Abstract
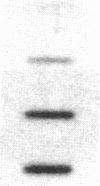
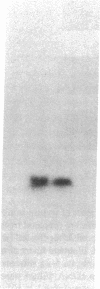
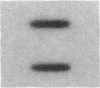
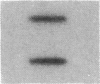
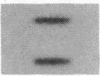
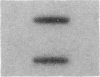
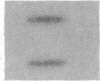
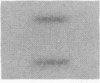
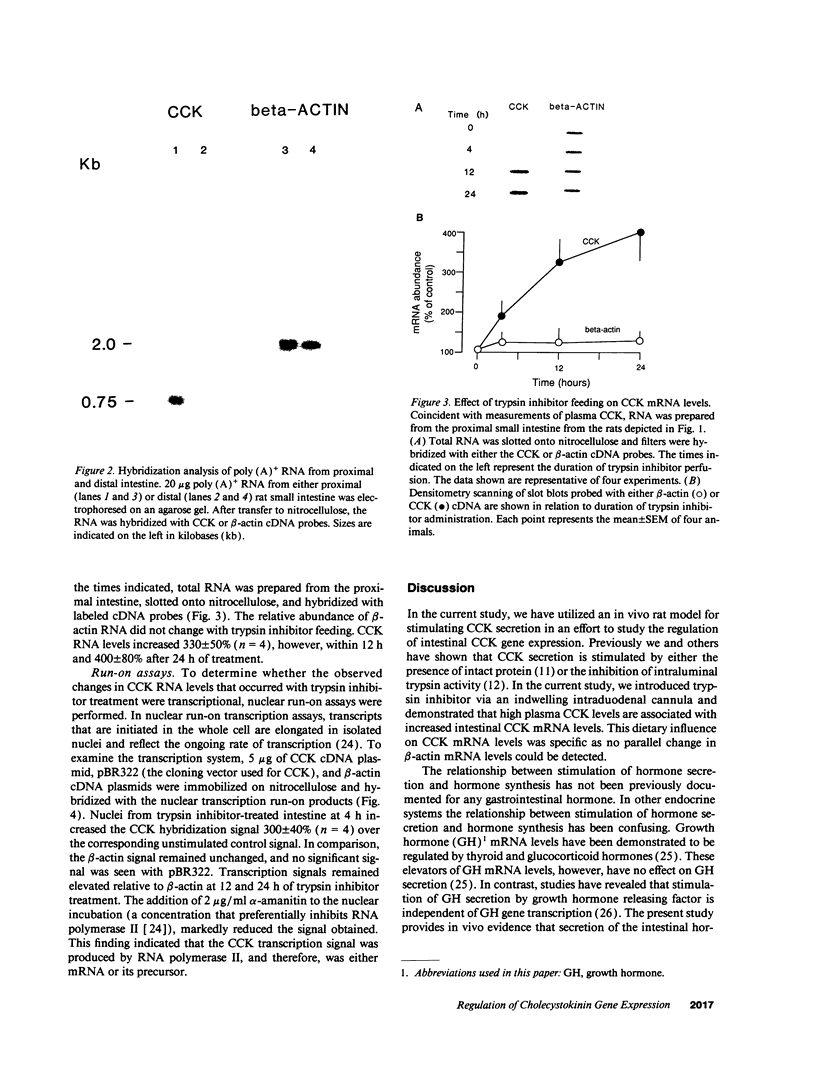
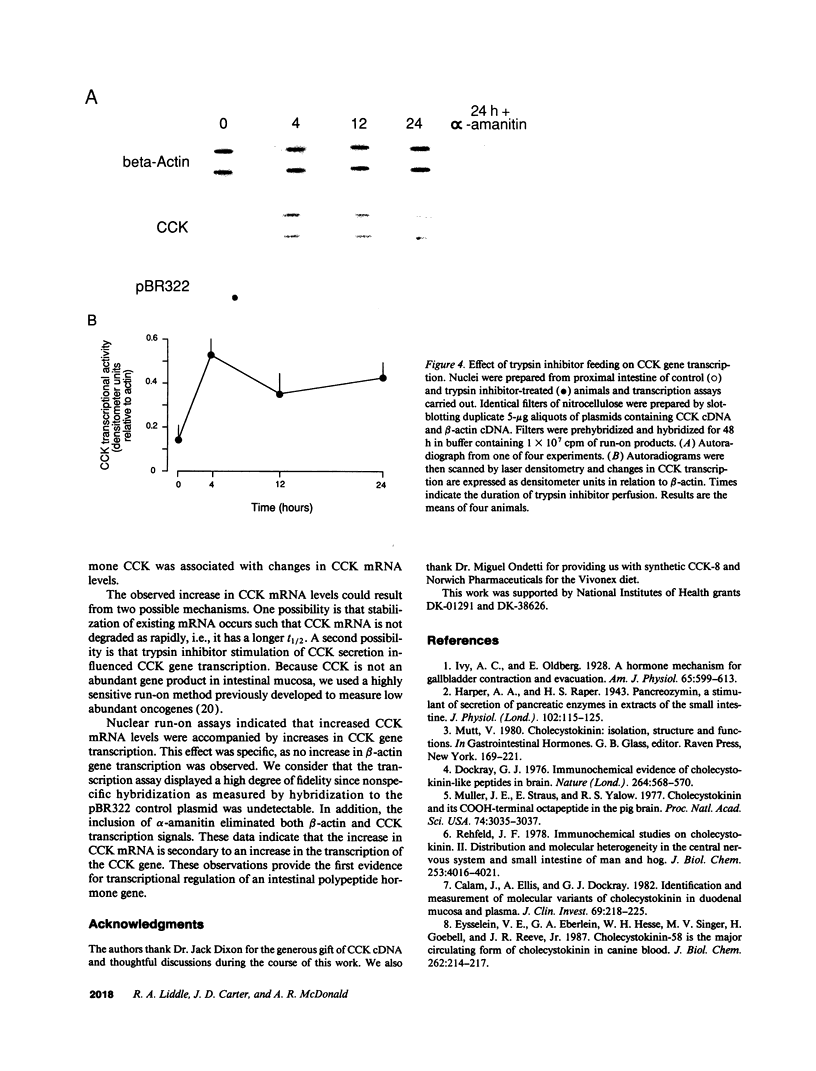
Cholecystokinin (CCK) is a gastrointestinal hormone produced by discrete endocrine cells in the upper small intestine and released after ingestion of a meal. The present study was designed to determine if enhanced CCK secretion is associated with increases in intestinal CCK mRNA levels. Rats, prepared with indwelling intraduodenal cannulae, were first fed an elemental diet that did not stimulate CCK release. Next, as a means of stimulating CCK secretion, soybean trypsin inhibitor was perfused for up to 24 h. Trypsin inhibitor administration increased plasma CCK levels from 0.9 +/- 0.1 to approximately 5 pmol/liter. RNA was prepared from the proximal small intestine at various times after trypsin inhibitor perfusion and mRNA levels analyzed by hybridization with a CCK cDNA probe. After 12 and 24 h of trypsin inhibitor treatment there were three- and fourfold increases, respectively, in CCK mRNA levels. In comparison, there was no change in beta-actin mRNA levels. To determine if regulation of CCK mRNA was at the level of CCK gene transcription, labeled transcripts from nuclear run-on incubations were hybridized to immobilized CCK cDNA. In trypsin inhibitor-treated rats, a two- to threefold increase in transcriptional activity was observed, whereas beta-actin gene transcription levels were unaltered. These studies indicate that stimulation of CCK secretion is associated with an increase in intestinal CCK mRNA content resulting from an increase in CCK gene transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M., Bilezikjian L. M., Vale W. W., Rosenfeld M. G., Evans R. M. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature. 1985 Mar 21;314(6008):279–281. doi: 10.1038/314279a0. [DOI] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Eberlein G. A., Hesse W. H., Singer M. V., Goebell H., Reeve J. R., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem. 1987 Jan 5;262(1):214–217. [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. 1972 May;140(1):6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- Green G. M., Nasset E. S. Importance of bile in regulation of intraluminal proteolytic enzyme activities in the rat. Gastroenterology. 1980 Oct;79(4):695–702. [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Liddle R. A., Green G. M., Conrad C. K., Williams J. A. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol. 1986 Aug;251(2 Pt 1):G243–G248. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A. R., Goldfine I. D. Glucocorticoid regulation of insulin receptor gene transcription in IM-9 cultured lymphocytes. J Clin Invest. 1988 Feb;81(2):499–504. doi: 10.1172/JCI113347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Rayford P. L., Pearse A. G., Buchan A. M., Thompson J. C. Identification of cholecystokinin-secreting cells. Lancet. 1975 Nov 22;2(7943):1016–1018. doi: 10.1016/s0140-6736(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]