Abstract
Malaria is a leading cause of pediatric mortality, and Uganda has among the highest incidences in the world. Increased morbidity and mortality are associated with delays to care. This qualitative study sought to characterize barriers to prompt allopathic care for children hospitalized with severe malaria in the endemic region of southwestern Uganda. Minimally structured, qualitative interviews were conducted with guardians of children admitted to a regional hospital with severe malaria. Using an inductive and content analytic approach, transcripts were analyzed to identify and define categories that explain delayed care. These categories represented two broad themes: sociocultural and structural factors. Sociocultural factors were 1) interviewee's distinctions of “traditional” versus “hospital” illnesses, which were mutually exclusive and 2) generational conflict, where deference to one's elders, who recommended traditional medicine, was expected. Structural factors were 1) inadequate distribution of health-care resources, 2) impoverishment limiting escalation of care, and 3) financial impact of illness on household economies. These factors perpetuate a cycle of illness, debt, and poverty consistent with a model of structural violence. Our findings inform a number of potential interventions that could alleviate the burden of this preventable, but often fatal, illness. Such interventions could be beneficial in similarly endemic, low-resource settings.
Introduction
Malaria is a leading cause of child mortality, responsible for approximately 1 million pediatric deaths each year. Uganda has among the highest incidences of malaria in the world; approximately 70,000 to 100,000 children die of this infection annually.1–4 Severe malaria infection has a pediatric mortality rate as high as 21%. Although artesunate therapy has been shown to significantly improve clinical outcomes, the use of this medication is not yet widespread in Uganda. Despite this optimal pharmaceutical treatment, severe and cerebral malaria still carry a mortality rate between 8.5% and 18%, respectively.5 Adjuvant therapies have been trialed, but none have been proven effective.6 Therefore, medications alone cannot address the significant impact of this preventable disease.
The lethal nature of severe malaria in children has been attributed in part to late presentation to health-care facilities. Delays to care are dangerous as this disease can progress rapidly over hours. A 2007 retrospective review of children hospitalized in western Kenya showed that 78% deaths from severe malaria occurred within 24 hours of hospital admission.7 Despite the rapidly progressive nature of this disease, delays to care are quite common. In 2000, the Ugandan Ministry of Health estimated that fewer than 8% guardians with children under 5 years of age sought allopathic care for malaria treatment within 24 hours of symptom onset.8 Other studies in Uganda and sub-Saharan Africa have shown similar patterns of delayed treatment-seeking behavior.9–12 Such delays to definitive care could result from the fact that malaria symptoms may be interpreted as reflecting “traditional illnesses,” caused by bewitching or family curses, for which homeopathic or spiritual remedies are sought first.12
In a low-resource setting, such as rural southwestern Uganda, many other factors may impede appropriate care. A 2010 epidemiological survey of the region showed decreasing burden of malaria in urban districts, but stable rates of infection in rural areas.13 In other rural regions of Africa, studies have indicated that economic and food insecurity, lack of access to transportation, and unequal distribution of health resources delay testing, diagnosis, and treatment of children with malaria.14–16 Such data underscore the discrepant global burden of this disease, which falls squarely upon the world's most vulnerable populations.17
It is clear that delayed diagnosis and treatment increase the risk of morbidity and mortality from severe malaria infection. Researches in other regions of Africa have highlighted several social and economic forces that have contributed to such delays; however, the scope and impact of these variables are not well understood, particularly as such forces can structure health-care-seeking behavior and limit access to health-care resources. To effectively develop interventions aimed at reducing the burden of this preventable disease, more research is needed to characterize the factors that frame treatment seeking and access to appropriate care in low-resource, malaria-endemic settings. To this end, we conducted a qualitative study aimed at identifying factors that explain delays to timely treatment of malaria among children in southwestern Uganda.
Methods
Study design.
Qualitative data collection was undertaken via minimally structured, face-to-face interviews with guardians of children 12 years of age or younger admitted to a regional referral hospital with some form of severe malaria. Given the resource-limited hospital context, we could not strictly adhere to the detailed laboratory guidelines used by the World Health Organization in the definition of severe malaria, which, for example, includes measures of renal function and arterial pH.18 Instead, we relied on a combination of clinical and laboratory findings available in our treatment setting and developed the following modified definition of severe malaria: confirmed Plasmodium parasitemia on peripheral smear with any of the following: 1) altered mental status; 2) convulsions; 3) severe anemia (hemoglobin < 5 g/dL, or symptomatic anemia requiring blood transfusion at the discretion of attending clinician); or 4) respiratory distress. We acknowledge that this approach does not specifically exclude alternative causes of illness or coinfections such as pneumonia or bacteremia, and that many children in this region have chronic parasitemia. However, these children are ostensibly hospitalized for severe malaria, based on their clinical condition and a positive blood smear, and receive treatment accordingly. As our study sought to identify factors causing delays to care, rather than treat or diagnose malaria itself, we did not believe that use of this liberal clinical definition would undermine our results.
The goal of the study was to better understand the factors contributing to delays in care for children hospitalized with severe malaria in southwestern Uganda. The study had three specific objectives: 1) to understand the nature of cultural and/or social factors that delay or impede timely treatment of malaria in children, 2) to characterize structural or economic barriers guardians faced when guardians attempted to escalate care to allopathic providers, and 3) to characterize the financial impact of illness on families with children hospitalized with severe malaria.
The research was approved by the Institutional Review Board, Partners Healthcare/Massachusetts General Hospital, Boston, MA; the Institutional Ethical Review Committee, Mbarara University of Science and Technology, Mbarara, Uganda; and the Ugandan National Council for Science and Technology, Kampala, Uganda.
Study setting.
Participants were enrolled at the general pediatrics ward of the Mbarara Regional Referral Hospital, a teaching hospital located approximately 250 km southwest of the Ugandan capital city of Kampala. The ward has an annual census of approximately 8,000 children. Approximately 600 children (7.5% of all admissions) are admitted each year for severe malaria infection. Annually, one-third of the children admitted to the ward test positive for malaria parasites on peripheral smear. The region surrounding Mbarara has a tropical climate, with bimodal rainfall, averaging 1,200 mm annually and occurring primarily between the months of September through January, and again from March through May. Admissions for severe malaria infection tend to peak during these rainy months.
Sampling, recruitment, and enrollment.
We used a purposive sampling strategy to guide enrollment, which sought to identify “information-rich” cases: individuals having knowledge and experience with our topics of interest.19 Guardians of the children admitted for severe malaria were therefore identified as the focus of recruitment, as they could speak directly to the process of seeking care, and the factors contributing to delays in care for their children. Guardians were eligible for participation in the study if they were aged 18 years or older, self-identified as the primary guardian of the admitted child, and were able to provide informed consent in one of the two languages spoken by study staff (Runyankole, the local language, and English). No remuneration was provided for those who chose to participate. Four hundred and twenty two guardians were eligible for inclusion between November 2013 and March 2014. Of them, 79 were approached for inclusion. The small percentage of those enrolled compared with those eligible for participation (18%) was due to an a priori cap placed on the number of interviews conducted per day to provide adequate time for translation, transcription, and quality review following each interview (see “Ensuring data quality” section below).
Four coauthors (Harriet Adrama, Jackline Tumuhairwe, Sheilla Mbabazi, and Kenneth Mworozi) carried out recruitment and enrollment on a rolling basis. Eligible participants were recruited only if their child's medical condition was stable or improving, to avoid adding stress to guardians facing potential death of their child. Eligible guardians were not recruited if 1) child was discharged before study staff was able to recruit the guardian; 2) numerous eligible guardians were present on the ward at the same time, and not enough study staff were available to recruit them; or 3) the admitted child expired or had a complicated hospital course. The recruitment process went as follows: a coauthor approached a potential participant on the pediatric ward, introduced himself/herself, provided background information on the purpose of the study, and inquired if the individual was interested in participating. If the individual expressed interest, then he/she was enrolled.
Enrollment generally took place on the same day as recruitment and consisted of informed written consent followed by a 1-hour long interview. Of the 79 guardians approached for participation, three declined to participate. One guardian was not enrolled because she did not speak one of the languages used for consent and interview. A total of 75 guardians were enrolled. In cases where the potential participant had limited literacy, consent forms were read aloud. Understanding of the consent process was verified with a questionnaire for all potential participants. The enrollment process and interviews were conducted in a private room, located down the hall from the pediatric ward.
The process of recruitment, enrollment, interview, translation, transcription, and quality review typically spanned 2–5 days from beginning to completion. This entire process was completed before a study staff member recruited another participant for enrollment. Qualitative data analysis was ongoing throughout the study period, as transcripts were reviewed for content in real time (see “Data analysis” section below). Enrollment was continued until data saturation was reached. While complete content saturation is realistically impossible, the concept of data saturation describes the point when interviews no longer reveal substantial new or relevant information, and analytical categories are sufficiently well developed based on content present in completed interviews.20 Following review of 75 interview transcripts, enrollment in the study was stopped.
Data collection.
Interviews were conducted between November 2013 and March 2014 by four of the coauthors who worked full-time in the region, had familiarity with local customs and cultural norms, and were fluent in the two languages used in this study. These researchers were trained in qualitative methods as well as ethical standards of human subjects research. Interviews were conducted in either Runyankole or English, based on the preference of the participant. To ensure reliability among interviewers, an interview guide was followed to facilitate consistent focus on the following core topics: 1) guardian knowledge of pediatric malaria symptoms; 2) course of the child's illness; 3) perceived barriers to the child's allopathic care; and 4) financial costs of the illness. Interviewers were encouraged to tailor questions to the responses provided by each participant. Interviews were audio recorded, and lasted approximately 1 hour. These were subsequently translated and transcribed into English by the interviewer.
Ensuring data quality.
The integrity of translations was ensured by review of transcripts by one of the coauthors (Juliet Mwanga-Amumpaire) who was fluent in both languages used in the study. She reviewed all English transcripts for potential translation errors and cross-checked transcripts with the original interview recordings in cases where she felt that original meaning may have been compromised by translation. Transcripts were also monitored by the primary author for clarity, attention to detail, grammar, and style. To ensure the highest quality study data, study staff translated and transcribed interviews within 24 hours of completion. These were reviewed by the primary author within 48 hours of transcript completion.
Data analysis.
Data were analyzed using an inductive approach to category construction and a content analysis framework.21 An inductive approach is one in which findings emerge through iteration, or repeated engagement, with a dataset. In this study, interview transcripts were reviewed for content pertaining to barriers to prompt treatment of children with malaria, and on the cost of illness borne by guardians and their families. On the basis of this content, a coding system was developed that represented common topics encountered in the transcript review. Codes were sharpened and refined throughout the data analysis period, as new transcripts were analyzed shortly after interview completion. This process continued until new interview data did not alter the definition or scope of the codes. Then, using this final set of codes, all interviews were re-coded. NVivo 10 software (QRS International Pty Ltd, Victoria, Australia) was used to organize study data, but not in the generation of codes.
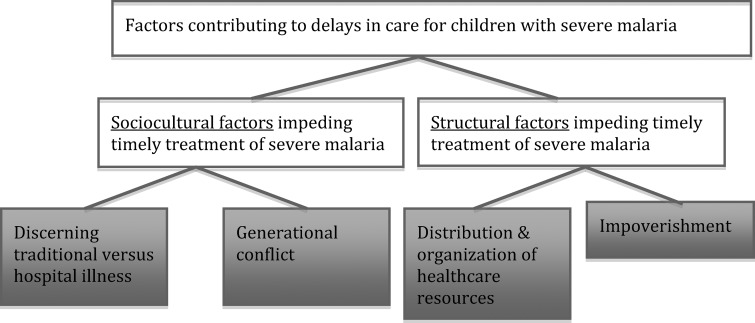
Content analysis involved a process of data reduction where core themes were identified within the dataset. Using this process, coded data were scrutinized, grouped, and re-grouped in an effort to elucidate patterns that might indicate an underlying theme or explanation for barriers to prompt treatment of children with severe malaria. Through this process, descriptive categories were developed to characterize factors contributing to delays in care for these children. These categories were “named” to describe a basis of explanation for the observed phenomenon. These categories were revised, elaborated, refined, and validated through data triangulation, where multiple interview sources were considered to develop a robust understanding of the phenomenon of interest. Example(s) from the dataset were selected that best illustrate the category. Four categories were developed and are presented here under two broad themes: 1) sociocultural factors and 2) structural factors that contribute to delayed treatment of children with severe malaria (see Figure 1).
Figure 1.
Schematic of results: themes and categories discussed in the text.
Results
Characteristics of the sample.
Our study included 75 participants; 76% (N = 57) of these individuals were female. Ages ranged from 18 to 60 years, with a mean age of 31 years. Average household size was 6.2 persons. Families came to Mbarara Regional Referral Hospital from the surrounding area of southwestern Uganda, ranging from 3 to 125 km away. Average monthly household income was 187,520 Ugandan shillings (UGX; approximately US$72). Highest level of education ranged from none to technical school graduate. Further details of these characteristics are provided in Table 1. The majority of guardians enrolled in the study were mothers (N = 54) or fathers (N = 17), but aunts (N = 1), uncles (N = 1), and grandmothers (N = 2) were also included.
Table 1.
Characteristics of study participants
| Female gender | 76% (N = 57) |
| Age (in years, range; mean) | 18–60; 31.1 |
| Household size (in persons, range; mean) | 3–14; 6.2 |
| Distance of residence from Mbarara (in kilometers, range; mean) | 3–125; 47.3 |
| Monthly household income (in UGX, range; mean) | 5,000–1,500,000; 187,520 |
| Highest educational level (range; median) | None–technical school; 5th grade |
UGX = Ugandan shillings.
Sociocultural factors that impede timely treatment.
The influences of interpersonal relationships and cultural norms intersect when a guardian recognizes that his or her child is ill and requires treatment. These sociocultural factors underlie the process of how and when care is sought. We identified two sociocultural factors that contributed to delays in care for children with severe malaria: 1) discerning between “traditional” and “hospital” illnesses and 2) generational conflict.
Discerning between traditional and hospital illness.
Participants described two mutually exclusive etiologies for the physical symptoms observed in their child. Traditional illnesses were those caused by bewitching, demons, family curses, or other factors that must be cured through herbal/traditional treatments. In contrast, hospital illnesses were those requiring treatment with western medicines for resolution. Of the families included in the study, 16 (21%) sought care from a traditional healer prior to arrival in our facility. Many more guardians struggled with the choice of whether to seek care initially from a traditional or allopathic provider. Guardians reported that if the first treatment modality failed, then they would try the other. The following quote describes a mother's decision to visit first a traditional healer before coming to the hospital, and the delay that resulted from that choice:
I did not know what to do. I decided to take her there (to the traditional healer) and check. When you take them there, she checks and tells you whether she has it (a traditional illness) or not. When I reached there, she told me she has the disease and treated her, but afterwards the child continued to become very sick. That's when I brought her here (to the hospital). (22 years old, mother)
Those who chose to seek allopathic care first emphasize the benefit of blood testing to inform diagnosis and guide treatment. This father describes his thought process in the face of discordant advice from family and friends about where to take his child for initial treatment:
[My friends and family] told me to take the child to traditional doctors, as she could have been bewitched, but I said, ‘No. Let me first take her to the hospital and they see …. if they check and find that it is not malaria, then I will know that it is witchcraft and go [to the healers]’. They found that it was malaria, and we continued with the hospital. (35 years old, father)
Generational conflict.
As suggested by the quotes presented above, there are conflicting influences directing guardians to either the traditional healers or allopathic providers. Guardians receive advice from family members, friends, and neighbors regarding care for their children. We also noted that, in this cultural setting, extended families are closely involved in childcare. While extended family members may not live in the same home, they often live in structures constructed on the same plot of land. As such, the health-care practices and beliefs of older family members can put children at risk of malaria infection. For example, parents report that grandparents provide care for their children in homes without insecticide-treated nets and routinely treat fevers with herbal home remedies.
In general, our data demonstrate that young parents struggle with advice from older members of society, who recommend initial treatment from traditional practitioners rather than from allopathic providers. These guardians—who often wish to seek allopathic care first—are torn between their own ideas about appropriate treatment of their child and the sociocultural expectation of showing respect and deference to members of the preceding generation, who suggest traditional medicine approaches. The following quotes illustrate this tension:
My parents were saying it is the traditional illness that is making him sick. I said, ‘But I have given the herbs you have been advising me to give, and he is not improving at all.’ They disagreed … then the child became very ill … when I told my husband, he agreed to take him to clinic for testing. (23 years old, mother)
My mother told me it was a [traditional illness], and I first went [to the healer] because a parent can't tell you to do something and you fail to do it. You have to obey. You first try what they tell you and when that fails, you can go to the hospital. (18 years old, father)
These types of conflicts uniquely affected younger caregivers in our study (under the age of 40 years) and caused delays to care, as they often deferred to the advice of their elders. Otherwise, there were no appreciable differences in care seeking between the older and younger caregivers in our study.
Structural factors that impede timely treatment.
Unique political, historic, and economic factors create and reinforce local institutions, infrastructure, and social inequities that influence access to, and engagement with, health-care resources. Considered together, these are structural factors that impact the process of seeking treatment of a sick child. Our data revealed two structural factors that contribute to delayed care for children in this region with severe malaria: 1) the distribution and organization of health-care resources, and 2) impoverishment.
Distribution and organization of health resources.
A mix of private and government clinics and hospitals provide health care for the inhabitants of rural southwestern Uganda. Private clinics are for-profit ventures that include both formal and informal facilities; many are single-room, one-person operations staffed by individuals without formal medical training.22 Most private clinics do not have blood testing capability, and practice empiric treatment of ailments without laboratory confirmation. Such facilities tend to be preferentially visited due to their proximity to villages. Health care at government facilities is free of charge, but these tend to be further away from the villages and are therefore less commonly visited first. A mother describes her experiences with the health-care services in this rural setting:
Sometimes, on the weekend, there are no health workers.… There is one clinic which tests blood in K___, but the one in the village does not … the others [clinics in the village] treat the disease while guessing whether this is malaria or not. They do not know what they are treating. (29 years old, mother)
Following initial visits to these local clinics, families may attempt to manage children at home using a combination of antipyretics, antibiotics, antimalarial and/or antiviral medications distributed by these facilities. When children deteriorate on these regimens, caretakers seek a higher level of care at larger clinics in neighboring towns, or at government hospitals. This leads to repeated treatment seeking over a span of days to weeks as a child's condition worsens.
The distribution of resources in this rural area also creates delays in diagnosis and treatment. Respondents describe experiences at both private and government facilities where health-care providers were not present, crucial medications were out of stock or past the expiration date, diagnostic testing was not performed, and blood products necessary for transfusion were unavailable to treat severe malarial anemia. A grandmother recounts her visits to multiple facilities in an effort to find appropriate care for her grandchild:
We took him to the clinic and after reaching there they told us they can't manage him. They don't have drips or blood. Because the child doesn't have blood (is anemic), they told us we have to take him to the town.… They said, ‘If we inject him he will die because the child is very sick’. We ran at night to town. After reaching there, another clinic they also refused; they can't manage him. ‘Take him to a referral hospital’. (60 years old, grandmother)
Impoverishment.
In this low-resource setting, people lack access to money and transportation that could quickly facilitate a higher level of care for their affected children; lack of funds prohibits timely escalation of care as guardians spend time raising money needed for the journey to Mbarara. The following quotes illustrate these experiences:
We first went to a private clinic … [the practitioner] checked him and referred us to Mbarara, but we did not have the money, and instead returned home. We spent about three days at home and the child worsened. Then we took him to the government clinic … because we did not have money for transportation [to Mbarara], we stayed in the village hoping that the child could improve. (28 years old, mother)
The government health center is far and the money for a [motorcycle taxi], … most of the times you don't have cash. Even that money that we used for transportation, I borrowed it from another woman. I told her that my child is bad off and then she gave it to me.… That's how we managed to come here [to Mbarara]. (35 years old, mother)
Lack of money to finance costs of transportation and allopathic care may influence the decision to seek care from local, traditional healers or informal private clinics rather than the government health centers located many kilometers away. In Uganda, one traditional practitioner exists for every 200–400 persons, while the density of trained, allopathic medical personnel is 1 per 20,000 persons.22 Furthermore, the cost of a motorized taxi can range from 2,500 to 200,000 UGX (US$1 = 2,500 UGX) depending on the distance traveled, time of day, and type of vehicle hired. Given that the average monthly income for rural households in Uganda is approximately 222,000 UGX (approximately US$83), the cost of travel to allopathic facilities is prohibitively high.23 One of our participants suggests that some people in her village prefer traditional medicine because they do not have the money to get to the hospitals:
Interviewer: Why do you think people go to traditional healers?
Respondent: I think if they lack money, they start to think whether they should take the child to the hospital or traditional healers. ‘Where will I get the money to take the child to hospital? All that money,’ they say, ‘let me take my child to traditional healers instead’. (20 years old, mother)
Financial impact of illness on families.
The economic impact of malaria in low-resource settings has been described as a cycle, where illness creates debt, leading to poverty, which then predisposes individuals to further illness.24 Our participants describe financial strain associated with their child's illness consistent with this type of cycle. While there is no charge for treatment in the public referral hospital where participants were enrolled, families must bear high costs of transportation to and from Mbarara, and—while a child is in the hospital—food for the hospitalized child and guardian. Funds needed to facilitate hospitalization are often borrowed from family members, neighbors, and village banks. In the context of preexisting impoverishment, this process creates sizeable debt for families. Furthermore, guardians report significant loss of earnings during hospitalization as they are taken away from income-generating activities. In addition, household economies in this region often depend on investments such as livestock or produce. When a child is admitted to the hospital, guardians are unable to attend to these investments, creating an avenue for loss of capital. A mother describes the impact of her child's hospitalization on her farm at home:
I was cultivating sweet potatoes; today I have not cultivated them. I have pigs, yesterday never fed and today not fed. I have hens; they have not got anyone to feed them. I also have goats; they are few but I have them. They are all suffering when I have the child [in the hospital].… If something has not eaten [one of my animals], it will die, or sometimes it will stray and [a thief] will kill it or take it. (28 years old, mother)
Despite the economic hardship created by illness and hospitalization, guardians prioritize their child's well-being over income-generating activities, and recognize the need for sacrifice to access appropriate care. The following quote from a mother illustrates the prioritization of her child's well-being despite devastating losses to her household economy as a result of the current illness:
It is harvest time and we are harvesting millet, and we left them in the garden to be eaten by the birds. Can you harvest when the child is sick? The sorghum is also still in the garden and even maize is being eaten by rodents. I tried to ask for a loan to pay back after the child has gotten better, and they told me ‘Can we give you the loan when your maize is being eaten by the rats?’ Can someone give you a loan when your maize is rotting? … The gardens are not more important than my child whom I produced and lost blood. I will dig other gardens. As a saying goes, “It is better to not to dig in this season than to be added on as soil (be buried in the ground after death).” (35 years old, mother)
Discussion
Our study revealed numerous factors contributing to delayed treatment of children with severe malaria in southwestern Uganda. We have categorized these broadly into sociocultural and structural factors. Here, based on our findings, we present potential interventions that could alleviate the burden of disease in this region. Although the goal of qualitative research is to produce a contextual, detailed understanding of a phenomenon rather than broadly generalizable results, we noted many similarities with other African studies on barriers to malaria control.9–12,14–16 As such, our findings and suggestions may be applicable to other rural areas of Africa. We also believe that forces we identify are not unique to the problem of malaria, and may be broadly applicable to understanding difficulties with access to appropriate health care in resource-poor contexts in general.
We found that late presentation of children with severe malaria to allopathic facilities was framed by two sociocultural factors: 1) interviewees' distinctions between traditional and hospital illnesses, which were thought of as mutually exclusive and 2) advice from family elders. The category to which any given illness is assigned drives subsequent treatment-seeking behavior by determining the context in which initial care is sought. Related to the process of seeking care, we note the influence of family elders in recommending traditional healers for initial assessment. As a result, symptoms such as coma, convulsions, respiratory distress, pallor, and fever are sometimes interpreted as signs of a traditional illness, rather than markers of severe malaria infection. As such, many children are initially seen by traditional healers in the village setting instead of by allopathic providers. In the case of severe malaria, where delays to care are associated with increased morbidity and mortality, the guardian's critical decision lies in which initial treatment modality to pursue.
Given these widespread practices, we suggest that educational programs targeting both village inhabitants (as highlighted in references 25, 26) and traditional practitioners (see reference 27) could facilitate testing and treatment prior to, or concurrent with, care from traditional healers. Traditional practitioners are important stakeholders in the management of pediatric malaria; educational programs could focus on integration of rapid diagnostic testing and/or antimalarial medications into existing healing practices. Such interventions must not subvert the current authority of traditional healers, but facilitate a dialogue where the technologies used in early diagnosis and treatment of malaria could be incorporated into their practices. Such an approach is likely to increase access to these potentially life-saving therapies.
Improving or increasing knowledge does not in itself lead to changes in health-care-seeking behavior, which is shaped by cultural traditions and constrained by economy and geography.28,29 As suggested above, educational content is more likely to be accepted if it can be integrated into current practices [for example, references 30–33 for HIV/AIDS]. Deference to one's elders is an important cultural value in this setting, and cross-generation relationships have an impact on the initial treatment received by a child with malaria. Integrating blood testing with traditional practices (such as herbal or spiritual treatments) may also be a way to avoid engendering familial conflict while ensuring early diagnosis and treatment of sick children.
We identified two significant structural issues that contribute to delays in care for children with severe malaria: 1) distribution of health-care resources and 2) impoverishment. These realities are deeply rooted in the politics, economy, and history of a region. The term structural violence has been used to describe such systematic, pervasive inequalities that perpetuate suffering and limit agency for certain groups.34,35 These inequities frame the experience of illness by constraining and restraining individuals' access to resources, and predispose certain groups to infectious vulnerability, disease progression, and complications. Structural violence therefore determines who has access to appropriate care, and who is likely to fall ill again despite treatment.
Consistent with this framework, our data demonstrate that inequitable distribution of health resources throughout the region, along with underlying poverty, restrict treatment options for children with symptoms of severe malaria. These children initially present to local clinics that do not have testing facilities, medications, or blood products necessary for appropriate treatment. Costs associated with travel to government clinics and referral hospitals are gravely prohibitive for families in this region. The agency of guardians is constrained by the structural realities they inhabit, and within which they navigate their search for appropriate health care for their children.
By acknowledging and understanding the structural realities within which illness occurs and is perpetuated, we can begin to explore potential solutions. For example, we noted poor distribution of health-care resources in village settings. Expansion of point-of-care testing (such as rapid diagnostic kits) and improving distribution of antimalarial medication to private village clinics, could facilitate early testing and appropriate treatment in the rural setting where many children initially presented. In some regions of southwestern Uganda, rapid diagnostic testing is currently carried out by community health workers (CHWs). We suggest supporting or creating programs that empower and expand the scope of these workers. CHWs may gain the trust of local populations by working closely with them, and are in a unique position to influence and educate communities. By teaching CHWs about the barriers faced by village inhabitants when seeking care, they may be able to provide concrete and pragmatic solutions about how and where to access appropriate treatment. For example, CHWs could direct guardians to health-care facilities with blood products for those children likely to require transfusion, or to clinics with ambulance services to speed referral to a referral hospital if necessary. Furthermore, for those children requiring transfer to a higher level of care, the implementation of a regional motorized ambulance system would reduce the financial barrier of transportation costs for these families.
While the high rate of pediatric malaria is only one of numerous problems in southwestern Uganda, our data indicate its relative importance to respondents as they prioritize the lives of their children above their family's financial and food security. As Sachs (2005) discusses, disease and poverty are inexorable.36 Reducing the burden of disease is a crucial step toward improving economic status, and vice versa. By addressing the factors that create barriers to care for children with severe malaria, in addition to decreasing morbidity and mortality from this preventable illness, we can potentially eliminate a common pathway that perpetuates the cycle of illness, debt, and poverty for families in this area.
The study has several limitations. We expect that we were not able to gather data about children with the worst outcomes. Guardians were enrolled after their children were hospitalized for severe malaria at Mbarara Regional Referral Hospital; therefore our sample only includes data from families who were able to successfully reach our facility and excludes those who could not. In addition, our study protocol precluded study staff from approaching families of children with deteriorating clinical course in the hospital. Therefore, our results may underestimate the severity of barriers to care for the most critically ill children. Second, our sample is composed of approximately three-quarters of female participants. As such, it is possible that our findings underrepresent the experiences of male guardians. We did not observe any significant thematic distinctions between responses given by our female and male participants; this suggests the smaller male sample has not influenced our findings. Finally, there were many eligible participants that could not be enrolled due to time-consuming nature of qualitative research. We attempted to address this limitation by continuing enrollment until we reached data saturation, suggesting that major themes pertaining to barriers to care were not excluded, and larger sample size would not have significantly changed our findings.
Conclusion
The morbidity and mortality of malaria infection is unequally borne by the world's most vulnerable populations. We identified several sociocultural and structural factors that contribute to delays in care for children with severe malaria in southwestern Uganda, which may be relevant to addressing the problem of malaria control, as well as improving access to health care in general, in other regions of Africa: 1) discerning between traditional versus hospital illness, 2) generational conflict, 3) distribution and organization of health resources, and 4) impoverishment. Interventions that consider the forces that frame the process of treatment seeking and determine access to health care are more likely to succeed and potentially address the insidious cycle of illness, debt, and poverty while concurrently decreasing the heavy burden of pediatric malaria in the region.
ACKNOWLEDGMENTS
We are grateful to all our participants for sharing their stories with us in an effort to improve the health care of children in this region. Radhika Sundararajan thanks Jeremy Beitler for his feedback on an early version of this manuscript.
Footnotes
Financial support: Funding for this project was provided by the Harvard-affiliated Emergency Medicine Residency Povinelli Research Award, as well as the Brigham and Women's Hospital Biomedical Research Institute/Center for Faculty Development.
Authors' addresses: Radhika Sundararajan, Department of Emergency Medicine, University of California, San Diego, CA, E-mail: rsundararajan@ucsd.edu. Juliet Mwanga-Amumpaire, Harriet Adrama, Jackline Tumuhairwe, Sheilla Mbabazi, Kenneth Mworozi, and Yap Boum II, Epicentre, Uganda Research Base, Mbarara, Uganda, E-mails: juliet.mwanga@epicentre.msf.org, harriet.adrama@gmail.com, tumuhairwejackline@gmail.com, sheilla.mbabazi@gmail.com, mworozikenneth@gmail.com, and yap.boum@epicentre.msf.org. Ryan Carroll, Department of Pediatrics, Massachusetts General Hospital, Boston, MA, E-mail: rcarroll4@partners.org. David Bangsberg, Massachusetts General Hospital, Center for Global Health, Boston, MA, E-mail: dbangsberg@partners.org. Norma C. Ware, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, E-mail: norma_ware@hms.harvard.edu.
References
- 1.Greenwood BM, Bojang K, Whitty CJM, Targett GA. Malaria. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization World Malaria Report 2013. 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/ Available at. Accessed September 18, 2014.
- 3.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 4.President's Malaria Initiative. Uganda: Malaria Operational Plan 2014. http://www.pmi.gov/docs/default-source/default- document-library/malaria-operational-plans/fy14/uganda_mop_fy14.pdf Available at. Accessed March 13, 2015.
- 5.Dondorp AM, Fanello CI, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ, AQUAMAT group Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna S. Adjunctive management of malaria. Curr Opin Infect Dis. 2012;25:484–488. doi: 10.1097/QCO.0b013e3283567b20. [DOI] [PubMed] [Google Scholar]
- 7.Obonyo CO, Vuvule J, Akhwale WS, Grobbee DE. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg. 2007;77:23–28. [PubMed] [Google Scholar]
- 8.Lutalo SKK, Bakyayita N, Lyimo E, Wanyana J, Sekitto G, Odiit A, Tumwesigire S. Report on Monitoring and Evaluation of Roll Back Malaria in the African Region: Baseline Data for Uganda. Kampala, Uganda: Uganda Ministry of Health; 2000. http://pdf.usaid.gov/pdf_docs/pnadu090.pdf Available at. [Google Scholar]
- 9.Maslove DM, Mnyusiwalla A, Mills EJ, McGowan J, Attaran A, Wilson K. Barriers to the effective treatment and prevention of malaria in Africa: a systematic review of qualitative studies. BMC Int Health Hum Rights. 2009;9:26. doi: 10.1186/1472-698X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winch PJ, Makemba AM, Kamazima SR, Lurie M, Lwihula GK, Premji Z, Minjas JN, Shiff CJ. Local terminology for febrile illnesses in Bagamoyo District, Tanzania and its impact on the design of a community-based malaria control programme. Soc Sci Med. 1996;42:1057–1067. doi: 10.1016/0277-9536(95)00293-6. [DOI] [PubMed] [Google Scholar]
- 11.Mwenesi H, Harpham T, Snow RW. Child malaria treatment practices among mothers in Kenya. Soc Sci Med. 1995;40:1271–1277. doi: 10.1016/0277-9536(94)00250-w. [DOI] [PubMed] [Google Scholar]
- 12.Kengeya-Kayondo JF, Seeley JA, Kajura-Bajenja E, Kabunga E, Mubiru E, Sembajja F, Mulder DW. Recognition, treatment seeking behaviour and perception of cause of malaria among rural women in Uganda. Acta Trop. 1994;58:267–273. doi: 10.1016/0001-706x(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 13.De Beadrap P, Nabsumba C, Grandesso F, Turyakira E, Schramm B, Boum Y, Etard J-F. Heterogenous decrease in malaria prevalence in children over a 6-year period in south-western Uganda. Malar J. 2011;10:132. doi: 10.1186/1475-2875-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKombie SC. Treatment seeking for malaria: a review of recent research. Soc Sci Med. 1996;43:933–945. doi: 10.1016/0277-9536(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 15.Beiersmann C, Aboubakary S, Wladarsch E, De Allegri M, Kouyaté B, Müller O. Malaria in rural Burkina Faso: local illness concepts, patterns of traditional treatment and influence on health-seeking behaviour. Malar J. 2007;6:106. doi: 10.1186/1475-2875-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houngbedji CA, N'Dri PB, Hürlimann E, Yapi RB, Silué KD, Soro G, Koudou BG, Acka CA, Assi SB, Vounatsou P, N'Goran EK, Fantodji A, Utzinger J, Raso G. Disparities of Plasmodium falciparum infection, malaria-related morbidity and access to malaria prevention and treatment among school-aged children: a national cross-sectional survey in Côte d'Ivoire. Malar J. 2015;14:7. doi: 10.1186/1475-2875-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Severe and complicated Malaria. Trans R Soc Trop Med Hyg. 1990;84((Suppl 2)):1–65. [PubMed] [Google Scholar]
- 19.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2013;40:1–12. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbin J, Strauss A. Basics of Qualitative Research. 3rd edition. London, United Kingdom: Sage Publications; 2008. [Google Scholar]
- 21.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 22.Konde-Lule J, Gitta SN, Lindfors A, Okuonzi S, Onama VON, Forsberg BC. Private and public health care in rural areas of Uganda. BMC Int Health Hum Rights. 2010;10:29. doi: 10.1186/1472-698X-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uganda National Household Survey Findings 2009/2010 Uganda Bureau of Statistics. http//:www.ubos.org/UNHS0910/chapter7.Average%20Monthly%20Household%20Income.html Available at. Accessed September 21, 2014.
- 24.Ingstad B, Munthali AC, Braathen SH, Grut L. The evil circle of poverty: a qualitative study of malaria and disability. Malar J. 2012;11:15. doi: 10.1186/1475-2875-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chourasia MK, Abraham VJ, John J. Household training vs. mass campaigns: a better method of health communication for preventing malaria. Trop Doct. 2014;44:196–200. doi: 10.1177/0049475514545201. [DOI] [PubMed] [Google Scholar]
- 26.Gomes MF, Warsame M, Nasemba N, Singlovic J, Kapinga A, Mwakuyse S, Mrango Z. Gaining time: early treatment of severe pediatric malaria in Tanzania. Drug Dev Res. 2010;71:92–98. [Google Scholar]
- 27.Makundi EA, Malebo HM, Mhame P, Kitua AY, Warsame M. Role of traditional healers in the management of severe malaria among children below five years of age: the case of Kilosa and Handeni Districts, Tanzania. Malar J. 2006;5:58. doi: 10.1186/1475-2875-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espino F, Manderson L, Acuin C, Domingo F, Ventura E. Perceptions of malaria in a low endemic area in the Philippines: transmission and prevention of disease. Acta Trop. 1997;63:221–239. doi: 10.1016/s0001-706x(96)00623-7. [DOI] [PubMed] [Google Scholar]
- 29.Williams HA, Jones CO. A critical review of behavioral issues related to malaria control in sub-Saharan Africa: what contributions have social scientists made? Soc Sci Med. 2004;59:501–523. doi: 10.1016/j.socscimed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Audet CM, Sidat M, Blevins M, Moon TD, Vergara A, Vermund SH. HIV knowledge and health-seeking behavior in Zambézia Province, Mozambique. SAHARA J. 2012;9:41–46. doi: 10.1080/17290376.2012.665257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodeker G, Kabatesi D, King R, Homsy J. A regional task force on traditional medicine and AIDS. Lancet. 2009;355:1284. doi: 10.1016/S0140-6736(05)74722-X. [DOI] [PubMed] [Google Scholar]
- 32.Bodeker G, Carter G, Burford G, Dvorak-Little M. HIV/AIDS: traditional systems of health care in the management of a global epidemic. J Altern Complement Med. 2006;12:563–576. doi: 10.1089/acm.2006.12.563. [DOI] [PubMed] [Google Scholar]
- 33.Homsy J, King R, Tenywa J, Kyeyune P, Opio A, Balaba D. Defining minimum standards of practice for incorporating African traditional medicine into HIV/AIDS prevention, care, and support: a regional initiative in eastern and southern Africa. J Altern Complement Med. 2004;10:905–910. doi: 10.1089/acm.2004.10.905. [DOI] [PubMed] [Google Scholar]
- 34.Farmer P. An anthropology of structural violence. Curr Anthropol. 2004;45:305–325. [Google Scholar]
- 35.Farmer PE, Nizeye B, Stulac S, Keshavjee S. Structural violence and clinical medicine. PLoS Med. 2006;3:e449. doi: 10.1371/journal.pmed.0030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs JD. The End of Poverty. London, United Kingdom: Penguin Press; 2005. [Google Scholar]