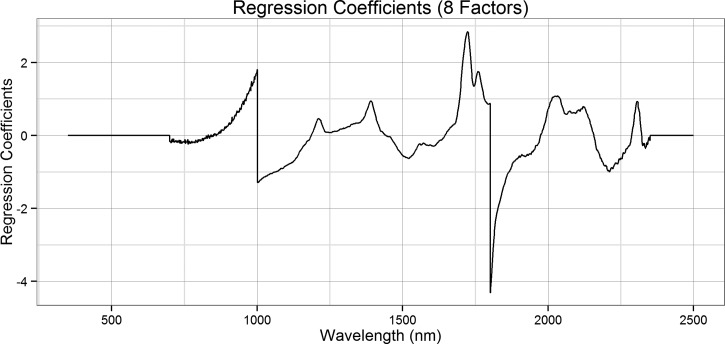
For each group of data, GRAMS IQ software was used to perform partial least squares (PLS) regression on the spectra in the 700- to 2,350-nm region. No color differences were expected between groups, thus the region below 700 nm was excluded. The region above 2,350 nm was noisy due to lack of sensor sensitivity, and thus this region above 2,350 nm was also excluded. Models were developed using a “leave-one-out” cross-validation method in which one sample from the calibration set is removed while the remaining samples in that set are used to develop an equation that will predict the removed sample. This process is repeated for each sample, and the best overall equation for predicting all samples is selected. The number of factors used to determine the “best” model for each calibration set was determined using the predicted residual error sum of squares (PRESS) and by viewing the regression coefficients (Figure 3). The best models for each data set contained eight factors based on these graphs, and these models were then used to predict the actual age of the validation and test sets using IQ Predict, a component of the GRAMS software suite. If more factors are selected, then the regression coefficient plot becomes noisy, indicating the model is over fitted.
Figure 3.
Regression coefficients for predicting the age of female Ae. aegypti using a model containing all experimental groups and eight partial least squares regression factors.
Specific age predictions for each mosquito were estimated using the best models, with the mean and 95% confidence intervals of the predicted ages reported in Table 1. In addition, the ability of the models to detect young and old mosquitoes was tested. Predicted ages of < 7 days were categorized as “young,” and the percentage of individuals with predicted and actual ages < 7 days was calculated. The same analysis was performed for “old” (≥ 7 days) individuals.
Results
Mosquitoes.
A total of 805 female Ae. aegypti mosquitoes, with 39–41 individuals of each age in each experimental group, were analyzed. Visual inspection of mosquitoes indicated that adult females fed infant cereal plus yeast as larvae were larger than those fed fish food, possibly due to the higher level of carbohydrates provided by the infant cereal plus yeast larval diet. No visual differences were observed between adults fed sugar versus those offered both blood and sugar.
Statistical analysis.
The eight-factor calibration models were used to predict the ages of all samples (Table 1). Overall, the models were able to predict the age of mosquitoes 4, 7, and 10 days old within 2 days, but very young (1-day old) and very old (13- and 16-day old) mosquitoes are less accurately predicted. When assessing the ability of the models to predict young versus old mosquitoes, the model containing all experimental groups detected the correct age group of the mosquito 90.2% of the time (Table 2). Though other models were able to predict validation or independent test set age groups with varying degrees of accuracy, the model containing all groups provided the most accurate overall result. The PRESS and regression coefficients (Figure 3) for this model demonstrate that the model is appropriate for the data, and that the data has not been over fit. The peaks in this regression coefficient plot are similar to those reported by Mayagaya and others,22 indicating that similar wavelengths, and thus similar functional groups, are used in the classification models.
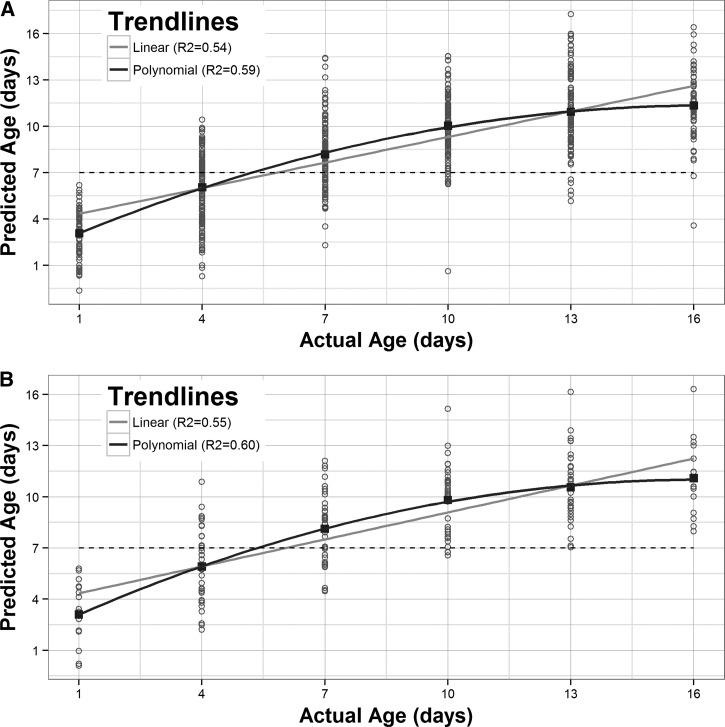
Interestingly, the predicted mean ages (Table 1) as well as the actual versus predicted plot (Figure 4) demonstrate that the sensitivity of any of these models to predict the exact age of the mosquito diminishes past the age of 10 days. Indeed, the highest mean prediction for a 16-day-old mosquito was only 12.5 days (Table 1), (IC_S+B). Fitting a second degree polynomial trendline to the data provided the best fit (Figure 4). The predicted ages (Table 2) and actual versus predicted age plot were similar for both the cross-validation output (Figure 4A) as well as the samples removed from the model for validation (Figure 4B), indicating that the model appropriately predicts the unknown samples.
Figure 4.
Predicted versus actual ages of mosquitoes using the most effective model, containing all experimental groups. (A) Samples used for cross-validation and (B) samples removed from model for validation.
Discussion
In this study, we assessed the ability of NIRS to effectively distinguish between young and old Ae. aegypti. This tool has been used to estimate the age of malaria vectors such as Anopheles gambiae,22,23 but had not previously been applied to dengue vectors. The ability to accurately determine the age of adult female Ae. aegypti is important for evaluating the impact of vector control interventions.27 The current available methods for age-grading are time consuming, difficult, and often costly, limiting their widespread applicability in the field. The availability of a fast, high-throughput, and simple technique to determine shifts in the age structure of mosquito populations will allow for more comprehensive evaluations of intervention impacts.
The effect of larval diet on adult mosquitoes was evident in comparing the near-infrared spectra of the two experimental groups that received different sources of larval nutrition. In addition, the larvae that had been provided infant cereal plus yeast were notably larger as adults than those fed fish food. In the field, variation in larval diet is very common. Ae. aegypti oviposit in any available water-holding containers,28 and the presence of nutrients in these containers varies, which can impact the size of the resulting adult mosquitoes. The difference between adults fed only sugar versus sugar and blood was also evident, based on the predictive ability of models including and excluding blood-fed mosquitoes for calibration (data not shown). When creating future calibration data sets for determining young versus old mosquitoes in the field, mosquitoes with varying larval and adult diets should be included to increase the resolution of the predictive ability of the model.
The best model developed herein included all four experimental groups, and predicted young and old mosquitoes effectively 79.7% and 96.6% of the time, respectively. Interestingly, though models predicted 10-day and older mosquitoes as old, none of the models were able to differentiate between 10-, 13-, or 16-day-old mosquitoes. This indicates that the measured functional groups between 700 and 2,350 nm change over time, but this change diminishes once mosquitoes reach a certain age. The cross-validation data from GRAMS show the nonlinear relationship of the predicted data. However, because a cutoff of 7 days is most appropriate for assessing a dengue vector's potential for virus transmission, the inability of NIRS to distinguish between 10 and 16 days would not have epidemiological significance for Ae. aegypti.
Although the application of NIRS for age-grading Ae. aegypti appears to be complex, these results are nonetheless encouraging. Focusing on categorizing mosquitoes as either young (< 7 days) or old (≥ 7 days) increases the predictive power of the models while maintaining epidemiological relevance. To successfully transfer this technique from the laboratory to the field, it will be necessary to develop calibration models that include mosquitoes with varying nutritional backgrounds. Because of the use of 7 days as the cutoff for young versus old, it is also important to look at different-aged mosquitoes that do not include 7-day-old females (i.e., days 1, 3, 6, 9, 12, and 15). Doing so will further refine the sensitivity of NIRS models to accurately categorize the age of field-collected Ae. aegypti. Future studies using field-collected pupae from natural habitats will further elucidate the ability of this technique to accurately determine the age group of field-collected female mosquitoes.
ACKNOWLEDGMENTS
We thank Dr. Michael Green for his insight and guidance on developing these models.
Disclaimers: Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Footnotes
Authors' addresses: Kelly Liebman, Isabel Swamidoss, Lucrecia Vizcaino, Audrey Lenhart, and Robert Wirtz, Centers for Disease Control and Prevention, CGH/DPDM/EB, Atlanta, GA, E-mails: wuq4@cdc.gov, gtz8@cdc.gov, vtb6@cdc.gov, ajl8@cdc.gov, and bew5@cdc.gov. Floyd Dowell, United States Department of Agriculture, Center for Grain and Animal Health Research, Manhattan, KS, E-mail: floyd.dowell@ars.usda.gov.
References
- 1.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue virus—mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 5.Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on blood. Am J Trop Med Hyg. 1997;57:235–239. doi: 10.4269/ajtmh.1997.57.235. [DOI] [PubMed] [Google Scholar]
- 6.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 7.Mazzarri MB, Georghiou GP. Characterization of resistance to organophosphate, carbamate and pyrethroid insecticdes in field populations of Aedes aegypti from Venezuela. J Am Mosq Control Assoc. 1995;11:315–322. [PubMed] [Google Scholar]
- 8.Rawlins SC, Wan JO. Resistance in some Caribbean populations of Aedes aegypti to several insecticides. J Am Mosq Control Assoc. 1995;11:59–65. [PubMed] [Google Scholar]
- 9.Montella IR, Martins AJV-M, Pereira PF, Lima JB, Braga IA, Valle D. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am J Trop Med Hyg. 2007;77:467–477. [PubMed] [Google Scholar]
- 10.Hemingway J, Boddington RG, Harris J, Dunbar SJ. Mechanisms of insecticide resistance in Aedes aegypti (L.) (Diptera: Culicidae) from Puerto Rico. Bull Entomol Res. 1989;79:123–130. [Google Scholar]
- 11.Pereira Lima JB, Da-Cunha MP, Da Silva Junior RC, Ribeiro Galardo AK, Da Silva Soares S, Aparecida Barga I, Pimentel Ramos R, Valle D. Resistance of Aedes aegypti to organophosphates in several municipalities in the state of Rio De Janeiro and Espirito Santo, Brazil. Am J Trop Med Hyg. 2003;68:329–333. [PubMed] [Google Scholar]
- 12.Rodriguez MM, Bisset JA, Fernandez D. Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007;23:420–429. doi: 10.2987/5588.1. [DOI] [PubMed] [Google Scholar]
- 13.Brengues C, Hawkes NJ, Chandre F, Mccarroll L, Duchon S, Guillet P, Manguin S, Morgan JC, Hemingway J. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17:87–94. doi: 10.1046/j.1365-2915.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 14.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:122–191. [PubMed] [Google Scholar]
- 15.Polovodova NS. The determination of physiological age of female Anopheles by number of gonotrophic cycles completed. Med Parazitol Parazitar Bolezni. 1949;18:352–355. [Google Scholar]
- 16.Desena ML, Clark JM, Edman JD, Symington SB, Scott TW, Clark GG, Peters TM. Potential for aging female Aedes aegypti (Diptera: Culicidae) by gas chromatographic analysis of cuticular hydrocarbons, including a field evaluation. J Med Entomol. 1999;36:811–823. doi: 10.1093/jmedent/36.6.811. [DOI] [PubMed] [Google Scholar]
- 17.Desena ML, Edman JD, Clark JM, Symington SB, Scott TW. Aedes aegypti (Diptera: Culicidae) age determination by cuticular hydrocarbon analysis of female legs. J Med Entomol. 1999;36:824–830. doi: 10.1093/jmedent/36.6.824. [DOI] [PubMed] [Google Scholar]
- 18.Gerade BB, Lee SH, Scott TW, Edman JD, Harrington LC, Kitthawee S, Jones JW, Clark JM. Field validation of Aedes aegypti (Diptera: Culicidae) age estimation by analysis of cuticular hydrocarbons. J Med Entomol. 2004;41:231–238. doi: 10.1603/0022-2585-41.2.231. [DOI] [PubMed] [Google Scholar]
- 19.Cook PE, Hugo LE, Iturbe-Ormaetxe IA, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O'Neill SL. The use of transcriptional profiles to predict adult mosquito age under field conditions. Insect Mol Biol. 2006;103:18060–18065. doi: 10.1073/pnas.0604875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugo LE, Cook PE, Johnson PH, Rapley LP, Kay BH, Ryan PA, Ritchie SA, O'Neill SL. Field validation of a transcriptional assay for the prediction of age of uncaged Aedes aegypti mosquitoes in Northern Australia. PLoS Negl Trop Dis. 2010;4:e608. doi: 10.1371/journal.pntd.0000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caragata EP, Poinsignon A, Moreira LA, Johnson PH, Leong YS, Ritchie SA, O'Neill SL, McGraw EA. Improved accuracy of the transcriptional profiling method of age grading in Aedes aegypti mosquitoes under laboratory and semi-field cage conditions and in the presence of Wolbachia infection. Insect Mol Biol. 2011;20:215–224. doi: 10.1111/j.1365-2583.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Mayagaya VS, Michel K, Benedict MQ, Killeen GF, Wirtz RA, Ferguson HM, Dowell FE. Non-destructive determination of age and species of Anopheles gambiae s.l. using near-infrared spectroscopy. Am J Trop Med Hyg. 2009;81:622–630. doi: 10.4269/ajtmh.2009.09-0192. [DOI] [PubMed] [Google Scholar]
- 23.Sikulu M, Killeen GF, Hugo LE, Ryan PA, Dowell KM, Wirtz RA, Moore SJ, Dowell FE. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasit Vectors. 2010;3:49. doi: 10.1186/1756-3305-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aw WC, Ballard JW. The effects of temperature and diet on age grading and population age structure determination in Drosophila. J Insect Physiol. 2013;59:994–1000. doi: 10.1016/j.jinsphys.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Dowell FE, Noutcha AE, Michel K. Short report: the effect of preservation methods on predicting mosquito age by near infrared spectroscopy. Am J Trop Med Hyg. 2011;85:1093–1096. doi: 10.4269/ajtmh.2011.11-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fern T. Independent validation sets. NIR News. 2013;24:19–20. [Google Scholar]
- 27.Cook PE, McMeniman CJ, O'Neill SL. Modifying insect population age structure to control vector-borne disease. Adv Exp Med Biol. 2008;627:126–140. doi: 10.1007/978-0-387-78225-6_11. [DOI] [PubMed] [Google Scholar]
- 28.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]