Abstract
In a study of children having polyparasitic infections in a Schistosoma haematobium–endemic area, we examined the hypothesis that S. haematobium–positive children, compared with S. haematobium–negative children (anti-soluble worm antigen preparation [SWAP] negative and egg negative) have increased systemic production of pro-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor [TNF]-α) and decreased down-regulatory IL-10. A total of 804 children, 2–19 years of age, were surveyed between July and December 2009 and tested for S. haematobium, Plasmodium falciparum, filariasis, and soil-transmitted helminth infections. Plasma levels of IL-6, TNF-α, and IL-10 were compared for S. haematobium–positive and S. haematobium–negative children, adjusting for malaria, filaria, and hookworm co-infections, and for nutritional status, age group, sex, and geographic location. IL-10 was significantly elevated among children infected with S. haematobium, showing bimodal peaks in 7–8 and 13–14 years age groups. IL-10 was also higher among children who were acutely malnourished, whereas IL-10 levels were lower in the presence of S. haematobium–filaria co-infection. After adjustment for co-factors, IL-6 was significantly elevated among children of 5–6 years and among those with P. falciparum infection. Lower levels of IL-6 were found in malaria–hookworm co-infection. High levels of TNF-α were found in children aged 11–12 years regardless of infection status. In addition, village of residence was a strong predictor of IL-6 and IL-10 plasma levels. In adolescent children infected with S. haematobium, there is an associated elevation in circulating IL-10 that may reduce the risk of later morbidity. Although we did not find a direct link between S. haematobium infection and circulating pro-inflammatory IL-6 and TNF-α levels, future T-cell stimulation studies may provide more conclusive linkages between infection and cytokine responses in settings that are endemic for multiple parasites and multiple co-infections.
Introduction
Data are scarce regarding the immune responses of children affected by chronic schistosomiasis and other overlapping parasitic infections, including malaria, filaria, and soil-transmitted helminth infections, experienced either sequentially or as polyparasitic disease. With an estimated 230 million people affected by schistosomiasis, and almost half of them being children,1 there remains a significant knowledge gap regarding the timing and mechanisms for the initiation of Schistosoma-associated morbidity. As a result, schistosomiasis treatment campaigns may not be targeting an appropriate age range to prevent advanced disease due to end-organ fibrosis.2
Laboratory and clinical studies suggest that adaptive host immune responses can modulate manifestations of schistosomiasis morbidity.3 However, production of pro-inflammatory cytokines is an essential part of chronic Schistosoma infection, and have been associated with malnutrition and anemia of inflammation, for example, by IL-6 stimulation of hepcidin production, a liver hormone responsible for sequestration of iron.4–6 In S. japonicum–endemic settings, end-organ damage, manifesting as liver fibrosis, has been associated with elevated pro-inflammatory cytokine responses highlighting the correlation between inflammation and fibrosis.5,7 Less is known about the link between S. haematobium–related cytokine response and infection-associated morbidity.8
The exact timing of the organ damage is largely unknown. Therefore, by analyzing the age profile of circulating pro-inflammatory (IL-6 and TNF-α) and immunomodulatory (IL-10) cytokines in the presence and absence of active S. haematobium infection, we aimed to identify the onset of inflammation and adaptive downregulation in chronically exposed children. To do this, we used a refined definition of S. haematobium infection that included either anti-parasite antibody positivity or egg patent infection. Our second objective was to correlate these cytokine levels with the presence or absence of schistosomiasis-associated morbidities such as anemia and undernutrition, and to explore interactions related to concurrent co-infections.
Methods
Children aged 2–19 years (N = 804) were surveyed between July and December 2009 from two S. haematobium–endemic villages in Matuga and Msambweni sub-counties (in former Coast Province), Kenya (Figure 1 ) as detailed elsewhere.9 The selected villages included low S. haematobium prevalence Vuga, and high prevalence Milalani (Table 1). Briefly, data collection included demography, urine filtration for S. haematobium eggs (one urine),10 and Kato-Katz11 stool examination for soil-transmitted helminths (one stool). Blood collection was performed by finger prick for the rapid antigen detection of P. falciparum (ICT Diagnostics, Sydney, Australia) and Wuchereria bancrofti filarial infection (Binax, Portland, ME).9 After centrifugation, plasma samples were kept frozen at −80°C.
Figure 1.
Map of the study villages in Kwale County, Kenya.
Table 1.
Population characteristics and cytokine distribution by village
| Milalani (N = 191) | Vuga (N = 613) | |
|---|---|---|
| Demography | ||
| Mean age ± SD in years (range) | 12.4 ± 3.2 (5–19) | 11.0 ± 3.7 (2–18)* |
| Female sex, n (%) | 96 (50) | 313 (51) |
| Morbidity | ||
| Mean hemoglobin ± SD (range) | 11.8 ± 1.9 (4.6–16.4) | 11.8 ± 1.4 (6.1–15.9) |
| Anemia, n (%) | 90 (47) | 251 (41) |
| Stunting (HAZ < −2) | 61 (32%) | 263 (43%)* |
| Wasting (BAZ < −2) | 21 (11%) | 188 (31%)* |
| Parasitic infections | ||
| Schistosoma haematobium | ||
| Mean egg count | 127 (0–1,000) | 54 (0–1,000)* |
| Any intensity positive, n (%) | 130 (68) | 155 (25)* |
| Light (< 50 eggs/10 mL urine) | 62 (32%) | 80 (13%) |
| Heavy (> 50 eggs/10 mL urine) | 68 (36%) | 75 (12%) |
| Anti-SWAP IgG4‡ | ||
| Positive, n (%) | 147 (77) | 253 (41)* |
| Malaria (P. falciparum), n (%) | 33 (17) | 77 (13) |
| Filaria (W. bancrofti), n (%) | 21 (11) | 97 (16) |
| Hookworms, n (%) | 43 (22) | 64 (10)* |
| Cytokines | ||
| Median IL-6 pg/mL (range) | 204 (2–165,586) | 298 (4–9,033)† |
| Median IL-10 pg/mL (range) | 180 (1–2,473) | 353 (2–424,996)† |
| Median TNF-α pg/mL (range) | 102 (7–769) | 104 (2–7,916) |
SD = standard deviation; HAZ = age-adjusted height Z-score; BAZ, age-adjusted body-mass index Z-score; IL = interleukin; TNF = tumor necrosis factor.
P < 0.01, significant difference between villages: χ2 test for categorical variables, Student's t test for continuous variables.
P < 0.001 for significant difference between villages, nonparametric testing by Mann–Whitney U test.
Antibody against Schistosoma adult Soluble Worm Antigen.
Hemoglobin was determined (Hemocue, Ångelholm, Sweden), and anemia and severe anemia were categorized according to World Health Organization (WHO) criteria for age and sex, and scored as present or absent for each child.12 Reference population Z scores were calculated for each subject's height-for-age (HAZ) and body mass index-for-age (BAZ) using international standards for comparison, taken from the WHO's Anthro program for ages 0–5 years and WHO Anthro-plus program for ages 5–19 years (WHO, Geneva, Switzerland).13 According to WHO standards, stunting was categorized as an observed HAZ that was two or more standard deviations (SDs) below average (HAZ score < −2). Children were categorized as clinically wasted if their BAZ was more than 2 SDs below average for their age (BAZ score < −2).13
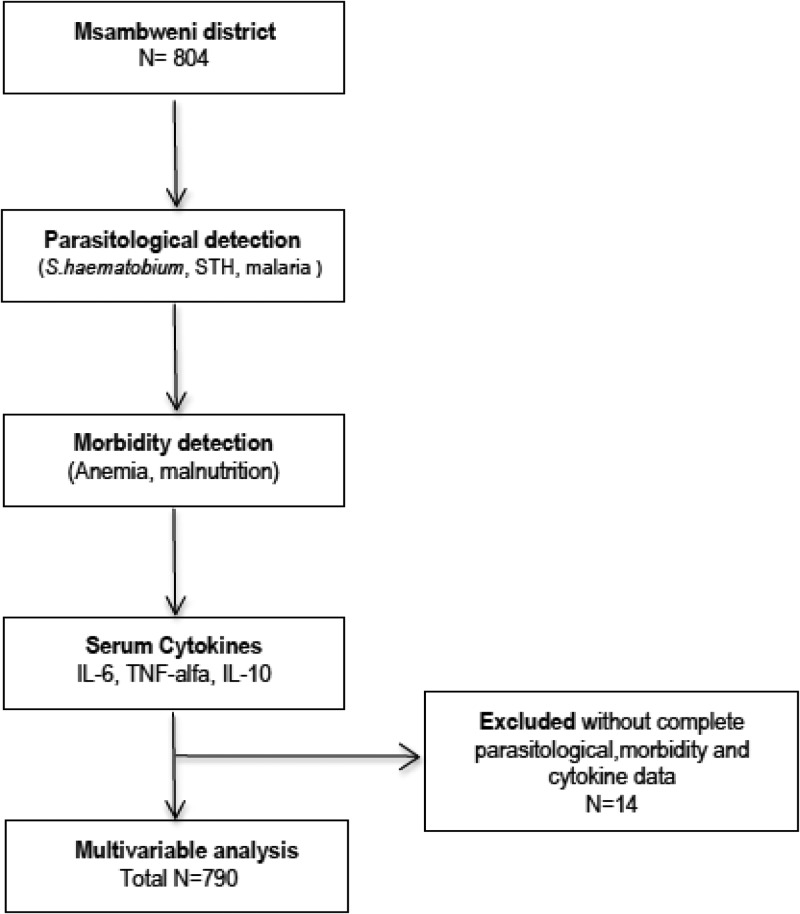
Ethical clearance was obtained from the Institutional Review Board at Case Western Reserve University and the Ethical Review Committee at the Kenya Medical Research Institute (KEMRI). Informed consent was obtained from each child's parent or guardian and verbal assent was obtained from children above 7 years of age. All infections detected during this study were treated in accordance with the national guidelines of Kenyan Ministry of Medical Services. Ultimately, 790 children provided full clinical and laboratory data (see Figure 2 for flow chart of enrollment), and these subjects with complete data were included in the data analysis presented in this article.
Figure 2.
Flow diagram with the design of the study.
Anti-Schistosoma IgG4 antibody detection assay.
To increase sensitivity for the detection of early, possibly egg-negative S. haematobium infection among younger children, we performed serologic testing for circulating anti-parasite IgG4 in all study subjects. Soluble worm antigen preparation (SWAP) (crude extract) was provided courtesy of Christopher King, Center for Global Health and Diseases, Cleveland, OH. Ninety-six-well plates were coated with 50 μL of 10 μg/mL SWAP diluted in enzyme-linked immunosorbent assay (ELISA) coating buffer and allowed to incubate at 4°C overnight. The plates were then blocked with ELISA blocking buffer and washed with ELISA wash buffer as described previously.14 Serum samples prepared in ELISA diluent buffer were then allowed to incubate on the antigen-coated plate at 4°C overnight.14 The plates were washed six times and incubated with 50 μL of a 1:2,000 dilution of mouse anti-human IgG4 (Jackson ImmunoResearch, West Grove, PA) for 2 hours. Plates were then washed and incubated with 50 μL of 1:1,000 dilution of goat anti-mouse, alkaline phosphatase–conjugated antibody (Jackson ImmunoResearch) for 1 hour. The plates were washed and incubated with alkaline phosphatase substrate in buffer (Sigma Aldrich, St. Louis, MO) for 15 minutes. After 15 minutes, the reaction was stopped with 50 μL of 5% EDTA. Bound IgG4 was then measured by the determination of optical density at 415 nm. The cutoff for a positive test was set at 2 SDs above the mean optical density of 20 negative control sera obtained from an area in northern Kenya endemic for multiple parasites, but not endemic for schistosomiasis or filariasis (courtesy Christopher King Laboratory, CWRU, Cleveland, OH).
Circulating cytokine detection and quantification assays.
Ninety-six-well Immulon IV ELISA plates were coated with 50 μL of 2.5 μg/mL primary antibody against IL-10, IL-6, and TNF-α (BD Diagnostics, San Jose, CA) diluted in ELISA coating buffer and allowed to incubate at 4°C overnight. After further washing and blocking of the plates, subject samples with standards and blanks were added in a 1:2 dilution and allowed to incubate at 4°C overnight. The plates were then washed and respective detection (biotinylated) antibodies were added, and the plates were incubated at room temperature for 45 minutes. The plates were rewashed and incubated in the presence of streptavidin alkaline phosphate for 30 minutes. The plates were then washed and incubated with alkaline phosphatase substrate buffer (Sigma Aldrich) for 1 hour. After 15 minutes, the reaction was stopped with 50 μL of 5% EDTA. Bound cytokines (IL-10, IL-6, and TNF-α) were then measured by the determination of optical density at 415 nm. There was a standard ELISA curve for each plate from the positive control standards, and the individual subject plasma levels (in pg/mL) were then interpolated from that curve.
Data entry and statistical analysis.
All data were entered in Microsoft Excel (Redmond, King County, WA) and analyzed using the R statistical package version 2.14.1 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS v.21 software (IBM Corp., Armonk, NY). Arithmetic and geometric mean (GMW) were calculated for S. haematobium infection intensity. For percentages, 95% confidence intervals (CI) were calculated by applying the exact method. S. haematobium mean egg count, hookworm egg count, antibody values, and cytokine values were found to have a non–Gaussian distribution and were compared using non-parametric statistics, or were log transformed for multivariable linear modeling. Categorical variables were created for malaria, hookworm, anemia, S. haematobium intensity of infection, wasting, and stunting. A case definition of schistosomiasis was created with aggregated variables S. haematobium egg and/or anti-SWAP positive. Analysis of variance (ANOVA) or χ2 testing was performed to assess the significance of differences detected among the study villages. Initial exploratory analysis included bivariate correlations between IL-6, IL-10, TNF-α levels, and the different covariates of interest: infection status, age, sex, village anemia, wasting, stunting, and other cytokines. Concurrent cytokine level predictors were also included in multivariable linear regression models.
Results
Location-related differences.
A total of 804 children were surveyed from two villages (see Figure 1 and Table 1). Milalani children were slightly older (mean age 12.4 versus 11.0, P < 0.01), but the male and female composition of the two community survey samples was not significantly different (Table 1). In high-prevalence Milalani (N = 191), 68% of the children had egg-patent S. haematobium infection in contrast to 25% of children in Vuga (N = 613). Schistosoma sero-prevalence was again markedly higher in Milalani (77%) in contrast to Vuga (41%). More high-intensity S. haematobium infections (based on standardized urine filtration egg counts) were present in Milalani and the mean egg count per subject was higher in Milalani (Table 1). Other parasitic infections including malaria, hookworm, and filariasis were found in both villages (Table 1). Whereas levels of detected malaria and filarial infection were not significantly different between the two villages, hookworm was more common in Milalani (22% prevalence versus 10% in Vuga, P < 0.01) (Table 1).
In terms of clinical morbidity, the prevalence of anemia was not significantly different between the two villages: 47% in Milalani versus 41% in Vuga. However, a significantly greater proportion of children were malnourished in Vuga, with 43% of them being stunted and 31% wasted, compared with 32% and 11%, respectively, in Milalani.
For the cytokine outcomes measured, we observed overall higher levels of pro-inflammatory IL-6 and down-regulatory IL-10 among children from Vuga village as compared with Milalani. Levels of TNF-α did not vary significantly between locations.
Cytokine age-related distribution.
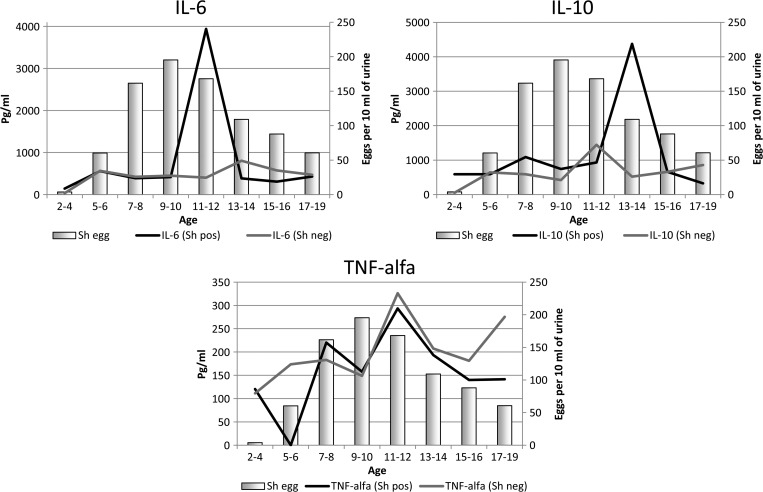
The age-related distribution of the cytokines analyzed by infection status is shown in Figure 3 . After controlling for possible confounders (sex, malaria, anemia, schistosomiasis, hookworm, wasting, stunting, and other cytokines), age remained a significant independent predictor of IL-6 response, with children aged 5–6 years having higher levels of IL-6 (see Table 2). IL-10 levels were significantly lower in children aged 11–12 years and 17–19 years as compared with 13–14 years when the model was adjusted for possible confounders (see Table 3). TNF-α levels were significantly higher in 11- to 12-year-old children (see Table 4).
Figure 3.
Age-related changes in cytokine levels according to S. haematobium infection status (Anti-SWAP positive and/or egg positive), contrasted with age-group-specific intensity of infection.
Table 2.
Multivariable log-linear modeling for the association of IL-6 with potential explanatory variables
| Variables | Multivariable model for log10-IL6 level | ||
|---|---|---|---|
| B coefficient | 95% CI | P value | |
| IL-10* | −0.018 | (−0.079, 0.051) | 0.673 |
| TNF-α* | 0.262 | (0.201, 0.391) | < 0.0001 |
| Sex (male) | 0.028 | (−0.040, 0.094) | 0.424 |
| Village (Vuga) | 0.131 | (0.067, 0.240) | 0.001 |
| 5–6 years old | 0.091 | (0.007, 0.269) | 0.040 |
| Wasted† | −0.013 | (−0.113, 0.084) | 0.771 |
| Stunted‡ | 0.021 | (−0.060, 0.102) | 0.609 |
| Malaria | 0.112 | (0.015,0.210) | 0.024 |
| S. haematobium§ | 0.052 | (−0.024,0.129) | 0.180 |
| Hookworm | 0.029 | (−0.070,0.127) | 0.567 |
| Malaria–hookworm | −0.076 | (−0.503, −0.011) | 0.040 |
Final models adjusted for sex, age category, location, wasting, stunting, anemia, individual infections (S. haematobium, malaria, hookworm), and any significant interactions for infection by two or more parasites.
log10 of IL-10 and TNF-α.
Wasting: Body mass index-for-age Z score (BAZ) < −2 SD.
Stunting: Height-for-age Z score (HAZ) < −2 SD.
Positive infection status is defined by S. haematobium egg +/antibody +, egg –/antibody +, or egg +/antibody –.
Table 3.
Multivariable log-linear modeling for the association of IL-10 with potential explanatory variables
| Variables | Multivariable model for log10-IL-10 level | ||
|---|---|---|---|
| B coefficient | 95% CI | P value | |
| TNF-α* | 0.584 | (0.773, 0.947) | < 0.0001 |
| IL-6* | −0.013 | (−0.094, 0.061) | 0.673 |
| Sex (male) | 0.011 | (−0.059, 0.087) | 0.713 |
| Village (Vuga) | 0.123 | (0.092, 0.281) | < 0.0001 |
| 11–12 years | −0.104 | (−0.308, −0.057) | 0.004 |
| 17–19 years old | −0.121 | (−0.468, −0.146) | < 0.0001 |
| Wasted† | 0.107 | (0.001, 0.214) | 0.048 |
| Stunted‡ | −0.035 | (−0.123, 0.054) | 0.434 |
| Malaria | −0.083 | (−0.189, 0.022) | 0.121 |
| S. haematobium§ | 0.207 | (−0.288, −0.126) | < 0.0001 |
| Hookworm | 0.001 | (−0.105, 0.107) | 0.985 |
| Schisto–Filaria | −0.069 | (−0.350, −0.033) | 0.018 |
Final models adjusted for sex, age category, location, wasting, stunting, anemia, individual infections (S. haematobium, malaria, hookworm), and significant interactions for infection by two or more parasites.
log10 of IL-6 and TNF-α.
Wasting: Body mass index-for-age Z score (BAZ) < −2 SD.
Stunting: Height-for-age Z score (HAZ) < −2 SD.
Positive infection status is defined by S. haematobium egg +/antibody +, egg –/antibody +, or egg +/antibody –.
Table 4.
Multivariable log-linear modeling for the association of TNF-α with potential explanatory variables
| Variables | Multivariable model for log10-TNF-α level | ||
|---|---|---|---|
| B coefficient | 95% CI | P value | |
| IL-10* | 0.561 | (0.343, 0.420) | < 0.0001 |
| IL-6* | 0.176 | (0.106, 0.207) | < 0.0001 |
| Sex (male) | −0.038 | (−0.081, 0.016) | 0.149 |
| Village (Vuga) | −0.024 | (−0.088, 0.039) | 0.126 |
| 11–12 years old | 0.135 | (0.078, 0.244) | < 0.0001 |
| Wasted† | −0.063 | (−0.134, 0.009) | 0.087 |
| Stunted‡ | 0.020 | (−0.041, 0.077) | 0.545 |
| Malaria | 0.026 | (−0.038, 0.105) | 0.356 |
| S. haematobium§ | 0.056 | (−0.006, 0.105) | 0.080 |
| Hookworm | −0.025 | (−0.103, 0.040) | 0.389 |
Final models adjusted for sex, age category, location, wasting, stunting, anemia, individual infections (S. haematobium, malaria, hookworm), and significant interactions for infection by two or more parasites.
log10 of IL-10 and IL-6.
Wasting: Body mass index-for-age Z score (BAZ) < −2 SD.
Stunting: Height-for-age Z score (HAZ) < −2 SD.
Positive infection status is defined by S. haematobium egg +/antibody +, egg –/antibody +, or egg +/antibody –.
Cytokine levels and infection.
Overall, median IL-6 levels were higher (P = 0.008, Mann–Whitney U test), and IL-10 levels were also higher (P = 0.003), among children infected with S. haematobium (antibody positive and/or egg positive) when compared with those not infected by S. haematobium. However, after adjustment for co-factors, the S. haematobium effect on IL-6 was no longer significant (Table 2), whereas the S. haematobium effect on IL-10 remained significant (Table 3). Nevertheless, we observed that IL-6 was particularly elevated among S. haematobium–infected children aged 11–12 years (compared with S. haematobium–negative children of the same and other age groups), and IL-10 was highest among S. haematobium–infected children in the 13- to 14-year age group (compared with S. haematobium–uninfected children in that and other age groups) (see Figure 2). We also noted that 11- to 12-year-old children had higher TNF-α levels regardless of S. haematobium–infection status.
After adjusting for age, sex, location, and other variables, children affected by malaria were younger (5–6 years old) and had significantly higher IL-6 levels (Table 2) although those co-infected with malaria and hookworm infection had relatively lower IL-6 levels, suggesting a significant immunomodulation between these two infections. Although IL-10 levels were significantly higher in S. haematobium–infected children, they were lower among those who had S. haematobium–filarial co-infection (Table 3). There was overall a positive correlation between IL-6 and TNF-α levels, and TNF-α and IL-10 levels, respectively.
Cytokine levels and morbidity outcomes.
Morbidity status measured as stunting (chronic malnutrition), wasting (acute malnutrition), or anemia was not associated with significant differences in measured circulating cytokines in our cross-sectional survey. The exception was IL-10 levels that were significantly elevated among wasted children.
Discussion
Our results show an overall association between down-regulatory IL-10 cytokine and S. haematobium infection, but with relatively lower levels in early puberty (11–13 years old) and late adolescence (17–19 years old). Contrary to what was expected, circulating levels of pro-inflammatory cytokines (IL-6 and TNF-α) were not associated with urogenital schistosomiasis, even though granulomatous inflammation with T-cell activation is a hallmark of S. haematobium infection.3,15 Children with malaria had a significant higher pro-inflammatory IL-6 levels, particularly in the younger ones (5–7 years old), although lower levels were seen in children co-infected with both malaria and hookworm. Striking inter-village differences were found for cytokine levels and morbidity outcomes, suggesting a clustering effect possibly related to geographical transmission patterns and nutritional and/or socioeconomic disparities.16,17 This highlights the multi-dimensional approaches needed to understand schistosomiasis infection and disease in the context of other endemic parasitic infections,18 and the social determinants of health that remain so far unexplored in this field. The unforeseen lack of association between schistosomiasis-associated morbidities (anemia, wasting, and stunting) and pro-inflammatory cytokines previously seen in other endemic settings5,18,19 might be explained by the intrinsic plasma level variability of cytokine levels when compared with laboratory responses seen in antigen T-cell-stimulating assays performed in other studies.18–20 Co-infections could have also played an important role. For example, interactions across the immune responses to co-infecting parasites have been suggested in studies in Mali, where immune responses to acute malaria were blunted in children co-infected with urogenital schistosomiasis whose baseline production of IL-6, IL-4, IL-10, and interferon (INF)-γ was initially high.21,22 In subsequent acute malaria infection, among 4- to 8-year-olds, the production of IL-6 was blunted in S. haematobium–positive children compared with those who were S. haematobium negative, suggesting a relative protective effect of S. haematobium co-infection on the outcomes of acute P. falciparum malaria.22 Age- or experience-related factors likely modify this interaction. A smaller study in Senegal found increased production of anti-fibrogenic INFγ in S. haematobium–infected children who had P. falciparum malaria.23 Increased levels of INFγ, seen in acute schistosomiasis24 have also been associated with protection against periportal fibrosis.25 The interesting inverse relationship between IL-6 and malaria–hookworm co-infection found in this study could potentially have the same immune mechanism as Plasmodium–Schistosoma, as hookworm and Schistosoma share some common host-regulatory pathways.26
In intestinal schistosomiasis, IL-10 is believed to have anti-inflammatory immunomodulatory effects as its greater presence is associated with reduced risk of periportal fibrosis from either S. mansoni or S. japonicum infection.7,27 Therefore, our findings of high IL-10 in children with urogenital schistosomiasis in mid-puberty suggest a possible acquired, age-related protective effect against further morbidity following the 13- to 14-year-old age mark.28 Protection against S. japonicum re-infection has also been described in association with sex hormone production during late puberty,29 and there may well be a cytokine–hormone interplay contributing to this effect. In the same geographical location as our study, a positive correlation has been found in adolescent males between structural urinary tract pathology and the simultaneous presence of low levels of IL-10 and high levels of TNF-α in response to S. haematobium antigens.18 Elevated pro-inflammatory TNF-α is associated with hepatic fibrosis in a S. mansoni–endemic area in Uganda and S. japonicum–endemic settings in the Philippines and China.6,20,27 Parasite-induced inflammation is thought to be part of the causal pathway of undernutrition and anemia.5,6,9 Our results, however, did not show any association between IL-6 or TNF-α and the wasting, stunting, or anemia in this group of children.
There are several limitations in our study. First, current serum cytokine levels taken in cross section may not accurately reflect the ongoing in vivo effects of infection related to different antigenic stimulation. Single time point cytokine detection in the circulation, rather than in the local tissue microenvironments, is an inherent limitation to this kind of study. The study was a single cross section, and therefore no post-treatment changes could be established to support parasite-specific associations. The effect of treatment on cytokine profile and its relationship to anemia and nutritional morbidity reversal would be an important question to address given that hepatic vascular remodeling has been shown to occur after treatment in studies in animal models of schistosomiasis.20,30,31 Exploring other cytokines not included in this study (i.e., IL-4, IL-5, and IL-13) and important in understanding the immune response to multiple parasitic infections should be integrated in future research efforts.20
In summary, we have shown age-specific variation in serum cytokine profiles among children with S. haematobium and other chronic parasitic infections. The high levels of IL-10 in young children with schistosomiasis could potentially protect them against developing inflammation-related morbidity. Further pairing of morbidity assessment studies with more refined T-cell stimulation assays will help to strengthen assessments for the appropriate timing of anti-schistosomal treatment for prevention or remission of infection-related organ fibrosis.
ACKNOWLEDGMENTS
We warmly thank the children and families that agreed to participate and also our energetic workers, in particular, Joyce Bongo, Phyllis Mutemi, and Nancy Halloway. Also, many thanks to the laboratory personnel and Nancy Erdey for her invaluable support with the ethical oversight.
Footnotes
Financial support: This study was supported by the U.S. National Institutes of Health through a T32 Ruth L. Kirschstein-National Service Research Award-Training Grant and R01–TW008067 from the Fogarty International Center.
Authors' addresses: Amaya L. Bustinduy, Center for Global Health and Diseases, Case Western Reserve University School of Medicine, Cleveland, OH, and the Institute of Immunity and Infection, St. George's University of London, London, UK, E-mails: bustinji06@gmail.com or Amaya.Bustinduy@doctors.org.uk. Laura J. Sutherland, Alicia Chang-Cojulun, Indu Malhotra, Adam S. DuVall, and Charles H. King, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH, E-mails: chikungunya.ljs@gmail.com, aliciachc18@gmail.com, ijm@case.edu, adam.s.duvall@gmail.com, and chk@case.edu. Jessica K. Fairley and Uriel Kitron, Department of Environmental Studies, Emory University, Atlanta, GA, E-mails: jfairleymd@gmail.com and ukitron@emory.edu. Peter L. Mungai, CWRU/DVBNTD, c/o CWRU/DVBNTD Filariasis-Schistosomiasis Research Unit, Msambweni, Coast, Kenya, E-mail: plmungai@yahoo.com. Eric M. Muchiri, Division of Vector Borne and Neglected Diseases, Ministry of Public Health and Sanitation, Nairobi, Kenya, E-mail: ericmmuchiri@gmail.com. Francis M. Mutuku, Department of Environmental Studies, Emory University, Atlanta, GA, and CWRU/DVBNTD, Diani, Kenya, E-mail: fmutuku73@gmail.com.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197–205. doi: 10.1016/j.pt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho HM, Leenstra T, Acosta LP, Su L, Jarilla B, Jiz MA, Langdon GC, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. Am J Trop Med Hyg. 2006;75:720–726. [PubMed] [Google Scholar]
- 6.Coutinho HM, McGarvey ST, Acosta LP, Manalo DL, Langdon GC, Leenstra T, Kanzaria HK, Solomon J, Wu H, Olveda RM, Kurtis JD, Friedman JF. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–536. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- 7.Alves Oliveira LF, Moreno EC, Gazzinelli G, Martins-Filho OA, Silveira AM, Gazzinelli A, Malaquias LC, LoVerde P, Leite PM, Correa-Oliveira R. Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infect Immun. 2006;74:1215–1221. doi: 10.1128/IAI.74.2.1215-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutapi F, Winborn G, Midzi N, Taylor M, Mduluza T, Maizels RM. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect Dis. 2007;7:139. doi: 10.1186/1471-2334-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustinduy AL, Parraga IM, Thomas CL, Mungai PL, Mutuku F, Muchiri EM, Kitron U, King CH. Impact of polyparasitic infections on anemia and undernutrition among Kenyan children living in a Schistosoma haematobium-endemic area. Am J Trop Med Hyg. 2013;88:433–440. doi: 10.4269/ajtmh.12-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54:159–162. [PMC free article] [PubMed] [Google Scholar]
- 11.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 12.World Health Organization . In: Worldwide Prevalence of Anaemia 1993–2005. WHO Database on Anaemia. Benoist B, McLean E, Egli I, Cogswell M, editors. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 13.De Onis M. World Health Organization, Department of Nutrition for Health and Development . WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 14.Maddison SE, Slemenda SB, Tsang VC, Pollard RA. Serodiagnosis of Schistosoma mansoni with microsomal adult worm antigen in an enzyme-linked immunosorbent assay using a standard curve developed with a reference serum pool. Am J Trop Med Hyg. 1985;34:484–494. doi: 10.4269/ajtmh.1985.34.484. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections: walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 16.Mutuku FM, King CH, Bustinduy AL, Mungai PL, Muchiri EM, Kitron U. Impact of drought on the spatial pattern of transmission of Schistosoma haematobium in coastal Kenya. Am J Trop Med Hyg. 2011;85:1065–1070. doi: 10.4269/ajtmh.2011.11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clennon JA, Mungai PL, Muchiri EM, King CH, Kitron U. Spatial and temporal variations in local transmission of Schistosoma haematobium in Msambweni, Kenya. Am J Trop Med Hyg. 2006;75:1034–1041. [PubMed] [Google Scholar]
- 18.King CL, Malhotra I, Mungai P, Wamachi A, Kioko J, Muchiri E, Ouma JH. Schistosoma haematobium-induced urinary tract morbidity correlates with increased tumor necrosis factor-alpha and diminished interleukin-10 production. J Infect Dis. 2001;184:1176–1182. doi: 10.1086/323802. [DOI] [PubMed] [Google Scholar]
- 19.Booth M, Mwatha JK, Joseph S, Jones FM, Kadzo H, Ireri E, Kazibwe F, Kemijumbi J, Kariuki C, Kimani G, Ouma JH, Kabatereine NB, Vennervald BJ, Dunne DW. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172:1295–1303. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 20.Coutinho HM, Acosta LP, Wu HW, McGarvey ST, Su L, Langdon GC, Jiz MA, Jarilla B, Olveda RM, Friedman JF, Kurtis JD. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195:288–295. doi: 10.1086/510313. [DOI] [PubMed] [Google Scholar]
- 21.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, Guindo A, Traore K, Daou M, Diarra I, Sztein MB, Plowe CV, Doumbo OK. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 22.Lyke KE, Dabo A, Sangare L, Arama C, Daou M, Diarra I, Plowe CV, Doumbo OK, Sztein MB. Effects of concomitant Schistosoma haematobium infection on the serum cytokine levels elicited by acute Plasmodium falciparum malaria infection in Malian children. Infect Immun. 2006;74:5718–5724. doi: 10.1128/IAI.01822-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier JP, Pillet S, Garraud O, N'Diaye AA, Capron A, Capron M, Riveau G. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol. 2004;26:365–369. doi: 10.1111/j.0141-9838.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 24.Montenegro SM, Miranda P, Mahanty S, Abath FG, Teixeira KM, Coutinho EM, Brinkman J, Goncalves I, Domingues LA, Domingues AL, Sher A, Wynn TA. Cytokine production in acute versus chronic human schistosomiasis mansoni: the cross-regulatory role of interferon-gamma and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J Infect Dis. 1999;179:1502–1514. doi: 10.1086/314748. [DOI] [PubMed] [Google Scholar]
- 25.Henri S, Chevillard C, Mergani A, Paris P, Gaudart J, Camilla C, Dessein H, Montero F, Elwali NE, Saeed OK, Magzoub M, Dessein AJ. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169:929–936. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 26.Supali T, Verweij JJ, Wiria AE, Djuardi Y, Hamid F, Kaisar MM, Wammes LJ, van Lieshout L, Luty AJ, Sartono E, Yazdanbakhsh M. Polyparasitism and its impact on the immune system. Int J Parasitol. 2010;40:1171–1176. doi: 10.1016/j.ijpara.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud V, Li J, Wang Y, Fu X, Mengzhi S, Luo X, Hou X, Dessein H, Jie Z, Xin-Ling Y, He H, McManus DP, Li Y, Dessein A. Regulatory role of interleukin-10 and interferon-gamma in severe hepatic central and peripheral fibrosis in humans infected with Schistosoma japonicum. J Infect Dis. 2008;198:418–426. doi: 10.1086/588826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, Fulford AJ. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118:101–105. doi: 10.1017/s0031182098003576. [DOI] [PubMed] [Google Scholar]
- 29.Kurtis JD, Friedman JF, Leenstra T, Langdon GC, Wu HW, Manalo DL, Su L, Jiz M, Jarilla B, Pablo AO, McGarvey ST, Olveda RM, Acosta LP. Pubertal development predicts resistance to infection and reinfection with Schistosoma japonicum. Clin Infect Dis. 2006;42:1692–1698. doi: 10.1086/504326. [DOI] [PubMed] [Google Scholar]
- 30.Andrade ZA, Baptista AP, Santana TS. Remodeling of hepatic vascular changes after specific chemotherapy of schistosomal periportal fibrosis. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):267–272. doi: 10.1590/s0074-02762006000900041. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho EM, Barros AF, Barbosa A, Jr., Oliveira SA, Silva LM, Araujo RE, Andrade ZA. Host nutritional status as a contributory factor to the remodeling of schistosomal hepatic fibrosis. Mem Inst Oswaldo Cruz. 2003;98:919–925. doi: 10.1590/s0074-02762003000700011. [DOI] [PubMed] [Google Scholar]