Abstract
Q fever in French Guiana is correlated with the rainy season. We found a 1- to 2-month lagged correlation between Q fever incidence and the number of births of three-toed sloth. This result strengthens the hypothesis that the three-toed sloth is the wild reservoir of Q fever in French Guiana.
Coxiella burnetii, the agent of Q fever, is an emerging pathogen in French Guiana. It is currently responsible for 24% community-acquired pneumonia in this region.1 The epidemiology of the infection is quite unusual because most of the cases have been described in areas where no classical reservoir is found,2 leading to the hypothesis that a wild reservoir exists.3 C. burnetii is present in high concentrations in the birth products of its usual reservoirs. For this reason, in the south of France, Tissot-Dupont and others found a correlation between Q fever incidence and the mistral wind 1 month before, shortly after the onset of the main lambing season.4 We recently detected C. burnetii by quantitative polymerase chain reaction (qPCR) in spleen samples, feces, and ticks from a dead three-toed sloth found next to a Q fever patient residence in Cayenne, French Guiana.5 This result suggests that this animal could be the reservoir of Q fever in French Guiana. The seasonality of Q fever cases in Cayenne has been studied by Gardon and others,3 and they observed a lag of 1–3 months between the monthly rainfall and Q fever incidence between 1996 and 2000. In addition, the reproductive behavior of the three-toed sloth (Bradypus tridactylus)6 has been described by Taube and others who have shown that their whelping period took place during the rainy season. The aim of this study was to investigate whether the seasonality of Q fever described at the beginning of the epidemics was still present in Cayenne in recent years and whether this seasonality was correlated to the number of births of the three-toed sloth during the rainy season.
Climate data (relative humidity, rainfall, temperature, and wind) in Cayenne between 2008 and 2012 measured at three meteorological stations was collected from the official website of Meteo France. Details of all Q fever cases diagnosed during the same period in the infectious disease ward of Cayenne were retrospectively collected. The presumed dates and number of births of three-toed sloths per months between 1994 and 1995 were collected from the study by Taube and others. Correlations between meteorological data and Q fever incidence were analyzed by Almon distributed lag analysis using STATISTICA software (Statsoft Inc., Tulsa, OK). The best coefficient of correlation and the best fit were sought while varying the length of the time lag. For correlations between the number of three-toed sloth births per month for 1 year and Q fever incidence, we pooled data from all 4 years to obtain Q fever incidence by month and carried out time-series analysis7 (cross-correlations) using the R software (R Foundation for Statistical Computing, Vienna, Austria).
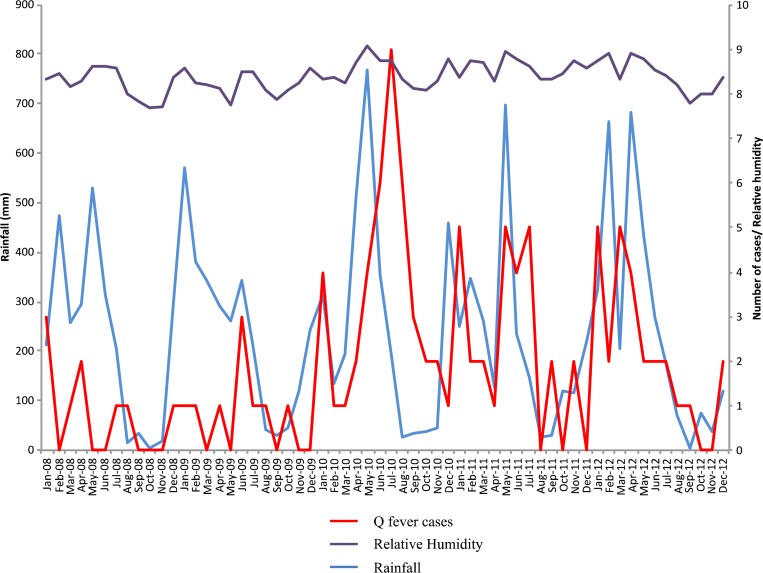
A total of 113 acute Q fever cases were reported in Cayenne between 2008 and 2012 (Figure 1) with a mean incidence of 22/100,000 inhabitants during this period. An increase in the incidence of Q fever from 5/100,000 in 2008 to 26/100,000 in 2012 was observed with a peak in 2010 (41/100,000) and specifically in July 2010 (Figure 1). When data from all years were pooled, we observed the highest incidence in January (18 cases) and July (18 cases) and a very low incidence during the month of October (3 cases), which is the end of the dry season. From 2008 to 2012, the annual rainfall increased from 2659 mm in 2008 to 3061 mm in 2012 with a peak of 768 mm observed in May 2010 (Figure 1). We observed the same trend in relative humidity values between 2008 (82.0%) and 2012 (84.0%) with a peak of 90.0% in May 2010 (Figure 1). A higher mean annual temperature (27.46°C) was observed in 2010, but the average annual temperature and wind speed remained constant between 2008 and 2012 (27°C and 2.5 m/s, respectively). The Almon distributed lag analysis showed a statistically significant positive correlation between Q fever incidence and rainfall (R2 = 0.58; t = 2.6; P = 0.01) and between Q fever incidence and relative humidity (R2 = 0.72; t = 2.6; P = 0.04) for a lag of 2 months. No correlation was observed between Q fever incidence and wind speed or Q fever incidence and temperature.
Figure 1.
Representation of monthly rainfall (mm), relative humidity (%/10), and number of Q fever cases per month between 2008 and 2012.
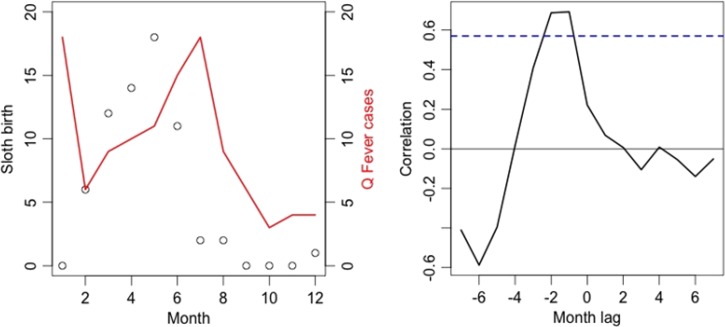
Taube and others had reported 66 presumed births of three-toed sloths in 1 year. Only one birth was observed between September and January. The number of births peaked at 18 (27%) in May and then decreased gradually to a stop in August. When the data from 5 years were pooled, we found a positive and statistically significant correlation between the number of three-toed sloths births per month and Q fever incidence per month for the observed lag of 1 and 2 months (Figure 2) with a correlation coefficient of 0.68 (P = 0.013) and 0.69 (P = 0.013), respectively.
Figure 2.
Left: representation of number of cases of Q fever per month in red (all years pooled) and number of births of the three-toed sloth per month between 1994 and 1995 (circles). Right: Graphic representation of the correlation coefficient value between the two variables at different lags (months).
Our work confirms that Q fever incidence in French Guiana exhibits seasonal variations that are correlated with the amount of monthly rainfall. High incidence is observed in July, 2 months following the peak in rainfall in May and January, following the increase in rainfall in November after the dry season (August–October). This 2 months lagged correlation is also observed with relative humidity. More interestingly, we observed a significant correlation between the 1 and 2 months lag between Q fever cases and the number of births of the three-toed sloths (Figure 2). The lag of the correlation varies between 1 and 2 months. The extreme lag of 2 months may depend on the route of transmission of C. burnetii to humans, and also be due to delays in the serological diagnosis of Q fever, for which diagnosis can only be done several weeks after the beginning of symptoms. Also, three-toed sloths live in the hills of rainforest next to houses in Cayenne, and the streaming of water after rainfall may help to bring down aerosols of C. burnetii in the plain of Cayenne where most houses are located. The limitation of our study is that in the absence of new data about the reproduction of three toed-sloths in French Guiana, we used historical data6 with the assumption that the reproductive behavior of the animal has not changed through the years. The correlation of whelping with the rainy season in three-toed sloths is supported by another study showing differences in the physical characteristics of semen of sloths between rainy and dry season, and the frequent observation of females with little young on their backs during the rainy season in Manaus, Brazil.8 This whelping seasonality is not observed in other species of sloths present in French Guiana, for example, in the two-toed sloth,6 which is the reservoir of Leishmania guyanensis.
The second peak of Q fever cases observed in January is not correlated with the observed whelping period of three-toed sloths. However, C. burnetii DNA had also been detected in ticks infesting a three-toed sloth. Further studies are needed to investigate the role of arthropods or unknown intermediate hosts in the epidemiological cycle of Q fever in French Guiana.
A few studies have been published on the seasonality of Q fever,4,9,10 and only one study has been reported from tropical environments. In Queensland, Australia, Harris and others found a clear seasonal peak of Q fever cases in May, 3 months following a peak in rainfall in February.11 The authors note that the rainfall season in this region is correlated with the time of parturient wildlife and with an increase in the marsupial population (Kangaroos and Wallabies), which are potential reservoirs of the disease. In French Guiana, the hypothesis of a wild reservoir has rapidly emerged, but large survey on wild mammals had remained negative for years.3 The recent detection of C. burnetii DNA in three-toed sloths5 and the observed seasonal correlation with their whelping period brings new support for the hypothesis that this animal is the wild reservoir of Q fever in French Guiana. Further case–control studies about exposure to three-toed sloths of Q fever patients in Cayenne are needed.
ACKNOWLEDGMENTS
We thank Jean-Louis Maridet and Thomas Beck.
Footnotes
Authors' addresses: Carole Eldin and Dider Raoult, Aix Marseille Université, URMITE UMR 7278, IRD 198, Marseille, France, E-mails: carole.eldin@gmail.com and didier.raoult@gmail.com. Aba Mahamat and Felix Djossou, Department of Infectious Diseases, Cayenne General Hospital, Cayenne, French Guiana, E-mails: mahamataba@gmail.com and felix.djossou@ch-cayenne.fr.
References
- 1.Epelboin L, Chesnais C, Boullé C, Drogoul A-S, Raoult D, Djossou F, Mahamat A. Q fever pneumonia in French Guiana: prevalence, risk factors, and prognostic score. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55:67–74. doi: 10.1093/cid/cis288. [DOI] [PubMed] [Google Scholar]
- 2.Eldin C, Mahamat A, Demar M, Abboud P, Djossou F, Raoult D. Q fever in French Guiana. Am J Trop Med Hyg. 2014;91:771–776. doi: 10.4269/ajtmh.14-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardon J, Héraud JM, Laventure S, Ladam A, Capot P, Fouquet E, Favre J, Weber S, Hommel D, Hulin A, Couratte Y, Talarmin A. Suburban transmission of Q fever in French Guiana: evidence of a wild reservoir. J Infect Dis. 2001;184:278–284. doi: 10.1086/322034. [DOI] [PubMed] [Google Scholar]
- 4.Tissot-Dupont H, Amadei M-A, Nezri M, Raoult D. Wind in November, Q fever in December. Emerg Infect Dis. 2004;10:1264–1269. doi: 10.3201/eid1007.030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davoust B, Marié J-L, Pommier de Santi V, Berenger J-M, Edouard S, Raoult D. Three-toed sloth as putative reservoir of Coxiella burnetii, Cayenne, French Guiana. Emerg Infect Dis. 2014;20:1760–1761. doi: 10.3201/eid2010.140694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taube E, Keravec J, Vié J-C, Duplantier J-M. Reproductive biology and postnatal development in sloths, Bradypus and Choloepus: review with original data from the field (French Guiana) and from captivity. Mammal Rev. 2001;31:173–188. [Google Scholar]
- 7.Mahamat A, Dussart P, Bouix A, Carvalho L, Eltges F, Matheus S, Miller MA, Quenel P, Viboud C. Climatic drivers of seasonal influenza epidemics in French Guiana, 2006–2010. J Infect. 2013;67:141–147. doi: 10.1016/j.jinf.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peres MA, Benetti EJ, Milazzotto MP, Visintin JA, Miglino MA, Assumpção MEOA. Collection and evaluation of semen from the three-toed sloth (Bradypus tridactylus) Tissue Cell. 2008;40:325–331. doi: 10.1016/j.tice.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Punda-Polić V, Luksić B, Capkun V. Epidemiological features of Mediterranean spotted fever, murine typhus, and Q fever in Split-Dalmatia County (Croatia), 1982–2002. Epidemiol Infect. 2008;136:972–979. doi: 10.1017/S0950268807009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellenbrand W, Breuer T, Petersen L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg Infect Dis. 2001;7:789–796. doi: 10.3201/eid0705.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris P, Eales KM, Squires R, Govan B, Norton R. Acute Q fever in northern Queensland: variation in incidence related to rainfall and geographical location. Epidemiol Infect. 2013;141:1034–1038. doi: 10.1017/S0950268812001495. [DOI] [PMC free article] [PubMed] [Google Scholar]