Abstract
Reducing the human reservoir of malaria parasites is critical for elimination. We conducted a community randomized controlled trial in Southern Province, Zambia to assess the impact of three rounds of a mass test and treatment (MTAT) intervention on malaria prevalence and health facility outpatient case incidence using random effects logistic regression and negative binomial regression, respectively. Following the intervention, children in the intervention group had lower odds of a malaria infection than individuals in the control group (adjusted odds ratio = 0.47, 95% confidence interval [CI] = 0.24–0.90). Malaria outpatient case incidence decreased 17% in the intervention group relative to the control group (incidence rate ratio = 0.83, 95% CI = 0.68–1.01). Although a single year of MTAT reduced malaria prevalence and incidence, the impact of the intervention was insufficient to reduce transmission to a level approaching elimination where a strategy of aggressive case investigations could be used. Mass drug administration, more sensitive diagnostics, and gametocidal drugs may potentially improve interventions targeting the human reservoir of malaria parasites.
Background
In the context of malaria control programs, antimalarial drugs have primarily been used to treat clinical cases, reducing illness through prompt diagnosis and treatment.1,2 In areas of high transmission, drug use for pregnant women, infants, and children has been expanded beyond the treatment of symptomatic individuals to include intermittent preventative therapy. With renewed emphasis on malaria elimination, the expansion of antimalarial drug use to clear infections from people regardless of symptoms or treatment-seeking behavior is regarded as a potential tool.3
In addition to clinical malaria cases, asymptomatic malaria infections likely play a significant role in malaria transmission.4 Individuals with an asymptomatic malaria infection have no reason to seek treatment, thus remaining infectious for longer periods of time than individuals with a symptomatic malaria infection who are more likely to seek treatment.5–9 Carriage of asymptomatic malaria infections is common,10–13 with such individuals capable of transmitting malaria gametocytes to mosquitoes.13–16
Antimalarial mass drug administration (MDA) has been used over the past 75 years in an attempt to clear both symptomatic and asymptomatic infections, often with the goal of interrupting malaria transmission.17 In higher transmission settings MDA has been shown to have a significant effect on reducing the malaria burden in some cases, especially when combined with high vector control coverage.17 However, the impact of MDA on malaria transmission has generally been short lived. Various concerns including implementation challenges of acceptability and coverage of MDA, in addition to the risk that prolonged use of MDA as a malaria control tool in the absence of elimination could potentially hasten the spread of drug-resistant parasites, have led to the search for effective and efficient alternatives to MDA.
Active parasite detection may be a potentially useful alternative to MDA, where individuals with a parasite infection, including those that are asymptomatic, are identified with a rapid diagnostic test (RDT) and then treated with an effective antimalarial such as an artemisinin-based combination therapy (ACT). Although RDTs have known limitations in sensitivity for infection detection, they are the only currently available tests capable of providing immediate results in field settings for treatment decisions.18 In addition to clearing asexual stage parasites, ACTs are effective against immature gametocytes, and have been shown to reduce the carriage time of gametocytes in infected individuals.1,2,19–21 When combined with sustained high vector control coverage, a population-wide mass test and treatment (MTAT) intervention may be useful in limiting onward transmission from infected individuals and thereby significantly reducing malaria transmission. The MTAT strategies have been hypothesized to be most effective in areas of low to moderate transmission if implemented repeatedly during the dry season when vector densities, parasite densities within individuals, and multiplicity of infection are presumably at their lowest.3,22–25
Zambia's national malaria strategic plan 2011–2015 declared a goal of five malaria free zones by 2015.26 The strategic plan highlights three phases of malaria elimination. The first phase focuses on sustaining high population coverage with vector control interventions coupled with expanding and strengthening the surveillance system. The second phase focuses on reducing the population-wide malaria burden through additional interventions beyond sustained high vector control coverage, including elimination of the parasite in the human population (i.e., using MTAT or other population-wide treatment strategies). The third phase focuses on transitioning health facility catchment areas (HFCA) to an aggressive active case investigation system for individual malaria cases once malaria transmission has been sufficiently decreased. In the second phase of the strategic plan, Southern Province was selected for implementation of three dry-season rounds of an MTAT intervention using RDTs and artemether-lumefantrine (AL; the nationally recommended ACT) to clear infections starting immediately after the peak transmission season of 2012. It was hypothesized that the 2012 MTAT intervention with AL would result in lowered confirmed malaria case incidence in the health system and lowered malaria parasite prevalence in children during the subsequent high transmission season; this area could then move to an aggressive active case investigation system that investigates incident malaria cases and responds as needed. This work presents the results of the impact evaluation of the 2012 MTAT intervention.
Methods
Study site description.
Although heterogeneous, Southern Province has generally modest malaria transmission with the highest levels along the shore of Lake Kariba. The overall mean parasite prevalence was estimated to be < 10% in children < 5 years of age in Malaria Indicator Surveys (MIS) conducted in 2006, 2008, and 2010.27–29 Malaria transmission in this area of Zambia is highly seasonal, with the peak occurring at the late end of the rainy season from March to May. Anopheles arabiensis and Anopheles funestus are the primary malaria vectors in the area.30 The Province has had sustained high coverage of insecticide-treated mosquito nets (ITNs) since 2007. Indoor residual spray (IRS) activities are conducted annually by the Zambia Ministry of Health National Malaria Control Center (NMCC). In Southern Province including the areas covered by the MTAT campaigns, IRS is implemented by District Health office staff. Spraying is done annually, usually in November or December at the end of the dry season and just before the onset of the rains. The IRS tends to be restricted to households with closer access to the available transportation network. Pyrethroids were the chemical used for IRS activities in Southern Province during 2011–2012.
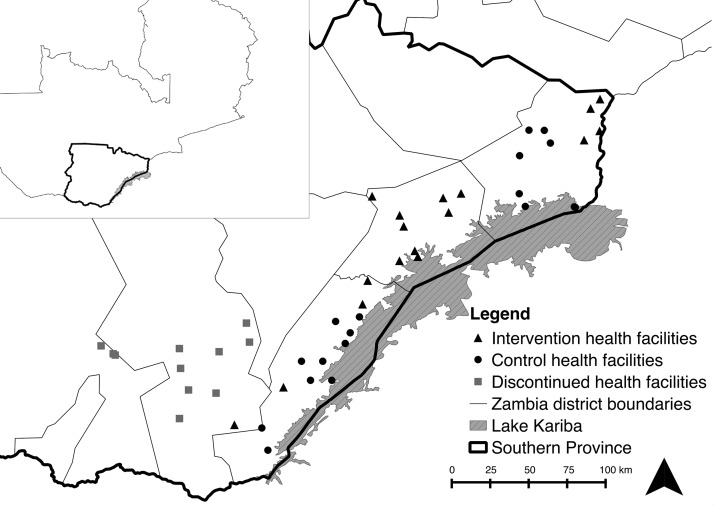
The MTAT intervention was implemented in Gwembe, Siavonga, Sinazongwe, and southern Kalomo districts along Lake Kariba (Figure 1). Subsistence farming is the principal economic activity for the majority of households, although there are some commercial farming activities including a large private cotton growing operation. Fishing is an important economic activity for many households located near the lake. Housing construction commonly consists of brick homes with thatched roofs.
Figure 1.
Map of study area.
Study design.
A community randomized step-wedge control trial design31–33 was used to assess the impact of the three dry-season MTAT rounds on reducing health facility malaria case incidence and parasite infection prevalence in children. An initial 46 HFCAs were available for inclusion in the study. As a result of logistical reasons for MTAT implementation and to ensure a buffer zone of 5 km of unhabituated land to mitigate contamination, the 46 HFCAs were organized into 18 contiguous randomization groups to be randomized to receive the MTAT intervention or serve as a control group. Each randomization group contained 2–3 HFCAs. Satellite imagery from Google Earth was used to create the randomization groupings and then confirmed by District Health Teams. Eight randomization groups were randomly selected to begin the MTAT intervention in the dry season of 2012 and 10 selected to serve as a contemporaneous control group, with the MTAT intervention scheduled to be implemented in subsequent years.
Intervention.
Beginning in December 2011 the MTAT intervention was piloted with a single round in 10 HFCAs to assess the feasibility and efficiency of implementation. During the dry season of 2012 three MTAT rounds were implemented in randomly selected HFCAs in the four districts, with the first round occurring in June–July, the second round occurring August–September, and the final round occurring October–November. The MTAT intervention was continued in an expanded number of HFCAs in 2013. After the first round in 2012 the MTAT intervention was discontinued in central Kalomo District because of very low infection rates; these areas were transitioned to a case investigation method for containing malaria transmission.
During each MTAT round, community health workers (CHWs) systematically went door to door and screened all individuals in their target areas using Ministry of Health (MoH) approved RDTs (SD Bioline Pf and ICT Mal Pf brands); both brands detect the histidine-rich protein 2 (HRP2). The CHWs conducted the screening alongside survey teams collecting household and individual level data. For those individuals testing positive, CHWs administered a treatment regimen of AL according to MoH treatment guidelines and referred individuals with symptoms of severe illness to the nearest clinic. In the event that a household member was absent from the home during the intervention, the MTAT teams returned three times at a different hour in an attempt to find that person at home for testing and treatment. Children < 3 months of age were excluded from the MTAT and pregnant women in their first trimester with a positive RDT were referred to the closest health center for malaria treatment, which consists of quinine in Zambia. There was no monitoring of adherence to treatment.
Study outcomes and data collection.
The primary outcome measure for the evaluation was malaria parasite prevalence among children 1–59 months of age in intervention and control groups measured during the peak malaria transmission season (April–May) in 2012 (before the MTAT) and again during the peak transmission season in 2013 (post MTAT) using a standardized malaria indicator household survey.34 The MIS uses a two-stage cluster design with primary sampling units (PSU-defined as standard enumeration areas from the last Zambia census) selected based on their relative population size. The RDTs, administered to all children 1–59 months of age in sampled households were used to ascertain malaria parasite prevalence for each survey round. Sixty PSUs were selected during each survey round, with 30 PSUs allocated each to intervention and control areas. A total sample size of 3,000 children < 5 years of age in 2,100 households > 2 rounds were sought to effectively measure a 50% reduction in malaria parasite prevalence between intervention and control groups, from an assumed 10% malaria parasite prevalence at baseline with a design effect of two.
The prevalence of malaria infection was measured through RDTs among all household residents ≥ 3 months of age as part of each MTAT round in 2012; control areas did not receive the MTAT and thus prevalence was not measured in those areas. Malaria prevalence as measured through RDTs from the first MTAT round in 2013 (June–July) within intervention areas is included for descriptive comparison to the first round in 2012.
The secondary outcome measure for the evaluation was confirmed and total outpatient malaria case incidence. Data for this outcome in intervention and control groups were obtained from the routine health management information system (HMIS) reported monthly by each health facility in the study area from January 2011–May 2013. This health facility HMIS system has been strengthened starting in 2009 with specific training and ongoing technical assistance for weekly cell phone reporting of malaria indicators. Monthly total and confirmed malaria case counts at each health facility were standardized as rates per 1,000 population using estimates of the mid-year population in each HFCA derived from the 2010 census.
Measurement of potential confounding factors.
Satellite imagery from moderate resolution imaging spectroradiometer (MODIS) provides remotely sensed data for deriving the enhanced vegetation indices (EVI) at a 250 m resolution. This metric served as an indicator of the propensity of an area to harbor mosquito habitats and by extension, malaria transmission.35 A digital elevation model constructed from the shuttle radar topography mission (SRTM) provided a measurement of elevation, also linked to malaria transmission. Remotely sensed data were linked to households for all analyses using the Raster package36,37 in R version 2.15.1.38 The EVI and elevation were dichotomized above and below the overall median.
For assessing differences in malaria parasite prevalence in children, potential confounding factors were measured at the individual and household level from the MIS. Relative household wealth tertiles were created using a principle components analysis based on asset ownership derived from the household questionnaire.39 Child age was dichotomized as < 24 months or ≥ 24 months. Household ITN ownership was derived from the household net roster, categorized as 0 or ≥ 1 ITN. Households were dichotomized as covered by IRS if they reported receiving IRS in the past 12 months.
For assessing differences in malaria outpatient case incidence, potential confounding factors were measured at the health center and monthly temporal level. The monthly laboratory testing rate was calculated as the proportion of suspected malaria cases tested for malaria using microscopy or RDT at each facility. Additionally, we created a dummy variable for month of the year to account for confounding caused by seasonality.
Data analysis.
We estimated the percent of the population receiving the MTAT intervention using a listing of all households in areas of the intervention and calculating the percent of households and individuals receiving the RDT and AL at the time of intervention. Baseline (2012) and follow-up (2013) study population and environmental characteristics of the intervention and control areas were compared using χ2 statistics to assess comparability and randomization balance (Table 1).
Table 1.
Baseline characteristics for children 1–59 months of age in intention-to-treat intervention and control sampled households
| Baseline (2012) | ||
|---|---|---|
| Intervention (N = 585) | Control (N = 226) | |
| Characteristic | % (95% CI) | % (95% CI) |
| Age in years | ||
| < 24 months | 56.6 (52.5–60.7) | 60.9 (56.9–64.7) |
| 24–59 months | 43.4 (39.3–47.5) | 39.2 (35.3–43.1) |
| Sex | ||
| Male | 48.5 (44.2–52.6) | 49.6 (45.0–54.1) |
| Female | 51.6 (47.4–55.8) | 50.4 (45.9–55.0) |
| Household wealth | ||
| Poorest | 32.1 (24.1–41.5) | 38.9 (25.7–54.1) |
| Middle | 33.9 (26.5–42.1) | 27.9 (19.8–37.7) |
| Richest | 34.0 (25.5–43.8) | 33.2 (21.5–47.4) |
| Household ≥ 1 ITN | 77.8 (70.3–83.8)* | 62.8 (54.6–70.4) |
| IRS in past 12 months | 44.4 (30.0–59.8)* | 15.0 (6.5–31.1) |
| Mean EVI (previous month) | 0.33 (0.31–0.35) | 0.35 (0.31–0.39) |
| Mean altitude (in meters) | 616.6 (546.4–686.9) | 573.4 (525.6.0–621.3) |
ITN = insecticide-treated mosquito nets; IRS = indoor residual spray; EVI = enhanced vegetation indices.
P < 0.05.
For the outcome of child malaria parasite prevalence, a logistic regression model was used to conduct a post-only comparison of malaria parasite prevalence between intervention and control groups after accounting for age, sex, household wealth, household ITN ownership, household IRS coverage, EVI, and altitude. Empirically estimated standard errors were used to account for correlated survey data at the PSU level. To account for potential contamination during campaign implementation wherein households targeted for MTAT did not receive it or vice versa, both intention-to-treat and per-protocol analyses were conducted. The intention-to-treat analysis defined a household as part of the MTAT intervention based on intervention planning maps, irrespective of whether the MTAT intervention was actually conducted in that area or not. The per-protocol analysis defined households exposed to MTAT if they were located within 100 m of recorded geo-coordinates taken of households receiving MTAT interventions in 2012, irrespective of intervention planning maps.
Confirmed outpatient case incidence at study health facilities was first compared descriptively between intervention and control groups (as monthly confirmed cases per 1,000 catchment population) and by season defined as wet (December–May) or dry (June–November). Because reporting of outpatient case incidence at study health facilities was imperfect, we calculated both raw and adjusted estimates of incidence for presentation of descriptive statistics by group and season. For adjusted estimates, we first calculated the percentage of reports received per month out of the total possible reports for each group or season; this percentage was weighted by average outpatient facility visits, so that the larger facilities carried more weight. Adjusted counts per month were then calculated by dividing the raw counts by this weighted reporting percentage. We then used random effects negative binomial regression to assess the impact of the MTAT intervention on both monthly confirmed and total malaria case incidence at the health facility level using a difference-in-differences (DiD) approach,40 with the primary effect estimate evaluated through a time (pre-post intervention period)—by a treatment group interaction term. Raw counts were used in this model, so some HFCA month values were missing. Monthly population estimates for each HFCA were included as the offset for incidence rates. The HFCA was included as a random effect in all models to account for unobserved heterogeneity and missing monthly counts. The pre-intervention period was categorized as January 2011–May 2012, and the post-intervention period was categorized as June 2012–May 2013. The DiD model additionally controlled for temporal autocorrelation through the inclusion of the previous months' cases, for seasonality with a categorical term for calendar month, and for differences in laboratory testing rates through monthly laboratory test counts at each facility.
Although the study was not powered to do so, an attempt was made to stratify all analyses by high (>10% initial prevalence) and low (≤ 10% initial prevalence) malaria transmission to assess whether malaria transmission modified the effectiveness of the MTAT intervention. Results are presented in the Supplement Appendix 1.
The study protocol was approved by the research ethics committees of the University of Zambia, PATH, and Tulane University and authorized by the MoH. Informed consent was obtained during each round of MTAT and during evaluation surveys.
Results
Baseline characteristics.
As measured by the 2012 MIS in the study area, just before the start of the MTAT intervention there was no difference between the intervention and control groups with respect to malaria parasite prevalence, EVI, altitude, sex ratio, child age, and household wealth (Table 1). Compared with control areas, intervention areas had higher vector control coverage in 2012, both in terms of household ownership of ≥ 1 ITN (78% versus 63%) and households being sprayed in the past 12 months with IRS (44% versus 15%, respectively).
MTAT intervention and RDT prevalence.
Between June and November in 2012 ∼85,000 individuals were tested three times for a malaria parasite infection in Gwembe, southern Kalomo, Siavonga, and Sinazongwe districts. Based on 92% of household members agreeing to participate in the MTAT intervention at each round, combined with MTAT intervention teams estimated to have reached 96% of targeted households, the MTAT intervention is estimated to have achieved a population coverage of 88% among the targeted population in MTAT designated areas.
The mean prevalence decreased substantially over each of the three MTAT rounds in 2012, which coincided with the progression of the dry season (Table 2). Starting from a mean prevalence of 23.2% at round 1, prevalence decreased to 10.9% at round 2, and 8.5% at round 3, representing a relative decrease of 60.5% in intervention areas over the course of the dry season in 2012. Over the course of 1 year, prevalence as measured during the MTAT interventions fell a relative 9.7%, from 23.2% in June 2012 to 20.9% in June 2013. This change in prevalence was not statistically significant (design-based χ2 = 0.70, P = 0.413).
Table 2.
Malaria infection prevalence as measured through RDTs during each round of MTAT
| District | Health facility catchment area | June–July 2012 | August–September 2012 | October–November 2012 | June–July 2013 | Absolute % change between June 2012 and June 2013 | Relative % change between June 2012 and June 2013 | Chi-square (P value) |
|---|---|---|---|---|---|---|---|---|
| Gwembe | Bbondo | 9.2% (7.9–10.9%) | 2.2% (1.7–2.8%) | 1.0% (0.7–1.4%) | 3.5% (2.9–4.2%) | −5.8% | −62.3% | 68.82 (< 0.001) |
| Chaambwe | 11.0% (8.9–13.7%) | 3.9% (3.2–4.7%) | 2.2% (1.7–2.8%) | 5.8% (5.1–6.7%) | −5.2% | −47.1% | 23.29 (< 0.001) | |
| Chabbobboma | 41.5% (40.5–42.6%) | 19.5% (18.5–20.5%) | 17.3% (16.5–18.2%) | 44.6% (43.5–45.8%) | +3.1% | +7.4% | 14.70 (< 0.001) | |
| Chipepo | 34.5% (32.6–36.4%) | 17.7% (16.0–19.6%) | 17.1% (15.7–18.7%) | 35.7% (34.9–37.5%) | +1.3% | +3.7% | 0.92 (0.338) | |
| Chisanga | 29.5% (27.4–31.8%) | 16.8% (14.8–19.1%) | 13.2% (11.2–15.6%) | NO DATA | NA | N/A | N/A | |
| Gwembe | 1.4% (1.0–2.0%) | 0.7% (0.5–1.2%) | 0.4% (0.2–0.7%) | 1.4% (1.0–2.0%) | 0.0% | 1.1% | 0.002 (0.965) | |
| Lukonde | 2.0% (1.5–2.7%) | 0.5% (0.3–0.9%) | 0.5% (0.3–0.9%) | 1.3 (1.0–1.8%) | −0.7% | −33.6% | 3.43 (0.064) | |
| Luumbo | 42.3% (38.7–45.9%) | 27.2% (25.8–28.7%) | 20.3% (19.0–21.6%) | 41.4% (40.0–42.8%) | −0.9% | −2.1% | 0.20 (0.654) | |
| Munyumbwe | 27.3% (26.3–28.4%) | 10.7% (10.0–11.5%) | 7.5% (6.9–8.1%) | 16.4% (15.5–17.3%) | −10.9% | −40.1% | 227.16 (< 0.001) | |
| Sinafala | 46.2% (44.7–47.8%) | 18.7% (17.4–20.0%) | 14.7% (13.6–15.8%) | 40.9% (39.4–42.4%) | −5.3% | −11.5% | 23.08 (< 0.001) | |
| Kalomo | Mapatizya | 5.2% (4.6–5.9%) | 4.1% (3.6–4.6%) | 2.2% (1.8–2.5%) | 3.4% (3.0–3.9%) | −1.8% | −34.87% | 24.19 (< 0.001) |
| Siavonga | Chipepo | 3.4% (3.0–3.8%) | 1.4% (1.1–1.7%) | 1.1% (0.9–1.4%) | 3.7% (3.3–4.2%) | +0.4% | +10.68% | 1.53 (0.216) |
| Kapululira | 2.8% (2.1–3.7%) | 3.3% (2.6–4.0%) | 0.9% (0.6–1.4%) | 3.3% (2.6–4.1%) | +0.5% | +17.3% | 0.82 (0.365) | |
| Lusitu | 3.3% (2.8–3.8%) | 1.7% (1.4–2.2%) | 1.4% (1.1–1.7%) | 4.7% (4.1–5.4%) | +1.4% | +42.4% | 11.93 (0.001) | |
| Mtendere | 5.8% (5.4–6.3%) | 3.4% (3.1–3.8%) | 2.4% (2.0–2.7%) | 6.2% (5.7–6.8%) | +0.4% | +6.4% | 1.07 (0.300) | |
| Sinazongwe | Chiyabi | 47.0% (45.1–48.8%) | 27.9% (26.3–29.5%) | 21.1% (19.5–22.7%) | 43.6% (42.0–45.2%) | −3.3% | −7.1% | 7.20 (0.007) |
| Sinamalima | 49.2% (48.3–50.2%) | 25.3% (24.5–26.2%) | 18.5% (17.8–19.2%) | 33.3% (32.4–34.2%) | −15.9% | −32.4% | 560.14 (< 0.001) | |
| Sulwegonde | 28.6% (27.1–30.0%) | 7.5% (6.7–8.4%) | 4.9% (4.2–5.6%) | 25.6% (24.3–27.0%) | −2.9% | −10.3% | 8.57 (0.003) | |
| Totals | 23.2% (13.3–37.2%) | 10.9% (6.3–18.3%) | 8.5% (4.9–14.4%) | 20.9% (13.3–31.2%) | −2.2% | −9.69% | 0.70 (0.413) |
RDTs = rapid diagnostic tests; MTAT = mass test and treatment.
Total statistics account for facility level clustering.
Malaria parasite prevalence.
During the high transmission season before the MTAT in 2012, malaria parasite prevalence in children 1–59 months of age was similar in the MTAT (34.5, 95% confidence interval [CI] = 26.0–44.2) and control (38.5%, 95% CI = 24.5–34.7) areas, with MTAT exposure defined by intention-to-treat (χ2 = 1.12, P = 0.66) or per-protocol (χ2 = 0.43, P = 0.77) (Table 3). During the high transmission season in 2013 after the MTAT intervention, the malaria parasite prevalence in children 1–59 months of age was significantly lower in MTAT areas (29.2%; 95% CI = 19.6–41.2%) as compared with control areas (44.0%; 95% CI = 34.8–53.8%), equating to a 53% decrease in the odds of a malaria parasite infection in MTAT areas (unadjusted crude odds ratio [OR]). After controlling for potential confounding factors in an intention-to-treat analysis this resulted in a significant decrease in the odds of a malaria parasite infection (Adjusted OR [AOR] = 0.47; 95% CI = 0.24–0.90) (Table 4). Per-protocol results were similar but did not reach statistical significance (AOR = 0.55, 95% CI = 0.29–1.06) (Table 5). Results from the analysis stratified by high and low transmission are presented in Supplement Appendix 1.
Table 3.
Baseline and follow-up malaria parasite prevalence among children 1–59 months of age, as measured by the baseline and follow-up household surveys during the peak malaria transmission season
| Malaria parasite prevalence in children 1–59 months of age | Baseline (2012) | Follow-up (2013) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | RDT Positive | % (95% CI) | Crude odds ratio | n | RDT positive | % (95% CI) | Crude odds ratio | |
| Intervention | 585 | 202 | 34.5 (26.0–44.2) | 0.84 (0.61–1.16) | 513 | 150 | 29.2 (19.6–41.2) | 0.53 (0.41–0.68)* |
| Control | 226 | 87 | 38.5 (24.5–54.7) | 511 | 225 | 44.0 (34.8–53.8) | ||
RDT = rapid diagnostic test; CI = confidence interval.
Standard errors for crude odds ratios are unadjusted to account for cluster survey sampling.
P < 0.01.
Table 4.
Adjusted intention-to-treat analysis of malaria parasite prevalence in children 1–59 months of age comparing intervention areas during the peak malaria transmission season after the MTAT intervention (N = 1,024)*
| Factor | Coefficient | OR (95% CI) | P value |
|---|---|---|---|
| Group | Control | Reference | |
| Intervention | 0.47 (0.24–0.90) | 0.022 | |
| Age | < 24 months | Reference | |
| ≥ 24 months | 1.70 (1.36–2.14) | < 0.001 | |
| Sex | Male | Reference | |
| Female | 1.18 (0.90–1.55) | 0.225 | |
| Wealth | Poorest | Reference | |
| Middle | 0.93 (0.59–1.47) | 0.757 | |
| Richest | 0.76 (0.46–1.26) | 0.286 | |
| ITN ownership | No ITN | Reference | |
| ≥ 1 ITN | 1.14 (0.73–1.77) | 0.550 | |
| IRS coverage | No IRS | Reference | |
| IRS | 0.99 (0.57–1.73) | 0.980 | |
| Vegetation index | Low EVI | Reference | |
| High EVI | 1.05 (0.65–1.70) | 0.837 | |
| Altitude | High altitude | Reference | |
| Low altitude | 2.40 (1.27–4.55) | 0.008 |
MTAT = mass test and treatment; CI = confidence interval; ITN = insecticide-treated mosquito net; IRS = indoor residual spray.
Standard errors are adjusted to account for survey cluster sampling.
Table 5.
Adjusted per protocol analysis of malaria parasite prevalence in children 1–59 months of age comparing intervention areas during the peak malaria transmission season after the MTAT intervention (N = 1,024)*
| Factor | Coefficient | OR (95% CI) | P value |
|---|---|---|---|
| Group | Control | Reference | |
| Intervention | 0.55 (0.29–1.06) | 0.072 | |
| Age | < 24 months | Reference | |
| ≥ 24 months | 1.69 (1.35–2.14) | < 0.001 | |
| Sex | Male | Reference | |
| Female | 1.18 (0.90–1.55) | 0.218 | |
| Wealth | Poorest | Reference | |
| Middle | 0.94 (0.60–1.48) | 0.794 | |
| Richest | 0.81 (0.48–1.37) | 0.429 | |
| ITN ownership | No ITN | Reference | |
| ≥ 1 ITN | 1.13 (0.73–1.74) | 0.588 | |
| IRS coverage | No IRS | Reference | |
| IRS | 0.98 (0.56–1.73) | 0.954 | |
| Vegetation index | Low EVI | Reference | |
| High EVI | 1.06 (0.66–1.71) | 0.807 | |
| Altitude | High altitude | Reference | |
| Low altitude | 2.33 (1.23–4.43) | 0.011 |
MTAT = mass test and treatment; OR = odds ratio; ITN = insecticide-treated mosquito net; IRS = indoor residual spray.
Standard errors are adjusted to account for survey cluster sampling.
HMIS-based outpatient malaria case incidence.
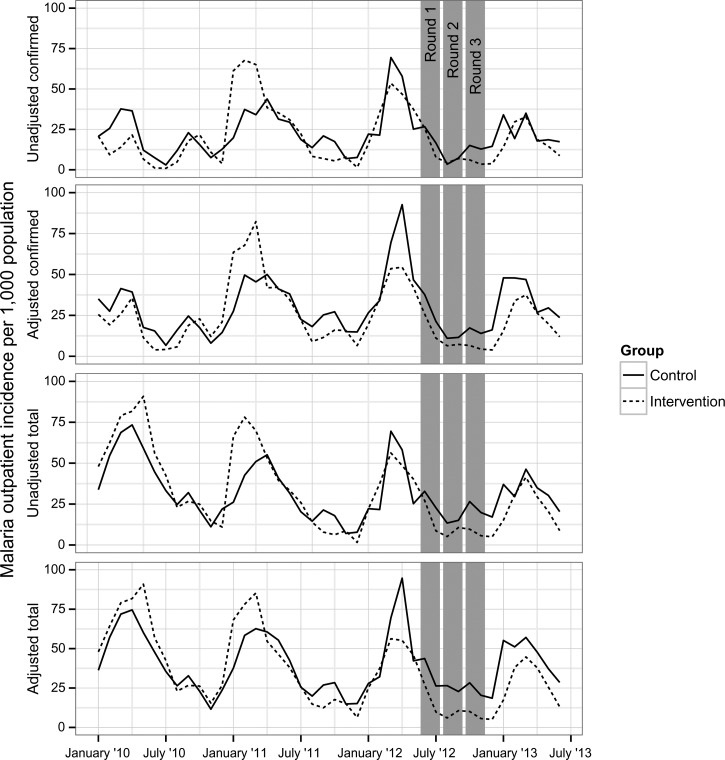
Unadjusted monthly confirmed case incidence in the preintervention period was 33.4 per 1,000 catchment population in control areas and 36.2 per 1,000 catchment population in intervention areas. Unadjusted monthly confirmed case incidence in the post-intervention period was 19.2 per 1,000 catchment population in control areas and 13.9 per 1,000 catchment population in intervention areas (Table 6). Based on the intention-to-treat analysis following the three dry-season rounds of MTAT in 2012, the seasonal peak of malaria incidence appeared to be mitigated in MTAT areas as measured by the monthly HMIS-derived total (suspected and confirmed) and confirmed outpatient case incidence (Figure 2 ). Unadjusted confirmed malaria case incidence fell in both the MTAT (Incidence rate ratio [IRR] = 0.69; 95% CI = 0.60–0.79) and control (IRR = 0.75; 95% CI = 0.66–0.85) areas between pre- and post-intervention time periods (Table 7). However, from the interaction term (pre-post intervention by treatment group), total outpatient malaria case incidence decreased significantly more in the areas that received the MTAT rounds compared with the contemporaneous control group (IRR = 0.65; 95% CI = 0.54–0.78), and marginally so for confirmed cases (IRR = 0.83; 95% CI = 0.68–1.01). This resulted in total and confirmed malaria case incidence decreasing in the MTAT intervention area by 35% (95% CI = 22–46%) and 17% (95% CI = −0.01 to 32%) more than control areas between the pre- and post-intervention periods, respectively. Results from the analysis stratified by high and low transmission are presented in Supplement Appendix 1.
Table 6.
Baseline and follow-up confirmed and total malaria case incidence as measured by facility health management information system data
| Baseline (January 2011–May 2012) | Follow-up (June 2012–May 2013) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total person-months* | HMIS-reported-cases | Monthly rate/1,000 | Crude IRR | Total person-months* | HMIS-reported cases | Monthly rate/1,000 | Crude IRR | |
| Confirmed malaria case incidence rate per 1,000 population | ||||||||
| Intervention | 1,859,775 | 67,313 | 36.2 | 1.08 (1.07–1.10) | 1,582,108 | 22,014 | 13.9 | 0.72 (0.71–0.73)† |
| Control | 2,018,386 | 67,407 | 33.4 | 1,712,697 | 32,940 | 19.2 | ||
| Total malaria case incidence rate per 1,000 population | ||||||||
| Intervention | 1,859,775 | 75,855 | 40.8 | 1.09 (1.08–1.11) | 1,582,108 | 27,140 | 17.2 | 0.61 (0.60–0.62)† |
| Control | 2,018,386 | 75,283 | 37.3 | 1,712,697 | 47,956 | 28.0 | ||
HMIS = health management information system; IRR = incidence rate ratio.
Standard errors for crude incident rate ratios are unadjusted to account for clustering at the health facility level.
Based on mid-year populations of health facility catchment areas.
P < 0.01.
Figure 2.
Intervention group incidence per 1,000 population with raw total suspected and confirmed cases, and total suspected and confirmed cases adjusted for reporting. Adjusted rates were computed by dividing raw rates by the percent of facilities reporting in a given month, weighted by facility size, and are therefore an indicator of data completeness.
Table 7.
Negative binomial regression analysis on monthly outpatient laboratory-confirmed malaria cases (either RDT or microscopy) and total malaria cases (both confirmed and clinical, intervention and control difference-in-difference analysis, 2011–2013*
| Factor | Coefficient | Confirmed malaria cases | Total malaria cases |
|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | ||
| Intervention group | Control | Reference | Reference |
| Intervention | 0.86 (0.67–1.11) | 0.92 (0.73–1.17) | |
| P = 0.257 | P = 0.556 | ||
| Time period | Pre-intervention | Reference | Reference |
| Post-intervention (after May 2012) | 0.89 (0.78–1.01) | 1.09 (0.97–1.22) | |
| P = 0.064 | P = 0.145 | ||
| Interaction | Time by intervention group interaction (DiD) | 0.83 (0.68–1.01) | 0.65 (0.54–0.78) |
| P = 0.072 | P < 0.001 |
RDT = rapid diagnostic test; IRR = incidence rate ratio; DiD = difference-in-differences.
Model controls for laboratory testing rates, previous months' incidence and calendar month (not shown).
Discussion
We conducted a community randomized controlled trial of three dry-season rounds of an MTAT intervention using RDTs and AL in Southern Province Zambia in 2012, an area of heterogeneous endemic malaria transmission and high sustained vector control coverage. The MTAT intervention was shown to have a significant but overall modest impact on decreasing the malaria infection burden in this area, as compared with a contemporaneous control group. The 2012 high season malaria infection prevalence was very similar in MTAT and control areas pre-MTAT (34.5% and 38.5%, respectively). Following the MTAT intervention, during the high transmission season in 2013, malaria infection prevalence was significantly lower in MTAT areas (29.2%), compared with control areas (44.0%). Outpatient total suspected and confirmed malaria cases incidence decreased by 35% and 17% more in the MTAT areas as compared with control areas, respectively. Although these analyses showed an effect of the MTAT intervention on malaria infection burden, the infection reduction was not sufficient to allow health facility staff to transition to an aggressive active case investigation intervention for further transmission containment and eventual elimination.
These results stand in slight contrast to a community randomized controlled trial from Burkina Faso that found no protective benefit of MTAT with AL on malaria incidence.41 This may have been caused by a number of factors, including higher malaria transmission in the Burkina Faso study area where dry season prevalence before MTAT implementation was estimated at 45% compared with our study in Zambia with dry season prevalence before MTAT implementation was estimated at 21.5%. The MTAT intensity and coverage as well as antimalarial adherence may have also led to differences in the outcomes of the two studies.
The reduction in malaria incidence and prevalence after a year of MTAT was observed despite a number of limitations in the design and implementation of the intervention. First, the RDTs used in the MTAT likely missed a substantial proportion of low density infections, leaving them untreated and allowing them to contribute to on-going transmission in the area.42,43 Second, AL was chosen for use in the 2012 and 2013 MTAT campaigns because it is national policy in Zambia for treating uncomplicated malaria. Although treatment with ACTs reduces immature gametocyte carriage, AL does not affect mature gametocytes, which can contribute to onward transmission from those treated individuals.20 Additionally, because of its very short half-life AL provides limited chemoprophylactic protection against reinfection.44 Third, the MTAT campaigns were estimated to have reached only 88% of the target population, whereas simultaneous qualitative investigations into AL adherence suggest that many individuals may not have completed a full 6-dose course of the drug (Silumbe and others, submitted for publication). Considering these three factors, if one assumes the 2012 MTAT achieved 88% population coverage, with perhaps 75% adherence to the full AL course among those reached by the MTAT campaign, and combined with the fact that RDTs may have missed up to 50% of infections, we surmise that as much as 67% of the infectious reservoir in the community may have been missed by the 2012 MTAT. This low level of effective coverage means that an insufficient proportion of the malaria parasite reservoir in the human population was reached to substantially reduce malaria transmission.23,24
We propose several ways to improve mass treatment interventions to more effectively reduce the reservoir of malaria parasites in the human population and reduce transmission. First, using an MDA approach, where individuals are provided an antimalarial irrespective of the results of a malaria diagnostic, may potentially circumvent challenges with diagnostic sensitivity and treat a larger portion of the parasite reservoir. Second, alternatives to AL are needed. Piperaquine has a much longer half-life than lumefantrine, up to 1 month, and therefore may potentially provide much better chemoprophylactic protection against reinfection, although still offering similar parasite clearance as artemether when combined with dihydro-artemesinin.45 If proven safe, other drugs such as single low dose primaquine, tafenoquine, and methylene blue could potentially provide immediate reductions in onward transmission among treated individuals as a result of their effect on mature gametocytes.46,47 Third, effective coverage of mass treatment campaigns must improve. Greater efforts must be made to reach targeted households through better training of field staff, improved mapping of target populations, and better sensitization of target communities to maximize participation. Although extremely labor-intensive and logistically difficult, future mass treatment campaigns should consider using directly observed therapy of all antimalarial courses to maximize adherence and parasite clearance. Shifting to a drug with a less complicated dosing regimen would also likely improve treatment compliance.
This study has a number of important limitations. First, significant differences in both ITN and IRS coverage between intervention and control areas were present before the study began. These differences occurred by chance likely because of the relatively small number of randomization units. We've attempted to account for these differences with covariates in the parasite prevalence models; a restricted randomization would have been a more robust design. Second, qualitative interviews with community members suggest fever treatment seeking may have decreased among those that received AL through the MTAT rounds because treatment doses were saved for future use, especially among those with no malaria symptoms during the MTAT (Silumbe and others, submitted for publication). Any decrease in fever treatment seeking in MTAT communities after the intervention may have led to an erroneous underestimate of suspected and confirmed malaria cases seen at local health facilities, in relation to HMIS trends observed pre-MTAT and in control areas post-MTAT, and therefore biased results away from the null in the analysis of incidence. Third, contamination in MTAT exposure likely occurred as a result of MTAT field workers unintentionally including some control households and excluding some MTAT households during the campaigns. The extent of potential contamination is not known, but we attempted to mitigate this error through running dual analyses with MTAT exposure dichotomized by both intention-to-treat and per-protocol methodology. Fourth, misclassification error in MTAT exposure may have resulted from individuals in MTAT and control HFCA seeking care for fevers in neighboring health centers in different randomization groups, which would have affected the analysis of health facility malaria case incidence. We surmise that both types of bias in MTAT exposure were limited compared with the overall large samples and large randomization areas, and although they might both lead to a bias toward the null hypothesis, such a bias is expected to be small. Fifth, a relatively large pilot conducted in eight HFCAs in late 2011 may have decreased the difference between health facility trends and parasite prevalence measured before and after the intervention started in June 2012. Sixth, the incidence data could not be adjusted for the imbalance in IRS at baseline caused by insufficient data on IRS coverage in each HFCA. Seventh, it is possible that RDT sensitivity decreased more in the MTAT group compared with the control group during the post-intervention follow-up survey. However, as parasite prevalence remained relatively high in both groups before and after the MTAT rounds, we feel such differences in RDT sensitivity would have had little, if any, effect on these results. And finally, the outcome of health facility incidence used in this study is subject to a number of issues including non-reporting and inconsistent testing of suspected malaria cases. In this area of Zambia the health facility reporting has been greatly strengthened through repeated trainings and feedback from the NMCC, giving greater confidence in the numbers reported than would perhaps be the case in other parts of the country. Furthermore, we have attempted to account for differences in testing and reporting rates in the analysis.
These data show that three rounds of dry-season MTAT with RDTs and AL were effective at reducing the malaria infection burden as measured through malaria parasite prevalence and health facility incidence. After a single year of the MTAT in the context of moderate malaria transmission, results showed that few health HFCAs included in the MTAT intervention reduced transmission sufficiently to switch from an MTAT strategy to a strategy of aggressive active case investigations. Improvements to interventions targeting the malaria parasite reservoir in the human population can be made. If the strategy must rely on diagnostic testing, more sensitive diagnostics capable of timely point-of-care results are needed to identify low density infections. Drugs, ideally with simple dosing regimens (preferably single-dose, but possibly with few doses), that are safe, effective at clearing asexual and sexual stage parasites, and that provide prophylactic protection against new infections are also needed. And finally, these results suggest MTAT strategies will likely work better in very low transmission settings, given that high coverage can be achieved.
Supplementary Material
ACKNOWLEDGMENTS
We thank the community of Southern Province Zambia for their participation in this research. The Zambian Ministry of Health, National Malaria Control Centre, and the Southern Province Provincial and District Health Management Teams are thanked for the support and dedicated work on this project. Feiko ter Kuile is thanked for his comments on earlier drafts of the manuscript.
Footnotes
Financial support: This research was funded by the Malaria Control and Evaluation Partnership in Africa (MACEPA), a PATH project, with funding from the Bill and Melinda Gates Foundation.
Authors' addresses: David A. Larsen, Department of Public Health, Food Studies and Nutrition, Syracuse University David B Falk College of Sport and Human Dynamics, Syracuse, NY, E-mail: dalarsen@syr.edu. Adam Bennett, Malaria Elimination Initiative, Global Health Group, University of California, San Francisco, CA, E-mail: bennetta@globalhealth.ucsf.edu. Kafula Silumbe, Megan Littrell, John M. Miller, and Richard W. Steketee, Malaria Control and Evaluation Partnership, a Program at PATH, Seattle, WA, E-mails: ksilumbe@path.org, mlittrell@psi.org, jmiller@path.org, and rsteketee@path.org. Busiku Hamainza, National Malaria Control Programme, Zambia Ministry of Health, Lusaka, Zambia, E-mail: bossbusk@gmail.com. Joshua O. Yukich, Joseph Keating, and Thomas P. Eisele, Center for Applied Malaria Research and Evaluation (CAMRE), Tulane University School of Public Health and Tropical Medicine, New Orleans, LA, E-mails: jyukich@tulane.edu, jkeating@tulane.edu, and teisele@tulane.edu.
References
- 1.Nájera JA. Malaria control: achievements, problems and strategies. Parassitologia. 2001;43:1–89. [PubMed] [Google Scholar]
- 2.Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med. 2005;60:563–573. doi: 10.1016/j.socscimed.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Ogutu B, Tiono A, Makanga M, Premji Z, Gbadoe A, Ubben D, Marrast A, Gaye O. Treatment of asymptomatic carriers with artemether-lumefantrine: an opportunity to reduce the burden of malaria? Malar J. 2010;9:30. doi: 10.1186/1475-2875-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GAT. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22:424–430. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sama W, Killeen G, Smith T. Estimating the duration of Plasmodium falciparum infection from trials of indoor residual spraying. Am J Trop Med Hyg. 2004;70:625–634. [PubMed] [Google Scholar]
- 7.Abdel-Wahab A, Abdel-Muhsin A-MA, Ali E, Suleiman S, Ahmed S, Walliker D, Babiker HA. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J Infect Dis. 2002;185:1838–1842. doi: 10.1086/340638. [DOI] [PubMed] [Google Scholar]
- 8.Nassir E, Abdel-Muhsin A-MA, Suliaman S, Kenyon F, Kheir A, Geha H, Ferguson HM, Walliker D, Babiker HA. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol. 2005;35:49–55. doi: 10.1016/j.ijpara.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 10.Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA. Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans R Soc Trop Med Hyg. 2000;94:472–476. doi: 10.1016/s0035-9203(00)90056-7. [DOI] [PubMed] [Google Scholar]
- 11.Muirhead-Thomson RC. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae); a random xenodiagnostic survey. Am J Trop Med Hyg. 1957;6:971–979. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- 12.Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouagna LC, Ferguson HM, Okech BA, Killeen GF, Kabiru EW, Beier JC, Githure JI, Yan G. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in western Kenya. Parasitology. 2004;128:235–243. doi: 10.1017/s003118200300444x. [DOI] [PubMed] [Google Scholar]
- 14.Schneider P, Bousema J, Gouagna L, Otieno S, van de Vegte-Bolmer M, Omar S, Sauerwein R. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–474. [PubMed] [Google Scholar]
- 15.Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, Zollner G, Sattabongkot J. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol. 2004;41:201–208. doi: 10.1603/0022-2585-41.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Ouedraogo A, Bousema T, Schneider P, de Vlas S, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nebie I, Roeffen W, Verhave J, Luty A, Sauerwein R. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE. 2009;4:e8410. doi: 10.1371/journal.pone.0008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidlein von L, Greenwood BM. Mass administrations of antimalarial drugs. Trends Parasitol. 2003;19:452–460. doi: 10.1016/j.pt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.MalERA Consultative Group on Diagnosis and Diagnostics, 2011. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 8:e1000396. doi: 10.1371/journal.pmed.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NP, Coleman RE, White NJ. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother. 2006;50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatton ML, Cheng Q. Interrupting malaria transmission: quantifying the impact of interventions in regions of low to moderate transmission. PLoS ONE. 2010;5:e15149. doi: 10.1371/journal.pone.0015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basáñez M-G, Ghani AC. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE. 2011;6:e20179. doi: 10.1371/journal.pone.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern SE, Tiono AB, Makanga M, Gbadoe A, Premji Z, Gaye O, Sagara I, Ubben D, Cousin M, Oladiran F, Sander O, Ogutu B. Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modeling and simulation analysis. Malar J. 2011;10:210. doi: 10.1186/1475-2875-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambia Ministry of Health, National Malaria Control Centre . National Malaria Strategic Plan 2011–2015. Lusaka, Zambia: Ministry of Health; 2011. [Google Scholar]
- 27.Zambia Ministry of Health . Zambia National Malaria Indicator Survey. Lusaka, Zambia: Ministry of Health; 2006. [Google Scholar]
- 28.Zambia Ministry of Health . Zambia National Malaria Indicator Survey. Lusaka, Zambia: Ministry of Health; 2008. [Google Scholar]
- 29.Zambia Ministry of Health . Zambia National Malaria Indicator Survey. Lusaka, Zambia: Ministry of Health; 2010. [Google Scholar]
- 30.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 31.Mdege ND, Man M-S, Taylor Nee Brown CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. J Clin Epidemiol. 2011;64:935–948. doi: 10.1016/j.jclinepi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roll Back Malaria Partnership and World Health Organization Malaria Indicator Survey (MIS) Toolkit. 2013. http://www.rbm.who.int/toolbox/tool_MISToolkit.html Available at. Accessed August 6, 2013.
- 35.Hay SI, Snow RW, Rogers DJ. From predicting mosquito habitat to malaria seasons using remotely sensed data: practice, problems and perspectives. Parasitol Today. 1998;14:306–313. doi: 10.1016/s0169-4758(98)01285-x. [DOI] [PubMed] [Google Scholar]
- 36.Hijmans RJ. Introduction to the“raster” package (version 2.0-08) 2012 [Google Scholar]
- 37.Hijmans RJ, van Etten J. Raster: geographic analysis and modeling with raster data 2012 [Google Scholar]
- 38.R Development Core Team R: A Language and Environment for Statistical Computing. 2010. http://wwwR-projectorg/ Available at. Accessed August 1, 2013.
- 39.Rutstein S, Johnson K. The DHS Wealth Index. 2004. [Google Scholar]
- 40.Angrist JD, Pischke J-S. Mostly Harmless Econometrics. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 41.Tiono AB, Ouédraogo A, Ogutu B, Diarra A, Coulibaly S, Gansané A, Sirima SB, O'Neil G, Mukhopadhyay A, Hamed KA. Controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J. 2013;12:79. doi: 10.1186/1475-2875-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237–1239. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouatcho JC, Goldring JPD. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–1505. doi: 10.1099/jmm.0.052506-0. [DOI] [PubMed] [Google Scholar]
- 44.Ashley EA, Stepniewska K, Lindegardh N, McGready R, Annerberg A, Hutagalung R, Singtoroj T, Hla G, Brockman A, Proux S, Wilahphaingern J, Singhasivanon P, White NJ, Nosten F. Pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop Med Int Health. 2007;12:201–208. doi: 10.1111/j.1365-3156.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- 45.Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009:CD007483. doi: 10.1002/14651858.CD007483.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA. 2011;108:e1214–e1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White NJ, Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J. 2012;11:418. doi: 10.1186/1475-2875-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.